Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects
Abstract
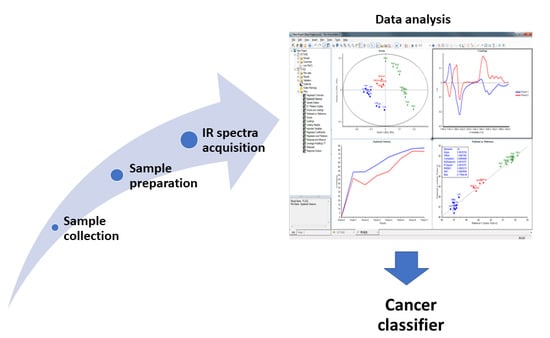
:1. Introduction
2. Wavenumber Range and Computational Models in Fourier Transform Infrared Spectroscopic Analysis of Biological Specimens
3. Sensitivity, Specificity and Accuracy in Cancer Detection
4. Classification, Staging and Grading for Cancer Management
5. Automated Cancer Diagnosis
6. Cancer Surgical Management
7. Monitoring of Cancer Treatment Response and Follow-Up
8. Fourier Transform Infrared Spectroscopic Analysis of Cancer-Derived Extracellular Vesicles
8.1. Diagnostic Value of Extracellular Vesicles
8.2. Analysis of Extracellular Vesicles Using Fourier Transform Infrared Spectroscopy
9. Challenges and Future Directions of Fourier Transform Infrared Spectroscopy in Clinical Use
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 October 2019).
- Duffy, M.J. Serum tumor markers in breast cancer: Are they of clinical value? Clin. Chem. 2006, 52, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.B.; Zhang, L.; Rosin, M. Advances in the diagnosis of oral premalignant and malignant lesions. J. Can. Dent. Assoc. 2002, 68, 617–621. [Google Scholar] [PubMed]
- Khanmohammadi, M.; Bagheri Garmarudi, A.; Samani, S.; Ghasemi, K.; Ashuri, A. Application of linear discriminant analysis and Attenuated Total Reflectance Fourier Transform Infrared microspectroscopy for diagnosis of colon cancer. Pathol. Oncol. Res. 2011, 17, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Bergner, N.; Romeike, B.F.; Reichart, R.; Kalff, R.; Krafft, C.; Popp, J. Tumor margin identification and prediction of the primary tumor from brain metastases using FTIR imaging and support vector machines. Analyst 2013, 138, 3983–3990. [Google Scholar] [CrossRef]
- Poste, G. Bring on the biomarkers. Nature 2011, 469, 156–157. [Google Scholar] [CrossRef]
- Holmström, B.; Johansson, M.; Bergh, A.; Stenman, U.-H.; Hallmans, G.; Stattin, P. Prostate specific antigen for early detection of prostate cancer: Longitudinal study. BMJ 2009, 339, b3537. [Google Scholar] [CrossRef] [Green Version]
- Seregni, E.; Coli, A.; Mazzucca, N. Circulating tumour markers in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, S15–S22. [Google Scholar] [CrossRef]
- Soletormos, G.; Nielsen, D.; Schioler, V.; Mouridsen, H.; Dombernowsky, P. Monitoring different stages of breast cancer using tumour markers CA 15-3, CEA and TPA. Eur. J. Cancer 2004, 40, 481–486. [Google Scholar] [CrossRef]
- Farlow, E.C.; Patel, K.; Basu, S.; Lee, B.S.; Kim, A.W.; Coon, J.S.; Faber, L.P.; Bonomi, P.; Liptay, M.J.; Borgia, J.A. Development of a multiplexed tumor-associated autoantibody-based blood test for the detection of non-small cell lung cancer. Clin. Cancer Res. 2010, 16, 3452–3462. [Google Scholar] [CrossRef] [Green Version]
- Lumachi, F.; Marino, F.; Orlando, R.; Chiara, G.B.; Basso, S.M. Simultaneous multianalyte immunoassay measurement of five serum tumor markers in the detection of colorectal cancer. Anticancer Res. 2012, 32, 985–988. [Google Scholar]
- Fan, X. Application of fourier transform infrared spectroscopy in life science. Life Sci. Res. 2003, 7, 83–87. [Google Scholar]
- Woernley, D.L. Infrared absorption curves for normal and neoplastic tissues and related biological substances. Cancer Res. 1952, 12, 516–523. [Google Scholar] [PubMed]
- Le Naour, F.; Sandt, C.; Peng, C.; Trcera, N.; Chiappini, F.; Flank, A.M.; Guettier, C.; Dumas, P. In situ chemical composition analysis of cirrhosis by combining synchrotron fourier transform infrared and synchrotron X-ray fluorescence microspectroscopies on the same tissue section. Anal. Chem. 2012, 84, 10260–10266. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, J.; Mueller, R.; Formanski, N.; Szlama, N.; Meerpohl, H.-G.; Eidt, M.; Bugert, P. Diagnosis of breast cancer with infrared spectroscopy from serum samples. Vib. Spectrosc. 2010, 52, 173–177. [Google Scholar] [CrossRef]
