Cryopreserved Human Natural Killer Cells Exhibit Potent Antitumor Efficacy against Orthotopic Pancreatic Cancer through Efficient Tumor-Homing and Cytolytic Ability
Abstract
:1. Introduction
2. Results
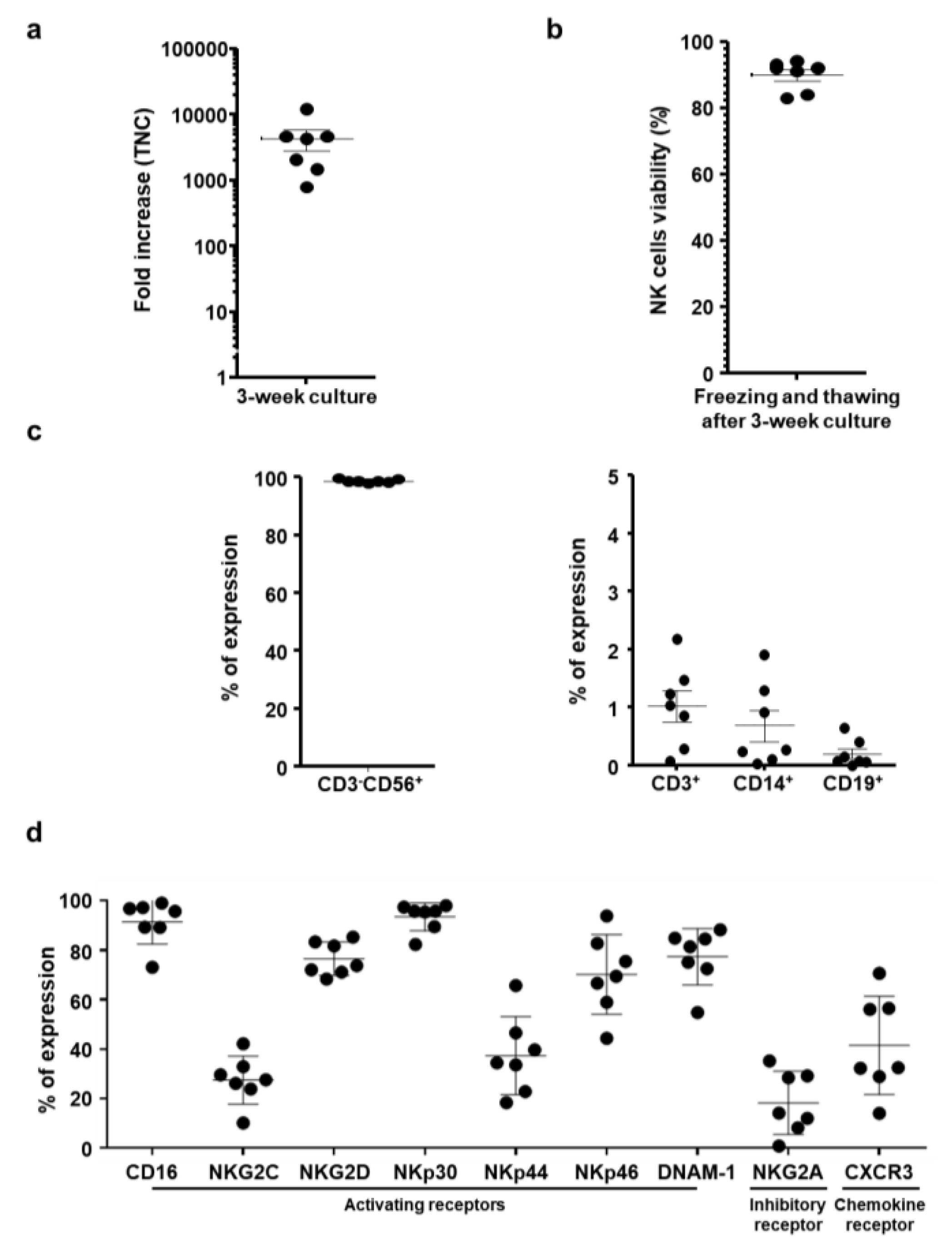
2.1. Characterization of Ex Vivo-Large-Scale Expanded and Frozen NK cells
2.2. Characteristic Changes in NK Cells Co-Cultured with Cancer Cells
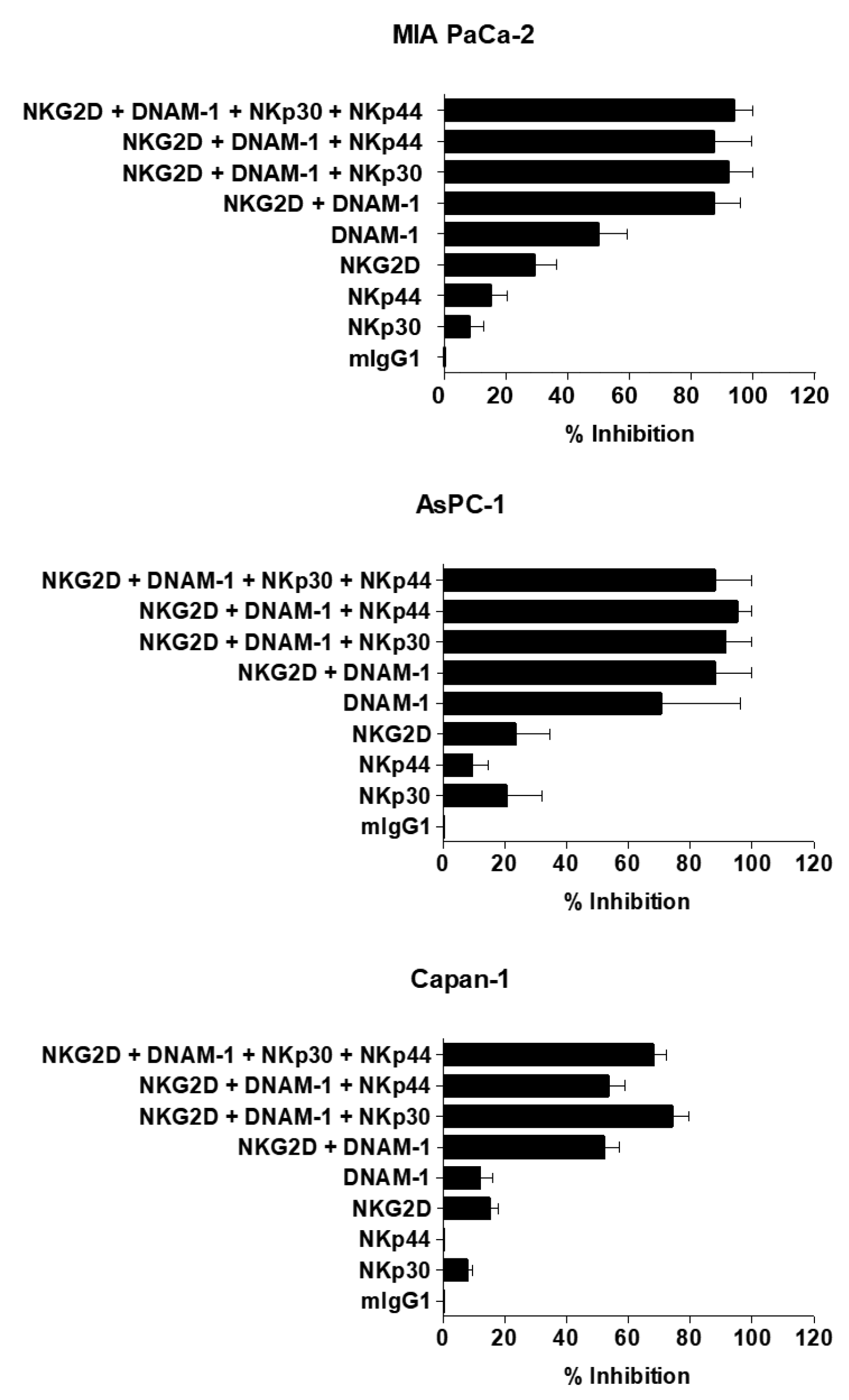
2.3. Cytotoxic Effect of NK Cells against Human Pancreatic Cancer Cell Lines
2.4. Potent Induction of Apoptotic Cancer Cell Death by NK Cells
2.5. Therapeutic Efficacy of NK Cells in an Orthotopic Pancreatic Tumor Model
2.6. Increased IFN-γ Expression and Decreased TGF-β Expression in Tumor Tissues Following NK Cell Treatment
2.7. Histological and Immunohistological Analyses of Orthotopic Pancreatic Tumors Treated with NK Cells
2.8. Biodistribution Profile of Systemically Administered NK Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Ex Vivo Expansion and Cryopreservation of NK Cells
4.3. Cell Culture
4.4. Immunostaining and Flow Cytometric Analysis
4.5. Intracellular Cytokines and CD107a Staining
4.6. Calcein-AM Release Cytotoxicity Assay
4.7. MTT Assay
4.8. Apoptosis Analysis by Flow Cytometer
4.9. In Vivo Antitumor Efficacy and Bioluminescence Imaging
4.10. Quantification of IFN-γ and TGF-β Expression
4.11. Histological and Immunohistochemical Analysis
4.12. Assessment of NK Cell Biodistribution
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Allemani, C.; Santaquilani, M.; Knijn, A.; Marchesi, F.; Capocaccia, R.; EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur. J. Cancer 2009, 45, 931–991. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Paulson, A.S.; Tran Cao, H.S.; Tempero, M.A.; Lowy, A.M. Therapeutic advances in pancreatic cancer. Gastroenterology 2013, 144, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ramanathan, R.K.; Borad, M.J.; Laheru, D.A.; Smith, L.S.; Wood, T.E.; Korn, R.L.; Desai, N.; Trieu, V.; Iglesias, J.L.; et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J. Clin. Oncol. 2011, 29, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef]
- Sideras, K.; Braat, H.; Kwekkeboom, J.; van Eijck, C.H.; Peppelenbosch, M.P.; Sleijfer, S.; Bruno, M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat. Rev. 2014, 40, 513–522. [Google Scholar] [CrossRef]
