The Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment
Abstract
1. Introduction
2. Results
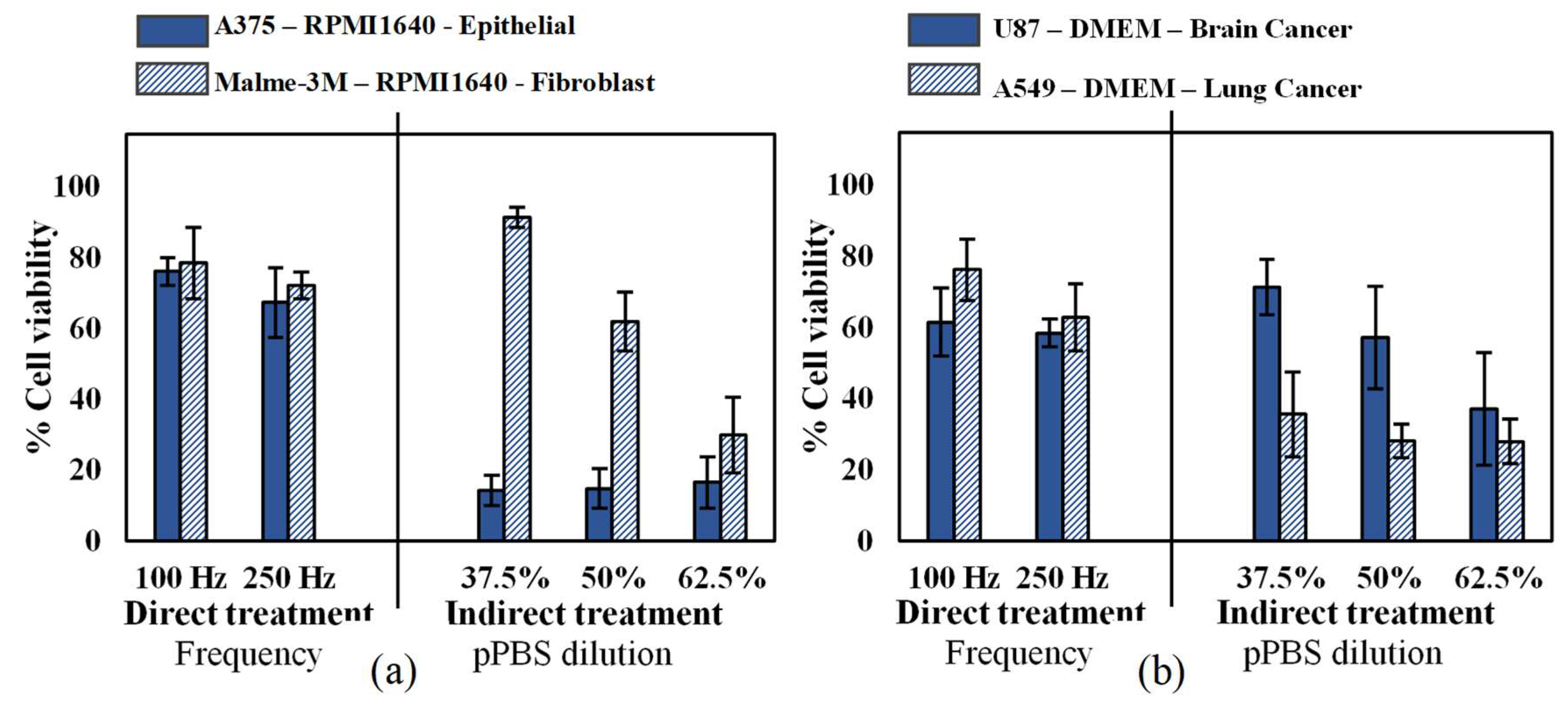
2.1. Influence of Cell Type and Cancer Type on Cell Viability
2.2. Influence of Cell Culture Media on Cell Viability
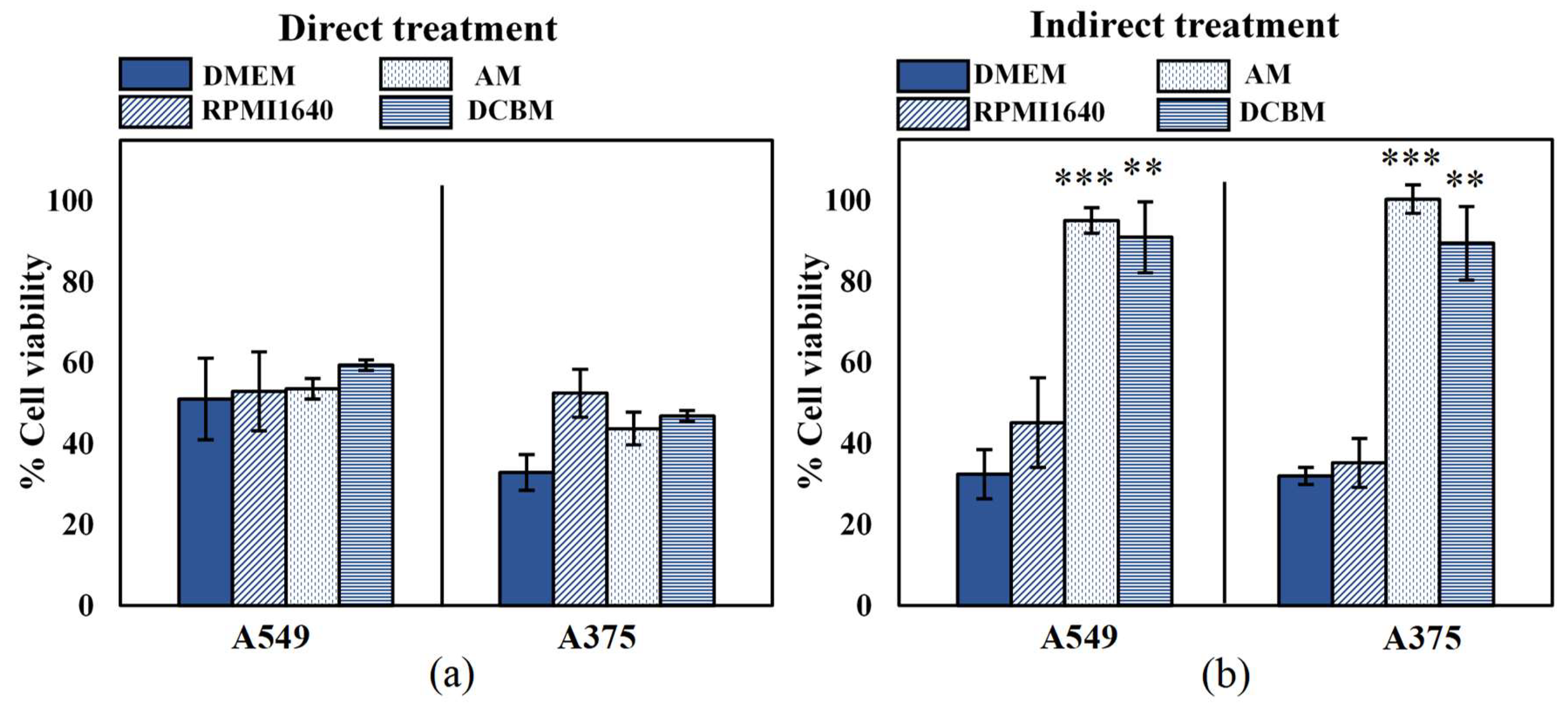
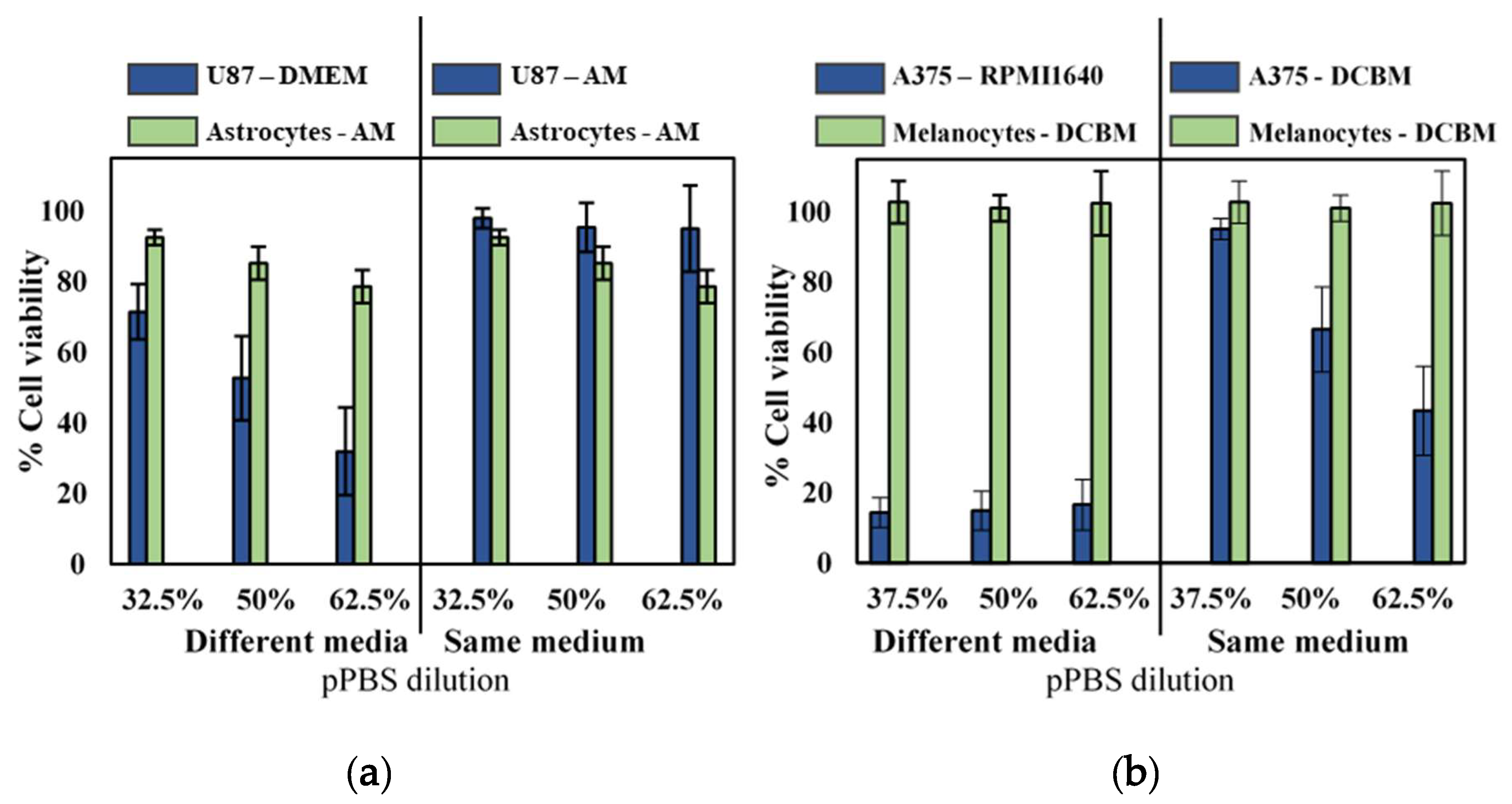
2.3. Influence of Cell Culture Media on Selectivity Evaluation of Indirect CAP Treatment
2.4. Influence of Cell Culture Media on Selectivity Evaluation of Direct CAP Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Plating
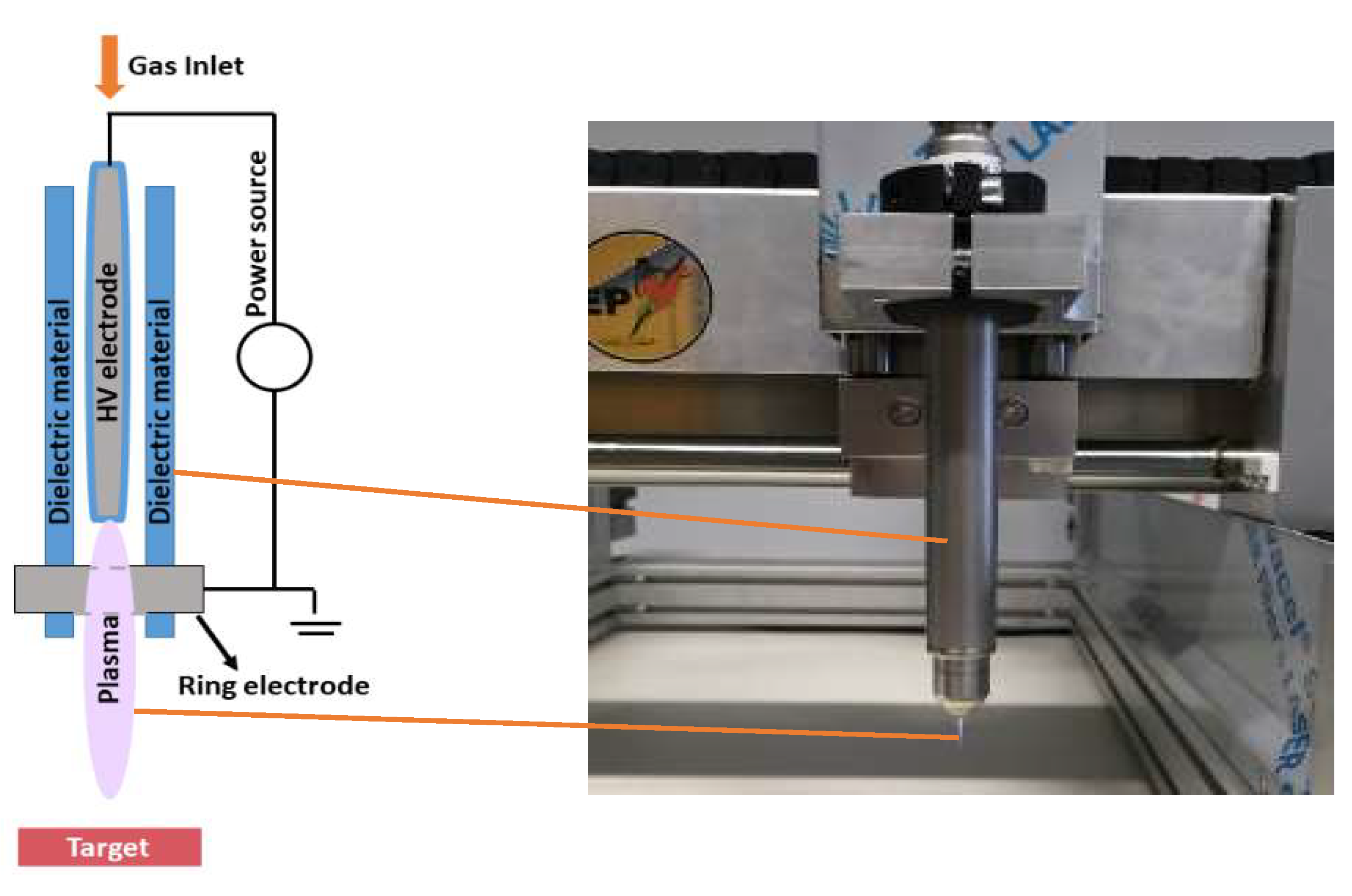
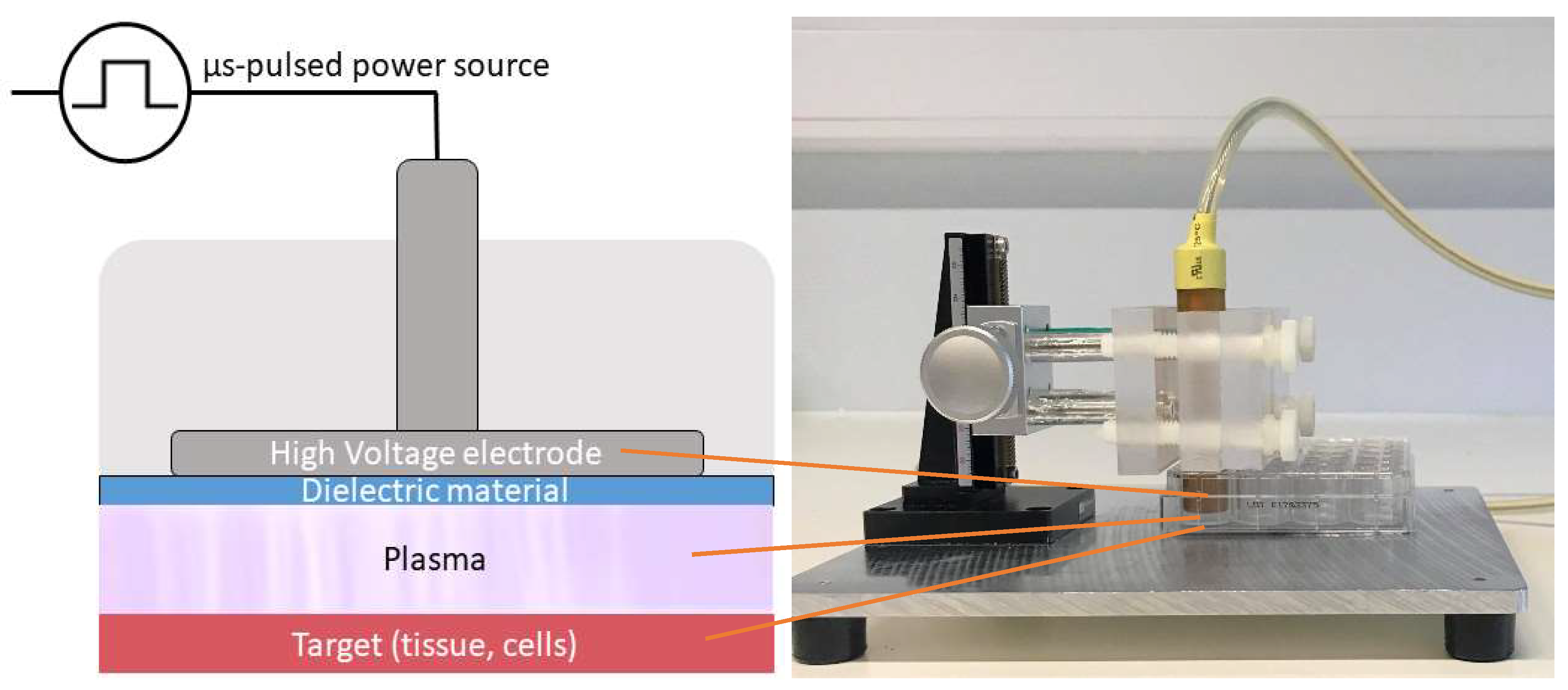
4.2. Plasma Sources
4.3. Indirect Plasma Treatment
4.4. Direct Plasma Treatment
4.5. Cell Viability Assay
4.6. Analysis of the Influence of the Cell Culture Medium
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of Production and Application in Dentistry and Oncology. Med. Gas Res. 2013, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, W.; Li, H.; Wang, X.; Lv, G.; Khohsa, M.L.; Guo, M.; Feng, K.; Wang, P.; Yang, S. Needle Deactivation of A549 Cancer Cells in Vitro by a Dielectric Barrier Discharge Plasma Needle. J. Appl. Phys. 2011, 109, 053305. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, T.H.; Bae, S.H.; Leem, S.H. Induction of Apoptosis in Human Breast Cancer Cells by a Pulsed Atmospheric Pressure Plasma Jet. Appl. Phys. Lett. 2010, 97, 203702. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of Plasma Treatment on Pancreatic Cancer Cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Plasma-Activated Medium Selectively Kills Glioblastoma Brain Tumor Cells by Down-Regulating a Survival Signaling Molecule, AKT Kinase. Plasma Med. 2011, 1, 265–277. [Google Scholar] [CrossRef]
- Yonson, S.; Coulombe, S.; Leveille, V.; Leask, R.L. Cell Treatment and Surface Functionalization Using a Miniature Atmospheric Pressure Glow Discharge. J. Phys. D. Appl. Phys. 2006, 39, 3508–3513. [Google Scholar] [CrossRef]
- Leduc, M.; Guay, D.; Leask, R.L.; Coulombe, S. Cell Permeabilization Using a Non-Thermal Plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Vermeylen, S.; De Waele, J.; Vanuytsel, S.; De Backer, J.; Ramakers, M.; Leyssens, K.; Marcq, E.; Van Der Paal, J.; Smits, E.L.J.; Dewilde, S.; et al. Cold Atmospheric Plasma Treatment of Melanoma and Glioblastoma Cancer Cells. Plasma Process. Polym. 2016, 13, 1195–1205. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.M. Response of Human Glioma U87 Xenografted on Mice to Non Thermal Plasma Treatment. Plasma Med. 2011, 1, 27–43. [Google Scholar] [CrossRef]