- Lima, K.M.; Gajjar, K.B.; Martin-Hirsch, P.L.; Martin, F.L. Segregation of ovarian cancer stage exploiting spectral biomarkers derived from blood plasma or serum analysis: ATR-FTIR spectroscopy coupled with variable selection methods. Biotechnol. Prog. 2015, 31, 832–839. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, X.; Li, W.; Wang, Y.; Chen, X.; Wang, X. Distinction of leukemia patients’ and healthy persons’ serum using FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 228–232. [Google Scholar] [CrossRef]
- Sheng, D.; Wu, Y.; Wang, X.; Huang, D.; Chen, X.; Liu, X. Comparison of serum from gastric cancer patients and from healthy persons using FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 365–369. [Google Scholar] [CrossRef]
- Zelig, U.; Mordechai, S.; Shubinsky, G.; Sahu, R.K.; Huleihel, M.; Leibovitz, E.; Nathan, I.; Kapelushnik, J. Pre-screening and follow-up of childhood acute leukemia using biochemical infrared analysis of peripheral blood mononuclear cells. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 827–835. [Google Scholar] [CrossRef]
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Clarke, N.W.; Gardner, P. Investigating FTIR based histopathology for the diagnosis of prostate cancer. J. Biophotonics 2009, 2, 104–113. [Google Scholar] [CrossRef]
- Großerueschkamp, F.; Kallenbach-Thieltges, A.; Behrens, T.; Bruning, T.; Altmayer, M.; Stamatis, G.; Theegarten, D.; Gerwert, K. Marker-free automated histopathological annotation of lung tumour subtypes by FTIR imaging. Analyst 2015, 140, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Kaznowska, E.; Depciuch, J.; Szmuc, K.; Cebulski, J. Use of FTIR spectroscopy and PCA-LDC analysis to identify cancerous lesions within the human colon. J. Pharm. Biomed. Anal. 2017, 134, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Sebbag, G.; Argov, S.; Mordechai, S.; Sahu, R.K. Early detection of colorectal cancer relapse by infrared spectroscopy in “normal” anastomosis tissue. J. Biomed. Opt. 2015, 20, 75007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Shi, X.; Zhang, Y. The use of FTIR-ATR spectrometry for evaluation of surgical resection margin in colorectal cancer: A pilot study of 56 samples. J. Spectrosc. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Krafft, C.; Wilhelm, K.; Eremin, A.; Nestel, S.; von Bubnoff, N.; Schultze-Seemann, W.; Popp, J.; Nazarenko, I. A specific spectral signature of serum and plasma-derived extracellular vesicles for cancer screening. Nanomedicine 2017, 13, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Yap, X.-L.; Ong, T.-A.; Lim, J.; Wood, B.; Lee, W.-L. Study of prostate cancer-derived extracellular vesicles in urine using IR spectroscopy. Prog. Drug Discov. Biomed. Sci. 2019, 2, a0000026. [Google Scholar]
- Zlotogorski-Hurvitz, A.; Dekel, B.Z.; Malonek, D.; Yahalom, R.; Vered, M. FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 685–694. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Ashton, K.M.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Potential of mid-infrared spectroscopy as a non-invasive diagnostic test in urine for endometrial or ovarian cancer. Analyst 2018, 143, 3156–3163. [Google Scholar] [CrossRef]
- Gok, S.; Aydin, O.Z.; Sural, Y.S.; Zorlu, F.; Bayol, U.; Severcan, F. Bladder cancer diagnosis from bladder wash by Fourier transform infrared spectroscopy as a novel test for tumor recurrence. J. Biophotonics 2016, 9, 967–975. [Google Scholar] [CrossRef]
- Untereiner, V.; Sockalingum, G.D.; Garnotel, R.; Gobinet, C.; Ramaholimihaso, F.; Ehrhard, F.; Diebold, M.D.; Thiefin, G. Bile analysis using high-throughput FTIR spectroscopy for the diagnosis of malignant biliary strictures: A pilot study in 57 patients. J. Biophotonics 2014, 7, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Lewis, K.E.; Ghosal, R.; Bayliss, S.; Lloyd, A.J.; Wills, J.; Godfrey, R.; Kloer, P.; Mur, L.A. Evaluation of FTIR spectroscopy as a diagnostic tool for lung cancer using sputum. BMC Cancer 2010, 10, 640. [Google Scholar] [CrossRef] [Green Version]