- Lutz, E.; Yeo, C.J.; Lillemoe, K.D.; Biedrzycki, B.; Kobrin, B.; Herman, J.; Sugar, E.; Piantadosi, S.; Cameron, J.L.; Solt, S.; et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011, 253, 328–335. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Chang, J.H.; Jiang, Y.; Pillarisetty, V.G. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine 2016, 95, e5541. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Conlon, K.; Bohac, G.C.; Barcenas, J.; Leslie, W.; Watkins, L.; Lamzabi, I.; Deng, Y.; Li, Y.; Plate, J.M. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J. Immunother. 2012, 35, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lanier, L.L. Natural killer cells and cancer. Adv. Cancer Res. 2003, 90, 127–156. [Google Scholar] [PubMed]
- Robertson, M.J.; Ritz, J. Biology and clinical relevance of human natural killer cells. Blood 1990, 76, 2421–2438. [Google Scholar] [PubMed]
- Chan, J.K.; Hamilton, C.A.; Cheung, M.K.; Karimi, M.; Baker, J.; Gall, J.M.; Schulz, S.; Thorne, S.H.; Teng, N.N.; Contag, C.H.; et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: A preclinical study. Clin. Cancer. Res. 2006, 12, 1859–1867. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339. [Google Scholar] [CrossRef]
- Margolin, K.A. Interleukin-2 in the treatment of renal cancer. Semin. Oncol. 2000, 27, 194–203. [Google Scholar]
- Rosenberg, S.A.; Lotze, M.T.; Yang, J.C.; Topalian, S.L.; Chang, A.E.; Schwartzentruber, D.J.; Aebersold, P.; Leitman, S.; Linehan, W.M.; Seipp, C.A.; et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl. Cancer Inst. 1993, 85, 622–632. [Google Scholar] [CrossRef]
- Semino, C.; Martini, L.; Queirolo, P.; Cangemi, G.; Costa, R.; Alloisio, A.; Ferlazzo, G.; Sertoli, M.R.; Reali, U.M.; Ratto, G.B.; et al. Adoptive immunotherapy of advanced solid tumors: An eight year clinical experience. Anticancer Res. 1999, 19, 5645–5649. [Google Scholar]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Raulet, D.H.; Held, W. Natural killer cell receptors: The offs and ons of NK cell recognition. Cell 1995, 82, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Ljunggren, H.G.; Karre, K. In search of the ’missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Farag, S.S.; Fehniger, T.; Ruggeri, L.; Velardi, A.; Caligiuri, M.A. Natural killer cells: Biology and application in stem-cell transplantation. Cytotherapy 2002, 4, 445–446. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T.; et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef]
- Law, T.M.; Motzer, R.J.; Mazumdar, M.; Sell, K.W.; Walther, P.J.; O’Connell, M.; Khan, A.; Vlamis, V.; Vogelzang, N.J.; Bajorin, D.F. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995, 76, 824–832. [Google Scholar] [CrossRef]