- Vandamme, M.; Rie, D.; Martel, E.; Robert, E.; Brulle, L.; Richard, S.; Pouvesle, J.; Pape, A.L. Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-Luc Orthotopic Pancreatic Carcinoma Model. PLoS ONE 2012, 7, e52653. [Google Scholar]
- Metelmann, H.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical Experience with Cold Plasma in the Treatment of Locally Advanced Head and Neck Cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Friedman, P.C.; Miller, V.; Fridman, G.; Lin, A.; Fridman, A. Successful Treatment of Actinic Keratoses Using Nonthermal Atmospheric Pressure Plasma: A Case Series. J. Am. Acad. Dermatol. 2017, 76, 349–350. [Google Scholar] [CrossRef]
- Miller, V.; Lin, A.; Fridman, G.; Fridman, A.; Friedman, P. Nanosecond-Pulsed DBD Plasma For A Clinical Trial Of Actinic Keratosis. Clin. Plasma Med. 2018, 9, 44. [Google Scholar] [CrossRef]
- Fridman, A.; Friedman, G. Plasma Medicine; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.; Laurencin-dalicieux, S.; Cousty, S. Use of Cold-Atmospheric Plasma in Oncology: A Concise Systematic Review. Ther. Adv. Med. Oncol. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-Cancer Therapies of 21st Century: Novel Approach to Treat Human Cancers Using Cold Atmospheric Plasma. Plasma Process. Polym. 2014, 11, 1128–1137. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for Cancer Treatment. Plasma Sources Sci. Technol. 2015, 24, 1–20. [Google Scholar] [CrossRef]