- Menzies, G.E.; Fox, H.R.; Marnane, C.; Pope, L.; Prabhu, V.; Winter, S.; Derrick, A.V.; Lewis, P.D. Fourier transform infrared for noninvasive optical diagnosis of oral, oropharyngeal, and laryngeal cancer. Transl. Res. 2014, 163, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rahbarghazi, R.; Jabbari, N.; Sani, N.A.; Asghari, R.; Salimi, L.; Kalashani, S.A.; Feghhi, M.; Etemadi, T.; Akbariazar, E.; Mahmoudi, M.; et al. Tumor-derived extracellular vesicles: Reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019, 17, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.J.; Singh, M.N.; Stringfellow, H.F.; Pollock, H.M.; Hammiche, A.; Grude, O.; Fullwood, N.J.; Pitt, M.A.; Martin-Hirsch, P.L.; Martin, F.L. FTIR microspectroscopy coupled with two-class discrimination segregates markers responsible for inter- and intra-category variance in exfoliative cervical cytology. Biomark. Insights 2008, 3, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Balan, V.; Mihai, C.T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational spectroscopy fingerprinting in medicine: From molecular to clinical practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef] [Green Version]
- Caine, S.; Heraud, P.; Tobin, M.J.; McNaughton, D.; Bernard, C.C. The application of Fourier transform infrared microspectroscopy for the study of diseased central nervous system tissue. Neuroimage 2012, 59, 3624–3640. [Google Scholar] [CrossRef]
- Kumar, S.; Srinivasan, A.; Nikolajeff, F. Role of infrared spectroscopy and imaging in cancer diagnosis. Curr. Med. Chem. 2018, 25, 1055–1072. [Google Scholar] [CrossRef]
- Griffiths, P.R.; De Haseth, J.A. Fourier Transform Infrared Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 171. [Google Scholar]
- Ramírez-Elías, M.G.; González, F.J. Raman spectroscopy for in vivo medical diagnosis. Raman Spectrosc. 2018, 293. [Google Scholar] [CrossRef] [Green Version]
- Kaznowska, E.; Łach, K.; Depciuch, J.; Chaber, R.; Koziorowska, A.; Slobodian, S.; Kiper, K.; Chlebus, A.; Cebulski, J. Application of infrared spectroscopy for the identification of squamous cell carcinoma (lung cancer). Preliminary study. Infrared Phys. Technol. 2018, 89, 282–290. [Google Scholar] [CrossRef]
- Zawlik, I.; Kaznowska, E.; Cebulski, J.; Kolodziej, M.; Depciuch, J.; Vongsvivut, J.; Cholewa, M. FPA-FTIR microspectroscopy for monitoring chemotherapy efficacy in triple-negative breast cancer. Sci. Rep. 2016, 6, 37333. [Google Scholar] [CrossRef] [Green Version]
- Kaznowska, E.; Depciuch, J.; Lach, K.; Kolodziej, M.; Koziorowska, A.; Vongsvivut, J.; Zawlik, I.; Cholewa, M.; Cebulski, J. The classification of lung cancers and their degree of malignancy by FTIR, PCA-LDA analysis, and a physics-based computational model. Talanta 2018, 186, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Depciuch, J.; Kaznowska, E.; Golowski, S.; Koziorowska, A.; Zawlik, I.; Cholewa, M.; Szmuc, K.; Cebulski, J. Monitoring breast cancer treatment using a Fourier transform infrared spectroscopy-based computational model. J. Pharm. Biomed. Anal. 2017, 143, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Depciuch, J.; Kaznowska, E.; Koziorowska, A.; Cebulski, J. Verification of the effectiveness of the Fourier transform infrared spectroscopy computational model for colorectal cancer. J. Pharm. Biomed. Anal. 2017, 145, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Šimundić, A.-M. Measures of diagnostic accuracy: Basic definitions. Med Biol. Sci. 2008, 22, 61. [Google Scholar]