- Burns, L.J.; Weisdorf, D.J.; DeFor, T.E.; Vesole, D.H.; Repka, T.L.; Blazar, B.R.; Burger, S.R.; Panoskaltsis-Mortari, A.; Keever-Taylor, C.A.; Zhang, M.J.; et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: A phase I/II trial. Bone Marrow Transplant. 2003, 32, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 1999, 17, 875–904. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, L.; Mancusi, A.; Capanni, M.; Martelli, M.F.; Velardi, A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr. Opin. Immunol. 2005, 17, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bernson, E.; Hallner, A.; Sander, F.E.; Wilsson, O.; Werlenius, O.; Rydstrom, A.; Kiffin, R.; Brune, M.; Foa, R.; Aurelius, J.; et al. Impact of killer-immunoglobulin-like receptor and human leukocyte antigen genotypes on the efficacy of immunotherapy in acute myeloid leukemia. Leukemia 2017, 31, 2552–2559. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.A.; Denman, C.J.; Rondon, G.; Woodworth, G.; Chen, J.; Fisher, T.; Kaur, I.; Fernandez-Vina, M.; Cao, K.; Ciurea, S.; et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: A phase i trial. Biol. Blood Marrow Transplant. 2016, 22, 1290–1298. [Google Scholar] [CrossRef]
- Bao, X.; Wang, M.; Zhou, H.; Zhang, H.; Wu, X.; Yuan, X.; Li, Y.; Wu, D.; He, J. Donor killer immunoglobulin-Like receptor profile bx1 imparts a negative effect and centromeric B-specific gene motifs render a positive effect on standard-risk Acute Myeloid leukemia/myelodysplastic syndrome patient survival after unrelated donor hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2016, 22, 232–239. [Google Scholar] [CrossRef]
- Impola, U.; Turpeinen, H.; Alakulppi, N.; Linjama, T.; Volin, L.; Niittyvuopio, R.; Partanen, J.; Koskela, S. Donor haplotype B of NK KIR receptor reduces the relapse risk in hla-identical sibling hematopoietic stem cell transplantation of AML patients. Front. Immunol. 2014, 5, 405. [Google Scholar] [CrossRef]
- Domogala, A.; Madrigal, J.A.; Saudemont, A. Cryopreservation has no effect on function of natural killer cells differentiated in vitro from umbilical cord blood CD3(+) cells. Cytotherapy 2016, 18, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Koehl, U.; Brehm, C.; Huenecke, S.; Zimmermann, S.Y.; Kloess, S.; Bremm, M.; Ullrich, E.; Soerensen, J.; Quaiser, A.; Erben, S.; et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front. Immunol. 2013, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, N.; Durett, A.G.; Sun, J.; Rollins, L.A.; Huye, L.L.; Fang, J.; Dandekar, V.; Mei, Z.; Jackson, K.; Vera, J.; et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012, 14, 1131–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, O.; Lee, Y.; Chung, H.; Her, J.H.; Kang, S.M.; Jung, M.Y.; Min, B.; Shin, H.; Kim, T.M.; Heo, D.S.; et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e53611. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.A.; Basse, P.H.; Hokland, M.; Goldfarb, R.H.; Nagelkerke, J.F.; Nannmark, U.; Kuppen, P.J. NK cells and the tumour microenvironment: Implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003, 24, 603–609. [Google Scholar] [CrossRef]
- Farag, S.S.; Fehniger, T.A.; Becknell, B.; Blaser, B.W.; Caligiuri, M.A. New directions in natural killer cell-based immunotherapy of human cancer. Expert Opin. Biol. Ther. 2003, 3, 237–250. [Google Scholar] [CrossRef]
- Bakker, A.B.; Wu, J.; Phillips, J.H.; Lanier, L.L. NK cell activation: Distinct stimulatory pathways counterbalancing inhibitory signals. Hum. Immunol. 2000, 61, 18–27. [Google Scholar] [CrossRef]
- Lanier, L.L. Follow the leader: NK cell receptors for classical and nonclassical MHC class I. Cell 1998, 92, 705–707. [Google Scholar] [CrossRef]
- Fei, X.F.; Zhang, Q.B.; Dong, J.; Diao, Y.; Wang, Z.M.; Li, R.J.; Wu, Z.C.; Wang, A.D.; Lan, Q.; Zhang, S.M.; et al. Development of clinically relevant orthotopic xenograft mouse model of metastatic lung cancer and glioblastoma through surgical tumor tissues injection with trocar. J. Exp. Clin. Cancer Res. CR 2010, 29, 84. [Google Scholar] [CrossRef]
- Weber, H.L.; Gidekel, M.; Werbajh, S.; Salvatierra, E.; Rotondaro, C.; Sganga, L.; Haab, G.A.; Curiel, D.T.; Cafferata, E.G.; Podhajcer, O.L. A Novel CDC25B Promoter-Based Oncolytic Adenovirus Inhibited Growth of Orthotopic Human Pancreatic Tumors in Different Preclinical Models. Clin. cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 1665–1674. [Google Scholar] [CrossRef]
- Dent, S.; Messersmith, H.; Trudeau, M. Gemcitabine in the management of metastatic breast cancer: A systematic review. Breast Cancer Res. Treat. 2008, 108, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ng, K.Y.; Lillehei, K.O. Cell-mediated immunotherapy: A new approach to the treatment of malignant glioma. Cancer Control 2003, 10, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713–758. [Google Scholar] [CrossRef] [PubMed]