- Maksudbek, Y.; Dayun, Y.; Cordeiro, R.M.; Bogaerts, A. Atomic Scale Simulation of H2O2 Permeation through Aquaporin: Toward the Understanding of Plasma-Cancer Treatment. J. Phys. D. Appl. Phys. 2018, 12, 125401. [Google Scholar]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The Role of Aquaporins in the Anti- Glioblastoma Capacity of the Cold Plasma-Stimulated Medium. J. Phys. D. Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Van Der Paal, J.; Verheyen, C.; Neyts, E.C.; Bogaerts, A. Hampering Effect of Cholesterol on the Permeation of Reactive Oxygen Species through Phospholipids Bilayer: Possible Explanation for Plasma Cancer Selectivity. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Van Der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Chemical Science Permeability of Cancer and Normal Cells Subjected to Oxidative Stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. Cold Atmospheric Plasma and Plasma-Activated Medium: Antitumor Cell Effects with Inherent Synergistic Potential. Plasma Med. 2019, 9, 57–88. [Google Scholar] [CrossRef]
- Zucker, S.N.; Zirnheld, J.; Bagati, A.; Disanto, T.M.; Des Soye, B.; Wawrzyniak, J.A.; Etemadi, K.; Nikiforov, M.; Berezney, R. Preferential Induction of Apoptotic Cell Death in Melanoma Cells as Compared with Normal Keratinocytes Using a Non-Thermal Plasma Torch. Cancer Biol. Ther. 2012, 13, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H. Cold Atmospheric Plasma Jet-Generated RONS and Their Selective Effects on Normal and Carcinoma Cells. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold Atmospheric Plasma for Selectively Ablating Metastatic Breast Cancer Cells. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold Atmospheric Plasma Treatment Selectively Targets Head and Neck Squamous Cell Carcinoma Cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, N.; Lupu, A.R. Tumoral and Normal Cells Treatment With High-Voltage Pulsed Cold Atmospheric Plasma Jets. IEEE Trans. Plasma Sci. 2010, 38, 1949–1955. [Google Scholar] [CrossRef]
- Yan, D.; Nourmohammadi, N.; Bian, K.; Murad, F.; Sherman, J.H.; Keidar, M. Stabilizing the Cold Plasma-Stimulated Medium by Regulating Medium’ s Composition. Sci. Rep. 2016, 6, 26016. [Google Scholar] [CrossRef]
- Heirman, P.; Van Boxem, W.; Bogaerts, A. Reactivity and Stability of Plasma-Generated Oxygen and Nitrogen Species in Buffered Water Solution: A Computational Study. Phys. Chem. Chem. Phys. 2019. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, W.; Van Der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-Cancer Capacity of Plasma-Treated PBS: Effect of Chemical Composition on Cancer Cell Cytotoxicity. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.; Wei, Y.; Li, J. A Flexible Cold Microplasma Jet Using Biocompatible Dielectric Tubes for Cancer Therapy A Flexible Cold Microplasma Jet Using Biocompatible Dielectric Tubes. Appl. Phys. Lett. 2010, 96, 203701. [Google Scholar] [CrossRef]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective Killing of Ovarian Cancer Cells through Induction of Apoptosis by Nonequilibrium Atmospheric Pressure Plasma Selective Killing of Ovarian Cancer Cells through Induction of Apoptosis by Nonequilibrium Atmospheric Pressure Plasma. Appl. Phys. Lett. 2012, 100, 113702. [Google Scholar] [CrossRef]
- Laroussi, M. Effects of PAM on Select Normal and Cancerous Epithelial Cells. Plasma Res. Express 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Velculescu, V.E.; Kern, S.E.; Hruban, R.H.; Hamilton, S.R.; Vogelstein, B.; Kinzler, K.W. Gene Expression Profiles in Normal and Cancer Cells. Science 1997, 276, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.; Abdollahifar, M.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect Cold Atmospheric-Pressure Plasma Methods in the B 16 F 10 Melanoma Cancer Cells Treatment. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Giordano, T.J.; Shedden, K.A.; Schwartz, D.R.; Kuick, R.; Taylor, J.M.G.; Lee, N.; Misek, D.E.; Greenson, J.K.; Kardia, S.L.R.; Beer, D.G.; et al. Organ-Specific Molecular Classification of Primary Lung, Colon, and Ovarian Adenocarcinomas Using Gene Expression Profiles. Am. J. Pathol. 2001, 159, 1231–1238. [Google Scholar] [CrossRef]
- Mallinjoud, P.; Villemin, J.; Mortada, H.; Espinoza, M.P.; Desmet, F.-O.; Samaan, S.; Chautard, E.; Tranchevent, L.-C.; Auboeuf, D. Endothelial, Epithelial, and Fibroblast Cells Exhibit Specific Splicing Programs Independently of Their Tissue of Origin. Genome Res. 2014, 24, 511–521. [Google Scholar] [CrossRef]
- Jagtap, J.C.; Chandele, A.; Chopde, B.A.; Shastry, P. Sodium Pyruvate Protects against H2O2 Mediated Apoptosis in Human Neuroblastoma Cell Line-SK-N-MC. J. Chem. Neuroanat. 2003, 26, 109–118. [Google Scholar] [CrossRef]
- Wang, X.; Perez, E.; Liu, R.; Yan, L.; Mallet, R.T.; Yang, S. Pyruvate Protects Mitochondria from Oxidative Stress in Human Neuroblastoma SK-N-SH Cells. Brain Res. 2007, 1132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Astrocyte Medium—Sciencell. Available online: https://www.sciencellonline.com/astrocyte-medium.html, (accessed on 20 August 2019).