- Baratloo, A.; Hosseini, M.; Negida, A.; El Ashal, G. Part 1: Simple definition and calculation of accuracy, sensitivity and specificity. Emergency 2015, 3, 48–49. [Google Scholar] [PubMed]
- van Stralen, K.J.; Stel, V.S.; Reitsma, J.B.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney Int. 2009, 75, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Given, M.; Scott, M.; Mc Grath, J.P.; Given, H.F. The predictive of tumour markers CA 15-3, TPS and CEA in breast cancer recurrence. Breast 2000, 9, 277–280. [Google Scholar] [CrossRef]
- van Haaften-Day, C.; Shen, Y.; Xu, F.; Yu, Y.; Berchuck, A.; Havrilesky, L.J.; de Bruijn, H.W.; van der Zee, A.G.; Bast, R.C., Jr.; Hacker, N.F. OVX1, macrophage-colony stimulating factor, and CA-125-II as tumor markers for epithelial ovarian carcinoma: A critical appraisal. Cancer 2001, 92, 2837–2844. [Google Scholar] [CrossRef]
- Yurkovetsky, Z.; Skates, S.; Lomakin, A.; Nolen, B.; Pulsipher, T.; Modugno, F.; Marks, J.; Godwin, A.; Gorelik, E.; Jacobs, I.; et al. Development of a multimarker assay for early detection of ovarian cancer. J. Clin. Oncol. 2010, 28, 2159–2166. [Google Scholar] [CrossRef]
- Cowherd, S.M. Tumor staging and grading: A primer. In Methods in Molecular Biology; Springer: Berlin, Germany, 2012; Volume 823, pp. 1–18. [Google Scholar]
- Greene, F.; Page, D.; Fleming, I.; Fritz, A.; Balch, C.; Haller, D.; Morrow, M. AJCC Cancer Staging Manual, 6th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966, 50, 125–128. [Google Scholar]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Jothi, J.A.A.; Rajam, V.M.A. A survey on automated cancer diagnosis from histopathology images. Artif. Intell. Rev. 2017, 48, 31–81. [Google Scholar]
- He, L.; Long, L.R.; Antani, S.; Thoma, G.R. Histology image analysis for carcinoma detection and grading. Comput. Methods Programs Biomed. 2012, 107, 538–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, P.S.; Thomas, S.C.; Nicolson, M.C.; Fyfe, M.N.; Kerr, K.M. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J. Thorac. Oncol. 2010, 5, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Roggli, V.L.; Vollmer, R.T.; Greenberg, S.D.; McGavran, M.H.; Spjut, H.J.; Yesner, R. Lung cancer heterogeneity: A blinded and randomized study of 100 consecutive cases. Hum. Pathol. 1985, 16, 569–579. [Google Scholar] [CrossRef]
- Peng, H. Bioimage informatics: A new area of engineering biology. Bioinformatics 2008, 24, 1827–1836. [Google Scholar] [CrossRef] [Green Version]
- Ollesch, J.; Drees, S.L.; Heise, H.M.; Behrens, T.; Brüning, T.; Gerwert, K. FTIR spectroscopy of biofluids revisited: An automated approach to spectral biomarker identification. Analyst 2013, 138, 4092–4102. [Google Scholar] [CrossRef]
- Ollesch, J.; Heinze, M.; Heise, H.M.; Behrens, T.; Brüning, T.; Gerwert, K. It’s in your blood: Spectral biomarker candidates for urinary bladder cancer from automated FTIR spectroscopy. J. Biophotonics 2014, 7, 210–221. [Google Scholar] [CrossRef]
- Hughes, C.; Baker, M.J. Can mid-infrared biomedical spectroscopy of cells, fluids and tissue aid improvements in cancer survival? A patient paradigm. Analyst 2016, 141, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.K. Postoperative pathologic assessment of surgical margins in oral cancer: A contemporary review. J. Oral. Maxillofac. Pathol. 2018, 22, 78–85. [Google Scholar] [CrossRef]
- Depciuch, J.; Kaznowska, E.; Zawlik, I.; Wojnarowska, R.; Cholewa, M.; Heraud, P.; Cebulski, J. Application of raman spectroscopy and infrared spectroscopy in the identification of breast cancer. Appl. Spectrosc. 2016, 70, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Sun, X.; Chao, Z.; Zhang, S.; Zheng, J.; Gurung, R.; Du, J.; Shi, J.; Xu, Y.; Zhang, Y. Evaluation of FTIR spectroscopy as diagnostic tool for colorectal cancer using spectral analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Y.; Wu, J.; Zhang, Y.; Sun, K. Detection of lung cancer tissue by attenuated total reflection-Fourier transform infrared spectroscopy—A pilot study of 60 samples. J. Surg. Res. 2013, 179, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [Green Version]