- Dominiecki, M.E.; Beatty, G.L.; Pan, Z.K.; Neeson, P.; Paterson, Y. Tumor sensitivity to IFN- γ is required for successful antigen-specific immunotherapy of a transplantable mouse tumor model for HPV-transformed tumors. Cancer Immunol. Immunother. 2005, 54, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Foon, K.A.; Sherwin, S.A.; Abrams, P.G.; Stevenson, H.C.; Holmes, P.; Maluish, A.E.; Oldham, R.K.; Herberman, R.B. A phase I trial of recombinant γ interferon in patients with cancer. Cancer Immunol. Immunother. 1985, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Storer, B.E.; Witt, P.L.; Alberti, D.; Tombes, M.B.; Arzoomanian, R.; Proctor, R.A.; McCarthy, D.; Brown, R.R.; Voss, S.D.; et al. Biological and clinical effects of intravenous tumor necrosis factor-alpha administered three times weekly. Cancer Res. 1991, 51, 1651–1658. [Google Scholar] [PubMed]
- Conti, F.; Dousset, B.; Coste, J.; Rosinski, M.; Cherruau, B.; Soubrane, O.; Houssin, D.; Calmus, Y. Correlation between daily cyclosporine dose and allograft injury in liver recipients with and without recurrent hepatitis C. Eur. J. Int. Med. 2003, 14, 185–191. [Google Scholar] [CrossRef]
- Stone, R. Sciencescope. Science 1995, 267, 443. [Google Scholar] [CrossRef]
- Pinkas, J.; Teicher, B.A. TGF-beta in cancer and as a therapeutic target. Biochem. Pharmacol. 2006, 72, 523–529. [Google Scholar] [CrossRef]
- Reiss, M. TGF-beta and cancer. Microbes Infect. Inst. Pasteur 1999, 1, 1327–1347. [Google Scholar] [CrossRef]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limon, P. The polarization of immune cells in the tumour environment by TGF β. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Villegas, F.R.; Coca, S.; Villarrubia, V.G.; Jimenez, R.; Chillon, M.J.; Jareno, J.; Zuil, M.; Callol, L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002, 35, 23–28. [Google Scholar] [CrossRef]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Che, X.; Iwashige, H.; Aridome, K.; Hokita, S.; Aikou, T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000, 88, 577–583. [Google Scholar] [CrossRef]
- Coca, S.; Perez-Piqueras, J.; Martinez, D.; Colmenarejo, A.; Saez, M.A.; Vallejo, C.; Martos, J.A.; Moreno, M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997, 79, 2320–2328. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Syme, R.M.; Bryan, T.L.; Gluck, S. Dendritic cell-based therapy: A review focusing on antigenic selection. J. Hematother. Stem Cell Res. 2001, 10, 601–608. [Google Scholar] [CrossRef]
- Gattinoni, L.; Powell, D.J., Jr.; Rosenberg, S.A.; Restifo, N.P. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. Immunol. 2006, 6, 383–393. [Google Scholar] [CrossRef]
- Grimm, E.A.; Mazumder, A.; Zhang, H.Z.; Rosenberg, S.A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 1982, 155, 1823–1841. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.; Lefterova, P.; Mehta, B.A.; Fernandez, L.P.; Huhn, D.; Blume, K.G.; Weissman, I.L.; Negrin, R.S. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp. Hematol. 1993, 21, 1673–1679. [Google Scholar]