- BEGMTM Bronchial Epithelial Cell Growth Medium BulletKitTM. Available online: https://bioscience.lonza.com/lonza_bs/CH/en/Primary-and-Stem-Cells/p/000000000000185308/BEGM-Bronchial-Epithelial-Cell-Growth-Medium-BulletKit, (accessed on 20 August 2019).
- Dermal Cell Basal Medium(ATCC® PCS-200-030™). Available online: https://www.lgcstandards-atcc.org/products/all/PCS-200-030.aspx?geo_country=be, (accessed on 20 August 2019).
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B.; Mammalian, P.; Exhibit, C.; Metabolism, A. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of Glucose Metabolization in Cancer Cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. 2011, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Redza-dutordoir, M.; Averill-bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochem. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Shaw, A.T.; Winslow, M.M.; Magendantz, M.; Ouyang, C.; Dowdle, J. Selective Killing of K-Ras Mutant Cancer Cells by Small Molecule Inducers of Oxidative Stress. PNAS. 2011, 108, 8773–8778. [Google Scholar] [CrossRef]
- Glasauer, A.; Sena, L.A.; Diebold, L.P.; Mazar, A.P.; Chandel, N.S. Targeting SOD1 Reduces Experimental Non–Small-Cell Lung Cancer. J. Clin. Investig. 2014, 124, 117–128. [Google Scholar] [CrossRef]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Chernets, N.; Han, J.; Alicea, Y.; Dobrynin, D.; Fridman, G.; Freeman, T.A.; Fridman, A.; Miller, V. Non-Equilibrium Dielectric Barrier Discharge Treatment of Mesenchymal Stem Cells: Charges and Reactive Oxygen Species Play the Major Role in Cell Death A. Plasma Process. Polym. 2015, 12, 1117–1127. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell Lines, Valuable Tools or Useless Artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Seebauer, C.; Wende, K.; Schmidt, A.; Metelmann, H.; Von Woedtke, T.; Bekeschus, S. Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study. Appl. Sci. 2019, 9, 2061. [Google Scholar] [CrossRef]
- Grizzle, W.E.; Bell, W.C.; Sexton, K.C. Issues in Collecting, Processing and Storing Human Tissues and Associated Information to Support Biomedical Research. Cancer Biomarkers 2010, 9, 531–549. [Google Scholar] [CrossRef]
- Xu, H.; Lyu, X.; Yi, M.; Zhao, W.; Song, Y.; Wu, K. Organoid Technology and Applications in Cancer Research. J. Hematol. Oncol. 2018, 1, 116. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Barrier, D.; Dbd, D.; Bibinov, N.; Rajasekaran, P.; Mertmann, P. Basics and Biomedical Applications of DBD. Available online: http://cdn.intechweb.org/pdfs/12799.pdf (accessed on 1 August 2019).

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscop, E.; Lin, A.; Van Boxem, W.; Van Loenhout, J.; De Backer, J.; Deben, C.; Dewilde, S.; Smits, E.; Bogaerts, A. The Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers 2019, 11, 1287. https://doi.org/10.3390/cancers11091287
Biscop E, Lin A, Van Boxem W, Van Loenhout J, De Backer J, Deben C, Dewilde S, Smits E, Bogaerts A. The Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers. 2019; 11(9):1287. https://doi.org/10.3390/cancers11091287
Chicago/Turabian StyleBiscop, Eline, Abraham Lin, Wilma Van Boxem, Jinthe Van Loenhout, Joey De Backer, Christophe Deben, Sylvia Dewilde, Evelien Smits, and Annemie Bogaerts. 2019. "The Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment" Cancers 11, no. 9: 1287. https://doi.org/10.3390/cancers11091287
APA StyleBiscop, E., Lin, A., Van Boxem, W., Van Loenhout, J., De Backer, J., Deben, C., Dewilde, S., Smits, E., & Bogaerts, A. (2019). The Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers, 11(9), 1287. https://doi.org/10.3390/cancers11091287