Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines and Cultures
2.3. Cell Viability Assay
2.4. Assessment of Apoptosis
2.5. Cell Cycle Analysis
2.6. Isobolographic and Statistical Analysis
3. Results
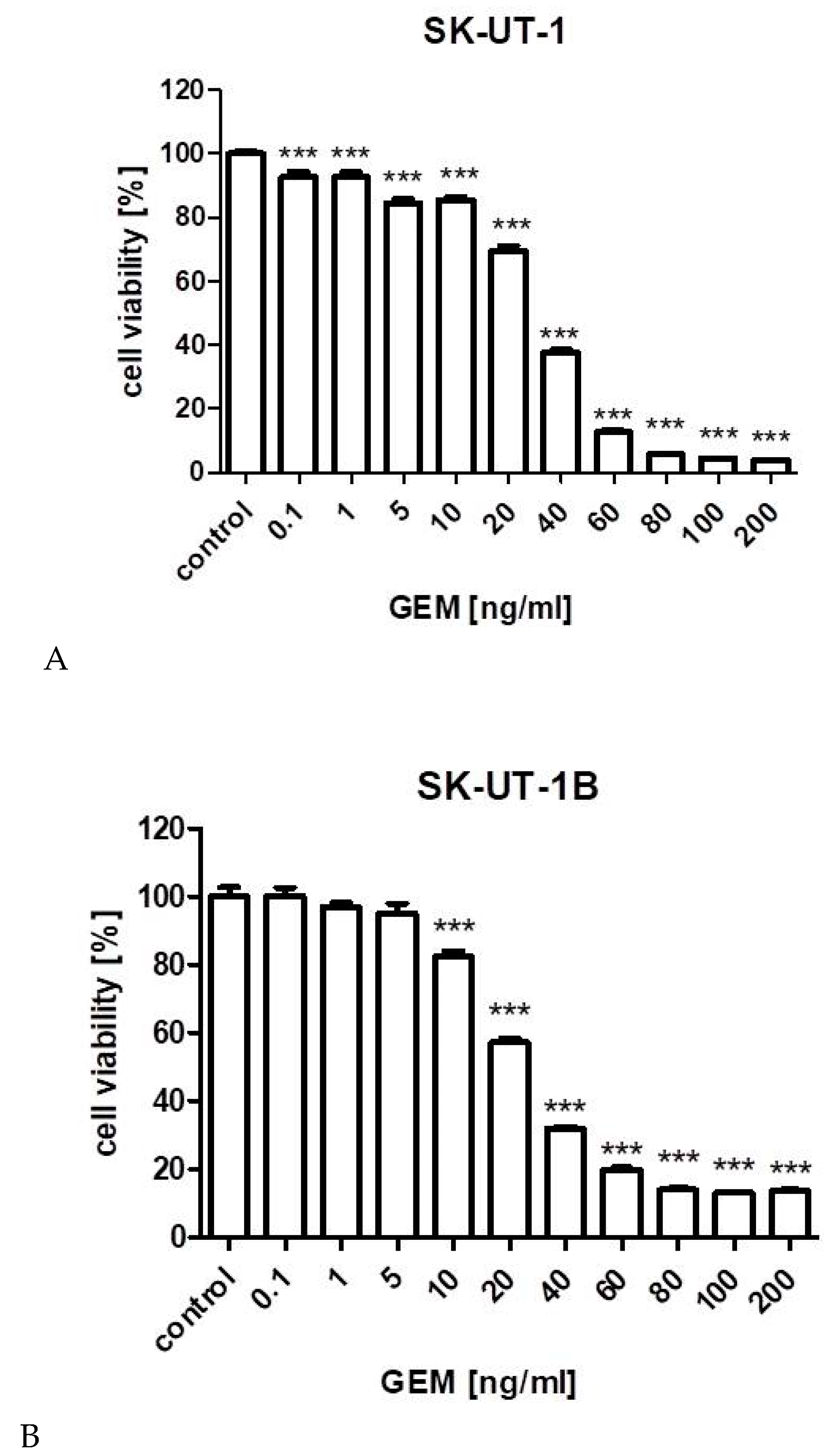
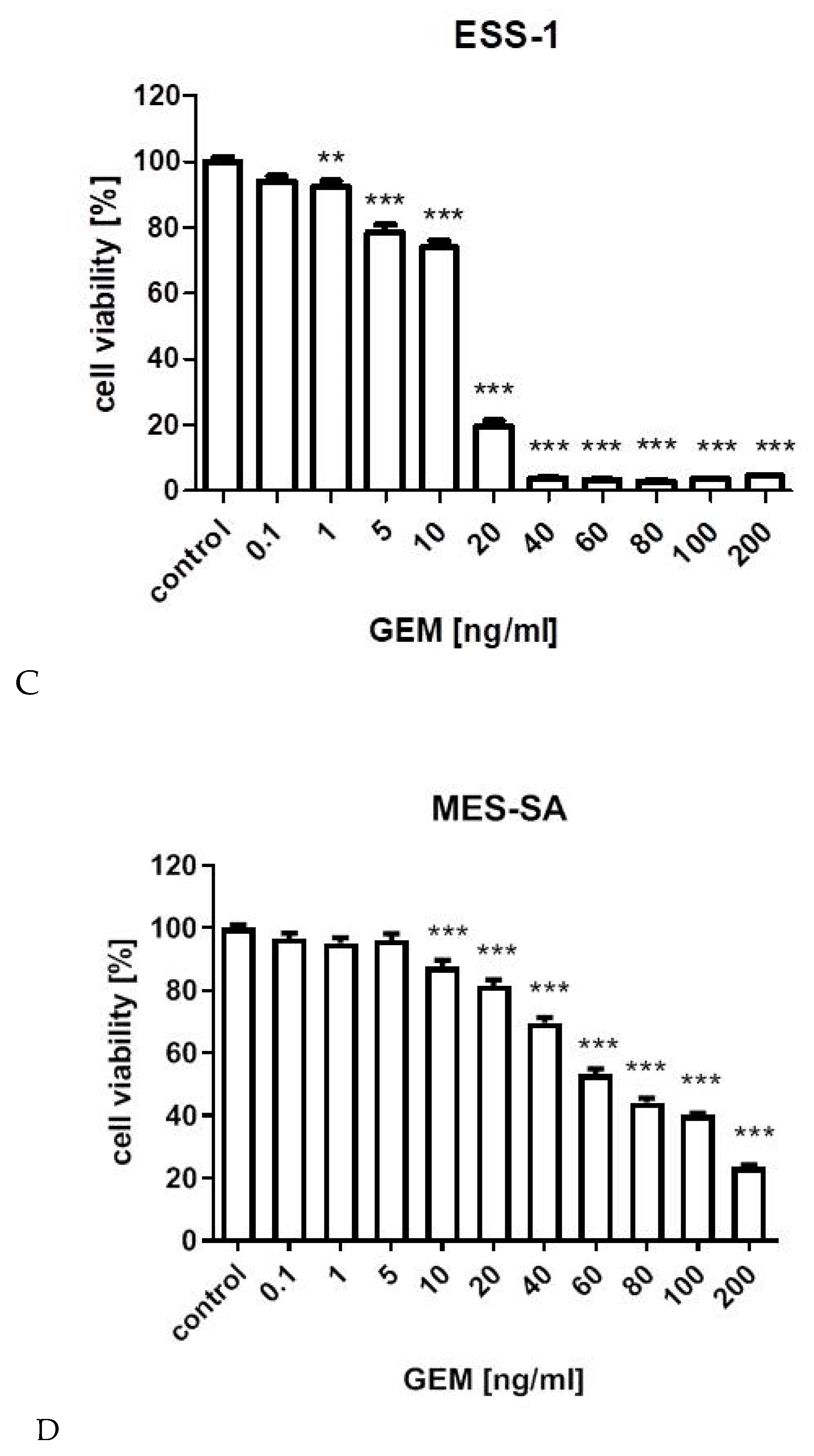