- Harrington, C.B.; Hansen, J.A.; Moskowitz, M.; Todd, B.L.; Feuerstein, M. It’s not over when it’s over: Long-term symptoms in cancer survivors—A systematic review. Int. J. Psychiatry Med. 2010, 40, 163–181. [Google Scholar] [CrossRef]
- Harrison, J.D.; Young, J.M.; Price, M.A.; Butow, P.N.; Solomon, M.J. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 2009, 17, 1117–1128. [Google Scholar] [CrossRef]
- Feiten, S.; Dünnebacke, J.; Friesenhahn, V.; Heymanns, J.; Köppler, H.; Meister, R.; Thomalla, J.; van Roye, C.; Wey, D.; Weide, R. Follow-up reality for breast cancer patients—Standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016, 76, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Frankland, J.; Brodie, H.; Cooke, D.; Foster, C.; Foster, R.; Gage, H.; Jordan, J.; Mesa-Eguiagaray, I.; Pickering, R.; Richardson, A. Follow-up care after treatment for prostate cancer: Evaluation of a supported self-management and remote surveillance programme. BMC Cancer 2019, 19, 368. [Google Scholar] [CrossRef]
- Kwast, A.B.; Drossaert, C.H.; Siesling, S. Breast cancer follow-up: From the perspective of health professionals and patients. Eur. J. Cancer Care 2013, 22, 754–764. [Google Scholar] [CrossRef]
- de Bock, G.H.; Bonnema, J.; van der Hage, J.; Kievit, J.; van de Velde, C.J. Effectiveness of routine visits and routine tests in detecting isolated locoregional recurrences after treatment for early-stage invasive breast cancer: A meta-analysis and systematic review. J. Clin. Oncol. 2004, 22, 4010–4018. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezin, A.; Zulli, A.; Kerrigan, S.; Petrovic, D.; Kruzliak, P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 2015, 48, 562–568. [Google Scholar] [CrossRef]
- Das, S.; Halushka, M.K. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc. Pathol. 2015, 24, 199–206. [Google Scholar] [CrossRef]
- Gong, J.; Jaiswal, R.; Dalla, P.; Luk, F.; Bebawy, M. Microparticles in cancer: A review of recent developments and the potential for clinical application. Semin. Cell Dev. Biol. 2015, 40, 35–40. [Google Scholar] [CrossRef]
- Lombardo, G.; Dentelli, P.; Togliatto, G.; Rosso, A.; Gili, M.; Gallo, S.; Deregibus, M.C.; Camussi, G.; Brizzi, M.F. Activated Stat5 trafficking Via endothelial cell-derived extracellular vesicles controls IL-3 pro-angiogenic paracrine action. Sci. Rep. 2016, 6, 25689. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Berezin, A.E. Impaired immune phenotype of endothelial cell-derived micro particles: The missing link between diabetes-related states and risk of cardiovascular complications. J. Data Min. Genom. Proteom. 2016, 7, 195–197. [Google Scholar] [CrossRef]
- França, C.N.; Izar, M.O.; do Amaral, J.B.; Tegani, D.M.; Fonseca, F.A.H. Microparticles as potential biomarkers of cardiovascular disease. Arq. Bras. Cardiol. 2015, 104, 169–174. [Google Scholar] [CrossRef]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [Green Version]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Morphological and molecular features of oral fluid-derived exosomes: Oral cancer patients versus healthy individuals. J. Cancer Res. Clin. Oncol. 2016, 142, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mezouar, S.; Mege, D.; Darbousset, R.; Farge, D.; Debourdeau, P.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin. Oncol. 2014, 41, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Wang, C.C.; Chang, H.C.; Tsai, T.H.; Chang, L.T.; Huang, K.T.; Leu, S.; Yen, C.H.; Liu, S.F.; Chen, C.H.; et al. Levels of circulating microparticles in lung cancer patients and possible prognostic value. Dis. Markers 2013, 35, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, A.F.; Lyubarova, R.; Tsiakos, J.; Palaia, T.; Deleon, J.R.; Ragolia, L. Circulating endothelial microparticles in diabetes mellitus. Mediat. Inflamm. 