- Sutlu, T.; Alici, E. Natural killer cell-based immunotherapy in cancer: Current insights and future prospects. J. Intern. Med. 2009, 266, 154–181. [Google Scholar] [CrossRef]
- Klingemann, H. Challenges of cancer therapy with natural killer cells. Cytotherapy 2015, 17, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Szmania, S.; Lapteva, N.; Garg, T.; Greenway, A.; Lingo, J.; Nair, B.; Stone, K.; Woods, E.; Khan, J.; Stivers, J.; et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J. Immunother. 2015, 38, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Putz, E.M.; Grundschober, E.; Prchal-Murphy, M.; Straka, E.; Kudweis, P.; Heller, G.; Bago-Horvath, Z.; Witalisz-Siepracka, A.; Cumaraswamy, A.A.; et al. STAT5 Is a key regulator in NK cells and acts as a molecular switch from tumor surveillance to tumor promotion. Cancer Discov. 2016, 6, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; Lundqvist, A. Targeting the tumor microenvironment to improve natural killer cell-based immunotherapies: On being in the right place at the right time, with resilience. Hum. Vaccines Immunother. 2016, 12, 607–611. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef]
- Campana, S.; De Pasquale, C.; Carrega, P.; Ferlazzo, G.; Bonaccorsi, I. Cross-dressing: An alternative mechanism for antigen presentation. Immunol. Lett. 2015, 168, 349–354. [Google Scholar] [CrossRef]
- Joly, E.; Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003, 4, 815. [Google Scholar] [CrossRef]
- Linsley, P.S.; Bradshaw, J.; Urnes, M.; Grosmaire, L.; Ledbetter, J.A. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J. Immunol. 1993, 150, 3161–3169. [Google Scholar]
- Valitutti, S.; Muller, S.; Salio, M.; Lanzavecchia, A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J. Exp. Med. 1997, 185, 1859–1864. [Google Scholar] [CrossRef]
- Huard, B.; Karlsson, L. KIR expression on self-reactive CD8+ T cells is controlled by T-cell receptor engagement. Nature 2000, 403, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroza-Pacheco, I.; Madrigal, A.; Saudemont, A. Interaction between natural killer cells and regulatory T cells: Perspectives for immunotherapy. Cell. Mol. Immunology 2013, 10, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Su, Z.Z.; Vozhilla, N.; Park, E.S.; Randolph, A.; Valerie, K.; Fisher, P.B. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005, 65, 9056–9063. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.L. NK cells mediate the anti-tumor effects of E1-deleted, type 5 adenovirus in a human tumor xenograft model. Oncol. Rep. 2000, 7, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Kapoor, V.; Jassar, A.S.; Kaiser, L.R.; Albelda, S.M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 6713–6721. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Kremer, V.; Childs, R.; Lundqvist, A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol. Immunother. 2015, 64, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Galani, I.E.; Suri-Payer, E.; Cerwenka, A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008, 68, 8437–8445. [Google Scholar] [CrossRef]
- Lavergne, E.; Combadiere, B.; Bonduelle, O.; Iga, M.; Gao, J.L.; Maho, M.; Boissonnas, A.; Murphy, P.M.; Debre, P.; Combadiere, C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003, 63, 7468–7474. [Google Scholar]
- Min, B.; Choi, H.; Her, J.H.; Jung, M.Y.; Kim, H.J.; Jung, M.Y.; Lee, E.K.; Cho, S.Y.; Hwang, Y.K.; Shin, E.C. Optimization of large-scale expansion and cryopreservation of human natural Killer cells for anti-tumor therapy. Immune Netw. 2018, 18, e31. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, E.; Min, B.; Li, Y.; Lian, C.; Hong, J.; Park, G.-m.; Yang, B.; Cho, S.Y.; Hwang, Y.K.; Yun, C.-O. Cryopreserved Human Natural Killer Cells Exhibit Potent Antitumor Efficacy against Orthotopic Pancreatic Cancer through Efficient Tumor-Homing and Cytolytic Ability. Cancers 2019, 11, 966. https://doi.org/10.3390/cancers11070966
Oh E, Min B, Li Y, Lian C, Hong J, Park G-m, Yang B, Cho SY, Hwang YK, Yun C-O. Cryopreserved Human Natural Killer Cells Exhibit Potent Antitumor Efficacy against Orthotopic Pancreatic Cancer through Efficient Tumor-Homing and Cytolytic Ability. Cancers. 2019; 11(7):966. https://doi.org/10.3390/cancers11070966
Chicago/Turabian StyleOh, Eonju, Bokyung Min, Yan Li, ChunYing Lian, JinWoo Hong, Gyeong-min Park, Bitna Yang, Sung Yoo Cho, Yu Kyeong Hwang, and Chae-Ok Yun. 2019. "Cryopreserved Human Natural Killer Cells Exhibit Potent Antitumor Efficacy against Orthotopic Pancreatic Cancer through Efficient Tumor-Homing and Cytolytic Ability" Cancers 11, no. 7: 966. https://doi.org/10.3390/cancers11070966
APA StyleOh, E., Min, B., Li, Y., Lian, C., Hong, J., Park, G.-m., Yang, B., Cho, S. Y., Hwang, Y. K., & Yun, C.-O. (2019). Cryopreserved Human Natural Killer Cells Exhibit Potent Antitumor Efficacy against Orthotopic Pancreatic Cancer through Efficient Tumor-Homing and Cytolytic Ability. Cancers, 11(7), 966. https://doi.org/10.3390/cancers11070966