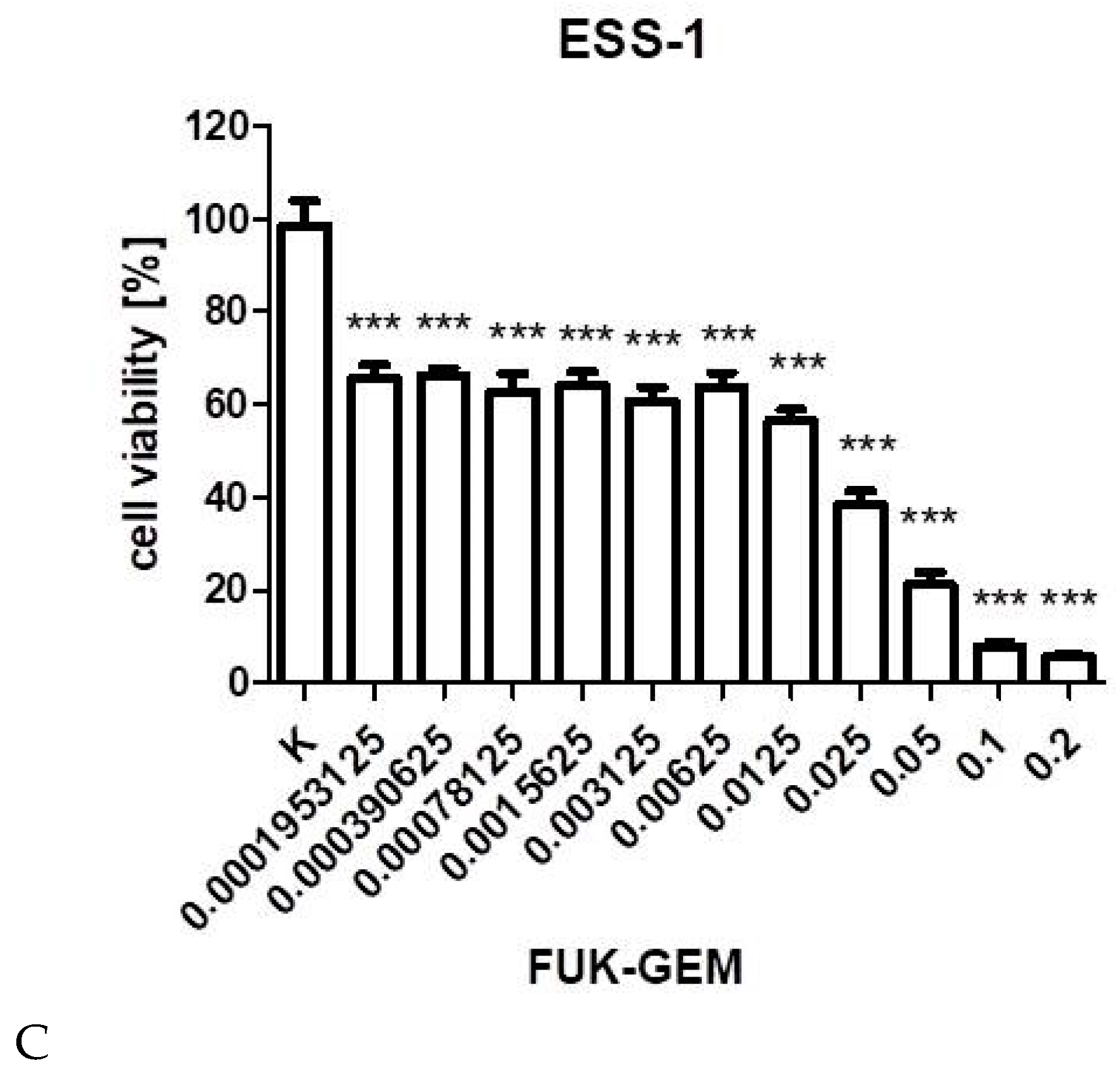
3.1. Cell Viability Assay
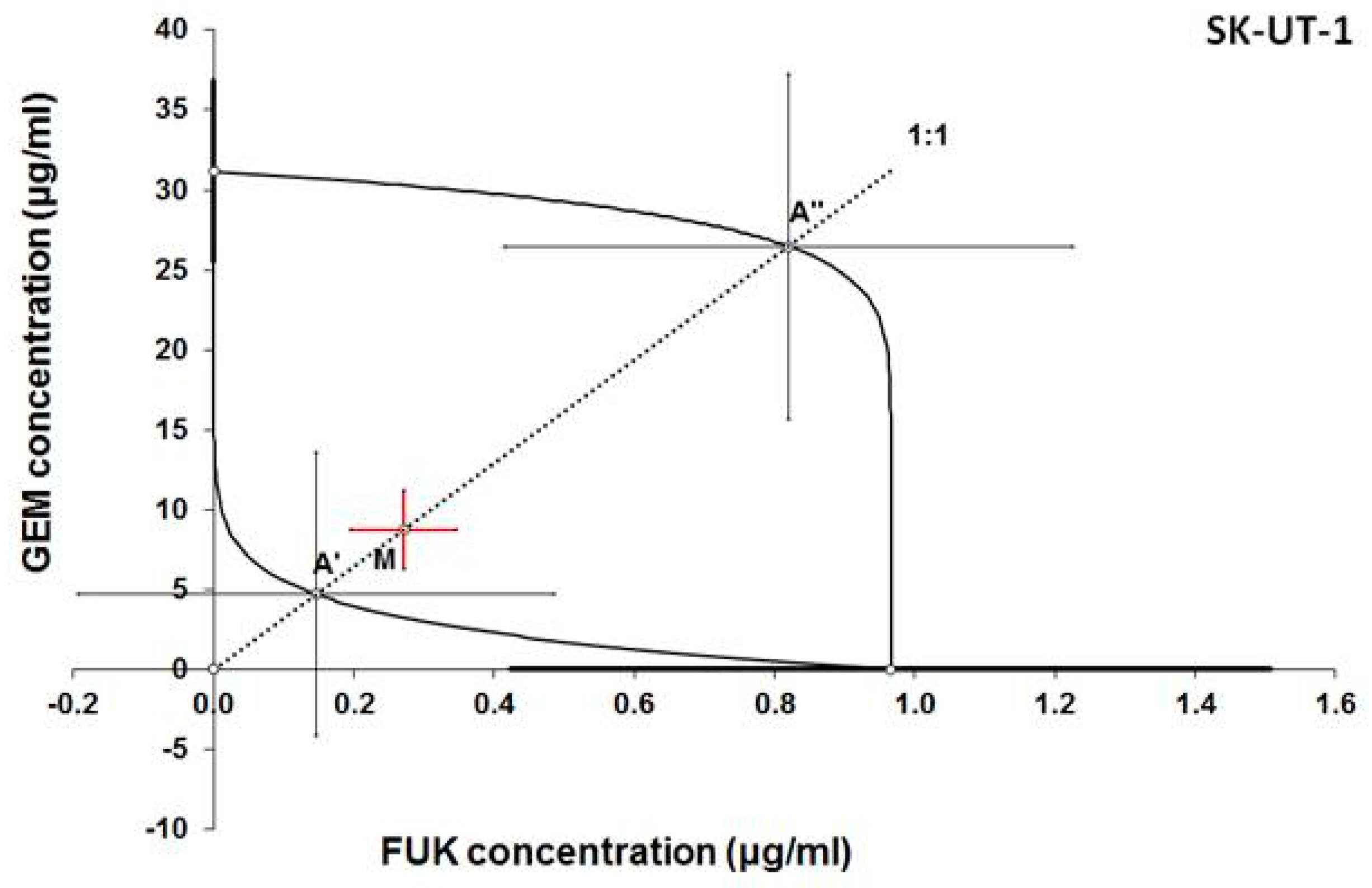
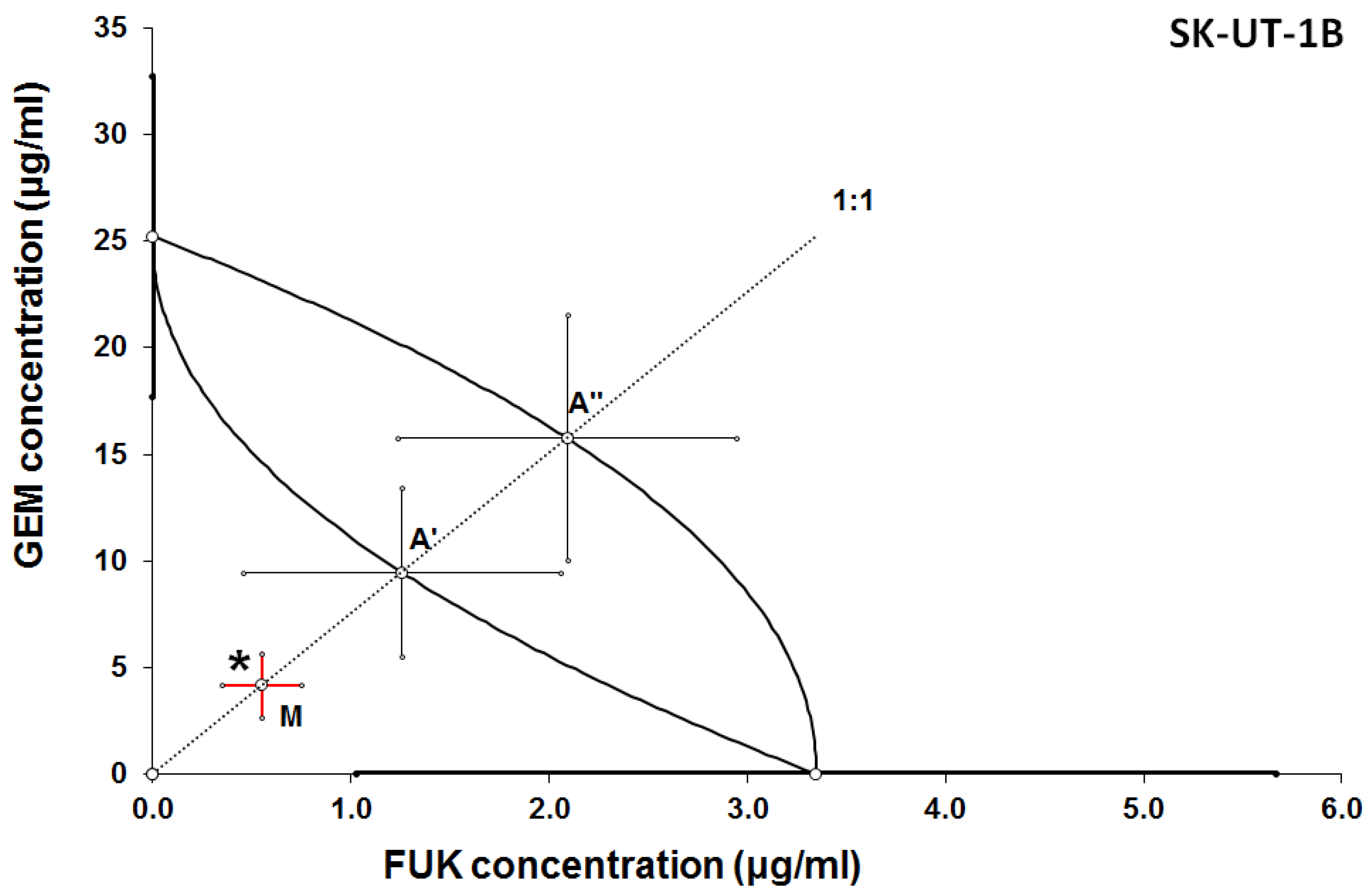
3.2. Isobolographic Anaysis
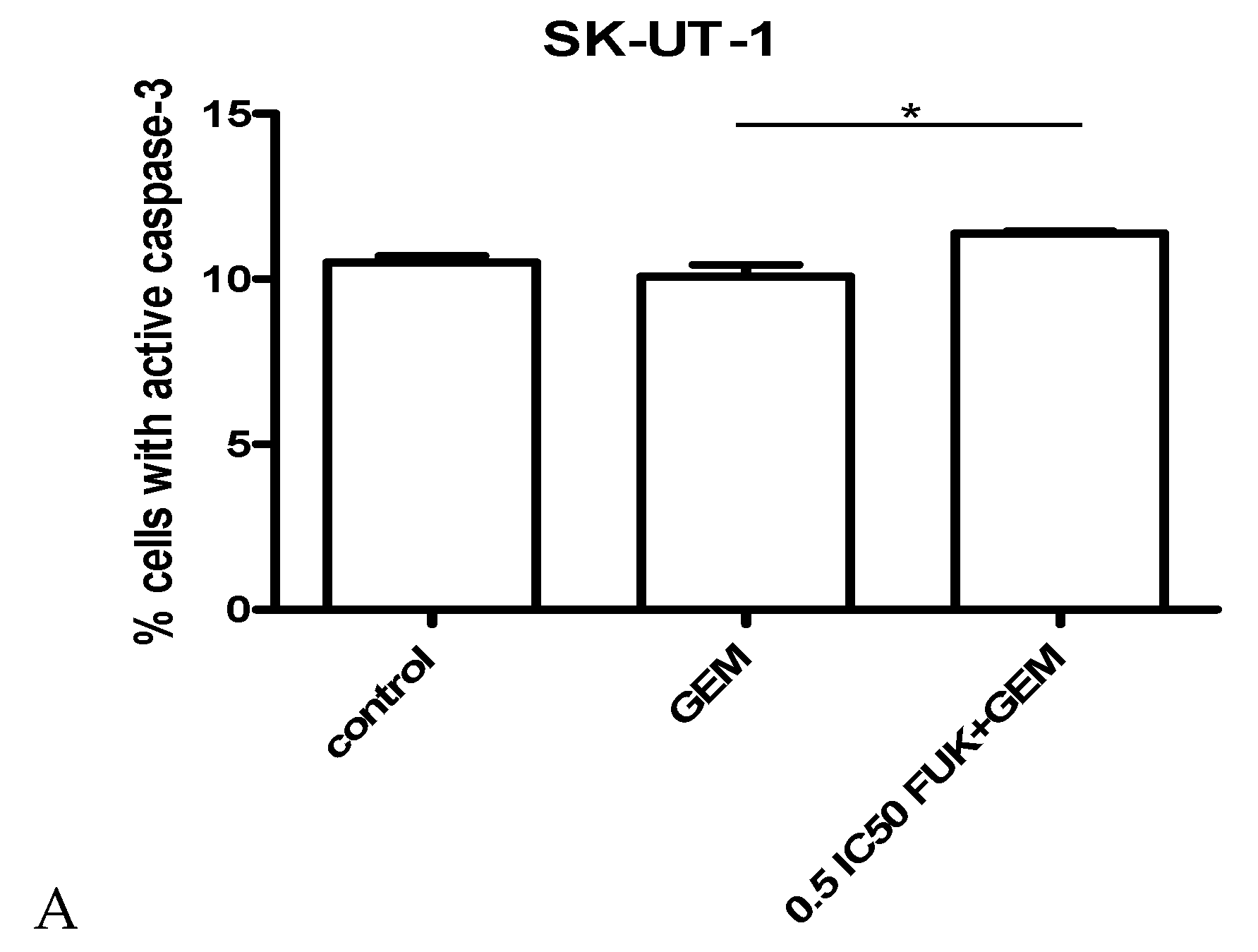
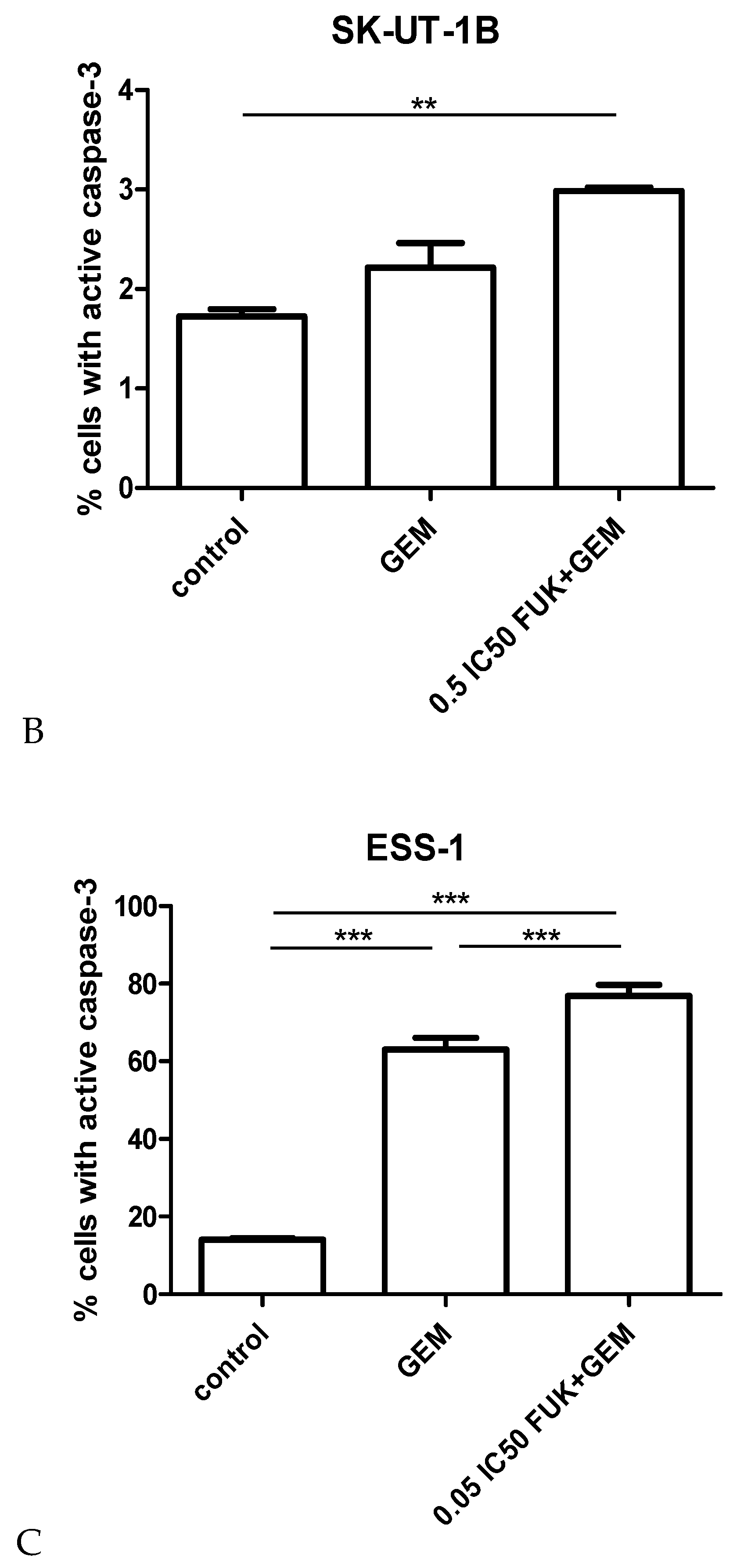
3.3. Assessment of Apoptosis
3.4. Cell Cycle
4. Discussion
- Analysis of the mechanisms of the MES-SA cell line relative resistance to fucoidan and gemcitabine that may lead to identification of such mechanisms in tumors and make progress for overcoming them.
- Due to similarities in clinical course between carcinosarcomas and endometrial carcinomas the results obtained on SK-UT-1 and SK-UT-1B cell lines might be replicated on carcinomas cell lines and extend indications for future practical applications.
- Studies on 3D cell cultures or animal models have to be performed in order to confirm the activity of proposed combination on tissue models and to assess how the tumor microenvironment affects it.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Khalfaoui, K.; du Bois, A.; Heitz, F. Current and future options in the management and treatment of uterine sarcoma. Ther. Adv. Med. Oncol. 2014, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.; Miah, A. Uterine sarcoma–current perspectives. Int. J. Womens Health 2017, 9, 597–606. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Uterine Neoplasms (Version: 3.2019-February 11, 2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 21 March 2019).