2010, 2010, 250476. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Guerin, A.P.; Leroyer, A.; Mallat, Z.; Nguyen, C.; Boddaert, J.; London, G.M.; Tedgui, A.; Boulanger, C.M. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 2005, 16, 3381–3388. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.F.; Ludlam, C.A. Plasma microparticles and vascular disorders. Br. J. Haematol. 2007, 137, 36–48. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Cortes, R. Extracellular vesicles as biomarkers of systemic lupus erythematosus. Dis. Markers 2015, 2015, 613536. [Google Scholar] [CrossRef] [Green Version]
- Schindler, S.M.; Little, J.P.; Klegeris, A. Microparticles: A new perspective in central nervous system disorders. Biomed. Res. Int. 2014, 2014, 756327. [Google Scholar] [CrossRef] [Green Version]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Duffy, M.J.; Sturgeon, C.M.; Soletormos, G.; Barak, V.; Molina, R.; Hayes, D.F.; Diamandis, E.P.; Bossuyt, P.M. Validation of new cancer biomarkers: A position statement from the European group on tumor markers. Clin. Chem. 2015, 61, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.J.; Hussain, S.R.; Lovergne, L.; Untereiner, V.; Hughes, C.; Lukaszewski, R.A.; Thiefin, G.; Sockalingum, G.D. Developing and understanding biofluid vibrational spectroscopy: A critical review. Chem. Soc. Rev. 2016, 45, 1803–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827. [Google Scholar] [CrossRef]
- Ristenpart, W.D.; Kim, P.G.; Domingues, C.; Wan, J.; Stone, H.A. Influence of substrate conductivity on circulation reversal in evaporating drops. Phys. Rev. Lett. 2007, 99, 234502. [Google Scholar] [CrossRef] [Green Version]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Esmonde-White, F.W.; Morris, M.D.; Roessler, B.J. Characterization of biofluids prepared by sessile drop formation. Analyst 2014, 139, 2734–2741. [Google Scholar] [CrossRef] [Green Version]
- Byrne, H.J.; Baranska, M.; Puppels, G.J.; Stone, N.; Wood, B.; Gough, K.M.; Lasch, P.; Heraud, P.; Sulé-Suso, J.; Sockalingum, G.D. Spectropathology for the next generation: Quo vadis? Analyst 2015, 140, 2066–2073. [Google Scholar] [CrossRef] [Green Version]
- Depciuch, J.; Kaznowska, E.; Szmuc, K.; Zawlik, I.; Cholewa, M.; Heraud, P.; Cebulski, J. Comparing paraffined and deparaffinized breast cancer tissue samples and an analysis of Raman spectroscopy and infrared methods. Infrared Phys. Technol. 2016, 76, 217–226. [Google Scholar] [CrossRef]
- Chaber, R.; Łach, K.; Depciuch, J.; Szmuc, K.; Michalak, E.; Raciborska, A.; Koziorowska, A.; Cebulski, J. Fourier Transform Infrared (FTIR) spectroscopy of paraffin and deparafinnized bone tissue samples as a diagnostic tool for Ewing sarcoma of bones. Infrared Phys. Technol. 2017, 85, 364–371. [Google Scholar] [CrossRef]
- Gajjar, K.; Trevisan, J.; Owens, G.; Keating, P.J.; Wood, N.J.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Fourier-transform infrared spectroscopy coupled with a classification machine for the analysis of blood plasma or serum: A novel diagnostic approach for ovarian cancer. Analyst 2013, 138, 3917–3926. [Google Scholar] [CrossRef]
- Beleites, C.; Neugebauer, U.; Bocklitz, T.; Krafft, C.; Popp, J. Sample size planning for classification models. Anal. Chim. Acta 2013, 760, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakidou, M.; Anastassopoulou, J.; Tsakiris, A.; Koui, M.; Theophanides, T. FT-IR spectroscopy study in early diagnosis of skin cancer. In Vivo 2017, 31, 1131–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theophilou, G.; Paraskevaidi, M.; Lima, K.M.; Kyrgiou, M.; Martin-Hirsch, P.L.; Martin, F.L. Extracting biomarkers of commitment to cancer development: Potential role of vibrational spectroscopy in systems biology. Expert Rev. Mol. Diagn. 2015, 15, 693–713. [Google Scholar] [CrossRef] [PubMed]

| Wavenumber (cm−1) | Assignment |
|---|---|
| 3080–2800 | Anti-symmetric and symmetric C–H stretches from proteins and lipids |
| 1745–1725 | Ester carbonyl of lipids |
| 1700–1500 | Amide I and II groups in peptide linkages of proteins |
| 1270–1080 | Anti-symmetric and symmetric C−O and P−O areas in DNA, RNA and phospholipids |
| 1200–900 | Carbohydrate vibrations of glucose, fructose and glycogen |
| Cancer Type | Title of Study | References |
|---|---|---|
| Colorectal Cancer | Application of linear discriminant analysis and attenuated total reflectance Fourier transform infrared microspectroscopy for diagnosis of colon cancer | [4] |
| The use of FTIR-ATR spectrometry for evaluation of surgical resection margin in colorectal cancer: a pilot study of 56 samples | [24] | |
| Early detection of colorectal cancer relapse by infrared spectroscopy in “normal” anastomosis tissue | [23] | |
| Use of FTIR spectroscopy and PCA-LDC analysis to identify cancerous lesions within the human colon | [22] | |
| Prostate Cancer | Study of prostate cancer-derived extracellular vesicles in urine using IR spectroscopy | [26] |
| Investigating FTIR based histopathology for the diagnosis of prostate cancer | [20] | |
| A specific spectral signature of serum and plasma-derived extracellular vesicles for cancer screening | [25] | |
| Leukemia | Distinction of leukemia patients’ and healthy persons’ serum using FTIR spectroscopy | [17] |
| Pre-screening and follow-up of childhood acute leukemia using biochemical infrared analysis of peripheral blood mononuclear cells | [19] | |
| Ovarian and/or Endometrial Cancers | Potential of mid-infrared spectroscopy as a non-invasive diagnostic test in urine for endometrial or ovarian cancer | [28] |
| Segregation of ovarian cancer stage exploiting spectral biomarkers derived from blood plasma or serum analysis: ATR-FTIR spectroscopy coupled with variable selection methods | [16] | |
| Lung Cancer | Evaluation of FTIR spectroscopy as a diagnostic tool for lung cancer using sputum | [31] |
| Marker-free automated histopathological annotation of lung tumor subtypes by FTIR imaging | [21] | |
| Oral, Oropharyngeal, and/or Laryngeal Cancer | Fourier transform infrared for noninvasive optical diagnosis of oral, oropharyngeal, and laryngeal cancer | [32] |
| FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer | [27] | |
| Gastric Cancer | Comparison of serum from gastric cancer patients and from healthy persons using FTIR spectroscopy | [18] |
| Breast Cancer | Diagnosis of breast cancer with infrared spectroscopy from serum samples | [15] |
| Bladder Cancer | Bladder cancer diagnosis from bladder wash by Fourier transform infrared spectroscopy as a novel test for tumor recurrence | [29] |
| MalignantBiliary Strictures | Bile analysis using high-throughput FTIR spectroscopy for the diagnosis of malignant biliary strictures: a pilot study in 57 patients | [30] |
| Skin Cancer | FT-IR spectroscopy study in early diagnosis of skin cancer | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, K.-Y.; Lee, W.-L. Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects. Cancers 2020, 12, 115. https://doi.org/10.3390/cancers12010115
Su K-Y, Lee W-L. Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects. Cancers. 2020; 12(1):115. https://doi.org/10.3390/cancers12010115
Chicago/Turabian StyleSu, Kar-Yan, and Wai-Leng Lee. 2020. "Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects" Cancers 12, no. 1: 115. https://doi.org/10.3390/cancers12010115
APA StyleSu, K.-Y., & Lee, W.-L. (2020). Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects. Cancers, 12(1), 115. https://doi.org/10.3390/cancers12010115