- Ricci, S.; Giuntoli, R.L., II; Eisenhauer, E.; Lopez, M.A.; Krill, L.; Tanner, E.J., III; Gehrig, P.A.; Havrilesky, L.J.; Secord, A.A.; Levinson, K.; et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol. Oncol. 2013, 131, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Maki, R.; Venkatraman, E.; Geller, G.; Lovegren, M.; Aghajanian, C.; Sabbatini, P.; Tong, W.; Barakat, R.; Spriggs, D.R. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J. Clin. Oncol. 2002, 20, 2824–2831. [Google Scholar] [CrossRef]
- Seddon, B.; Scurr, M.; Jones, R.L.; Wood, Z.; Propert-Lewis, C.; Fisher, C.; Flanagan, A.; Sunkersing, J.; A’Hern, R.; Whelan, J.; et al. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin. Sarcoma Res. 2015, 5, 13. [Google Scholar] [CrossRef]
- Hensley, M.L.; Miller, A.; O’Malley, D.M.; Mannel, R.S.; Behbakht, K.; Bakkum-Gamez, J.N.; Michael, H. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J. Clin. Oncol. 2015, 33, 1180–1185. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Bobiński, M.; Okła, K.; Bednarek, W.; Wawruszak, A.; Dmoszyńska-Graniczka, M.; Garcia-Sanz, P.; Wertel, I.; Kotarski, J. The Effect of Fucoidan, a Potential New, Natural, Anti-Neoplastic Agent on Uterine Sarcomas and Carcinosarcoma Cell Lines: ENITEC Collaborative Study. Arch. Immunol. Ther. Exp. 2019, 67, 125–131. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Karpiniec, S.S.; Dickinson, J.L. Fucoidan Suppresses the Growth of Human Acute Promyelocytic Leukemia Cells In Vitro and In Vivo. J. Cell. Physiol. 2016, 231, 688–697. [Google Scholar]
- Brown, K.; Dixey, M.; Weymouth-Wilson, A.; Linclau, B. The synthesis of gemcitabine. Carbohydr. Res. 2014, 387, 59–73. [Google Scholar] [CrossRef]
- Ducoulombier, A.; Cousin, S.; Kotecki, N.; Penel, N. Gemcitabine-based chemotherapy in sarcomas: A systematic review of published trials. Crit. Rev. Oncol. Hematol. 2016, 98, 73–80. [Google Scholar] [CrossRef]
- Tantari, M.; Barra, F.; Di Domenico, S.; Ferraioli, D.; Vellone, V.G.; De Cian, F.; Ferrero, S. Current state of the art and emerging pharmacotherapy for uterine leiomyosarcomas. Expert Opin. Pharmacother. 2019, 20, 713–723. [Google Scholar] [CrossRef]
- Harker, W.G.; MacKintosh, F.R.; Sikic, B.I. Development and characterization of a human sarcoma cell line, MES-SA, sensitive to multiple drugs. Cancer Res. 1983, 43, 4943–4950. [Google Scholar]
- Cell Lines Characteristics Cards. Available online: https://www.atcc.org (accessed on 17 December 2019).
- Wang, L.R.; Liu, J.; Huang, M.Z.; Xu, N. Comparison of pharmacokinetics, efficacy and toxicity profile of gemcitabine using two different administration regimens in Chinese patients with non-small-cell lung cancer. J. Zhejiang Univ. Sci. B 2007, 8, 307–313. [Google Scholar] [CrossRef]
- Grabarska, A.; Łuszczki, J.J.; Nowosadzka, E.; Gumbarewicz, E.; Jeleniewicz, W.; Dmoszyńska-Graniczka, M.; Kowalczuk, K.; Kupisz, K.; Polberg, K.; Stepulak, A. Histone Deacetylase Inhibitor SAHA as Potential Targeted Therapy Agent for Larynx Cancer Cells. J. Cancer 2017, 8, 19–28. [Google Scholar] [CrossRef]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of Interactions between Cisplatin and Two Histone Deacetylase Inhibitors in MCF7, T47D and MDA-MB-231 Human Breast Cancer Cell Lines-An Isobolographic Analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006, 319, 1–7. [Google Scholar] [CrossRef]
- Luszczki, J.J. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: A practical application. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 105–114. [Google Scholar] [CrossRef]
- Grabovsky, Y.; Tallarida, R.J. Isobolographic analysis for combinations of a full and partial agonist: Curved isoboles. J. Pharmacol. Exp. Ther. 2004, 310, 981–986. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Galmarini, C.M.; Dumontet, C. Gemcitabine resistance due to deoxycytidine kinase deficiency can be reverted by fruitfly deoxynucleoside kinase, DmdNK, in human uterine sarcoma cells. Cancer Chemother. Pharmacol. 2006, 58, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Coley, H.M.; Shotton, C.F.; Kokkinos, M.I.; Thomas, H. The effects of the CDK inhibitor seliciclib alone or in combination with cisplatin in human uterine sarcoma cell lines. Gynecol. Oncol. 2007, 105, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, O.H.; Lee, H.H.; Lee, B.Y. A 4-week repeated oral dose toxicity study of fucoidan from the Sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Clinical trial: NCT03422055 Study of Tolerance, Biodistribution and Dosimetry of Fucoidan Radiolabeled by Technetium-99m (NANO-ATHERO). Available online: https://clinicaltrials.gov/ct2/show/NCT03422055 (accessed on 31 December 2019).
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, D.; Zhao, X.; Jin, W.; Wang, J.; Zhang, Q. Microanalysis and preliminary pharmacokinetic studies of a sulfated polysaccharide from Laminaria japonica. Chin. J. Oceanol. Limnol. 2016, 34, 177–185. [Google Scholar] [CrossRef]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M.; Kongtawelert, P. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef]
- Mathew, L.; Burney, M.; Gaikwad, A.; Nyshadham, P.; Nugent, E.K.; Gonzalez, A.; Smith, J.A. Preclinical Evaluation of Safety of Fucoidan Extracts from Undaria pinnatifida and Fucus vesiculosus for Use in Cancer Treatment. Integr. Cancer Ther. 2017, 16, 572–584. [Google Scholar] [CrossRef]
- Burney, M.; Mathew, L.; Gaikwad, A.; Nugent, E.K.; Gonzalez, A.O.; Smith, J.A. Evaluation Fucoidan Extracts from Undaria pinnatifida and Fucus vesiculosus in Combination With Anticancer Drugs in Human Cancer Orthotopic Mouse Models. Integr. Cancer Ther. 2018, 17, 755–761. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Lee, E.F.; Harris, T.J.; Tran, S.; Evangelista, M.; Arulananda, S.; John, T.; Ramnac, C.; Hobbs, C.; Zhu, H.; Gunasingh, G.; et al. BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis. 2019, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Abudabbus, A.; Badmus, J.A.; Shalaweh, S.; Bauer, R.; Hiss, D. Effects of Fucoidan and Chemotherapeutic Agent Combinations on Malignant and Non-malignant Breast Cell Lines. Curr. Pharm. Biotechnol. 2017, 18, 748–757. [Google Scholar] [CrossRef]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar]
- da Silva, G.N.; de Castro Marcondes, J.P.; de Camargo, E.A.; da Silva Passos Junior, G.A.; Sakamoto-Hojo, E.T.; Salvadori, D.M.F. Cell cycle arrest and apoptosis in TP53 subtypes of bladder carcinoma cell lines treated with cisplatin and gemcitabine. Exp. Biol. Med. 2010, 235, 814. [Google Scholar] [CrossRef] [PubMed]
- Montano, R.; Khan, N.; Hou, H.; Seigne, J.; Ernstoff, M.S.; Lewis, L.D.; Eastman, A. Cell cycle perturbation induced by gemcitabine in human tumor cells in cell culture, xenografts and bladder cancer patients: Implications for clinical trial designs combining gemcitabine with a Chk1 inhibitor. Oncotarget 2017, 8, 67754–67768. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Choi, I.W.; Kim, G.Y.; Kim, B.W.; Kim, W.J.; Choi, Y.H. Fucoidan induces G1 arrest of the cell cycle in EJ human bladder cancer cells through down-regulation of pRB phosphorylation. Revista Brasileira De Farmacognosia 2015, 25, 246–251. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef]



| Cell line: | SKUT-1 * | SKUT-1B * | MES-SA | ESS-1 |
|---|---|---|---|---|
| Organism: | Homo sapiens, human | Homo sapiens, human | Homo sapiens, human | Homo sapiens, human |
| Tissue: | uterus | uterus/endometrium | uterus | uterus |
| Culture properties: | adherent | adherent | adherent | adherent |
| Disease: | grade III, mesodermal tumor (mixed); consistent with leiomyosarcoma | grade III, mesodermal tumor (mixed); consistent with leiomyosarcoma | grade III, recurrent, uterine leiomyosarcoma [14] | endometrial stromal sarcoma |
| Age: | 75 years | 75 years | 56 years | 76 years |
| Gender: | female | female | female | female |
| Ethnicity: | Caucasian | Caucasian | Caucasian | Caucasian |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobiński, M.; Okła, K.; Łuszczki, J.; Bednarek, W.; Wawruszak, A.; Moreno-Bueno, G.; Dmoszyńska-Graniczka, M.; Tarkowski, R.; Kotarski, J. Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells. Cancers 2020, 12, 107. https://doi.org/10.3390/cancers12010107
Bobiński M, Okła K, Łuszczki J, Bednarek W, Wawruszak A, Moreno-Bueno G, Dmoszyńska-Graniczka M, Tarkowski R, Kotarski J. Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells. Cancers. 2020; 12(1):107. https://doi.org/10.3390/cancers12010107
Chicago/Turabian StyleBobiński, Marcin, Karolina Okła, Jarogniew Łuszczki, Wiesława Bednarek, Anna Wawruszak, Gema Moreno-Bueno, Magdalena Dmoszyńska-Graniczka, Rafał Tarkowski, and Jan Kotarski. 2020. "Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells" Cancers 12, no. 1: 107. https://doi.org/10.3390/cancers12010107
APA StyleBobiński, M., Okła, K., Łuszczki, J., Bednarek, W., Wawruszak, A., Moreno-Bueno, G., Dmoszyńska-Graniczka, M., Tarkowski, R., & Kotarski, J. (2020). Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells. Cancers, 12(1), 107. https://doi.org/10.3390/cancers12010107





