Preoperative Assessment of Upper Extremity Secondary Lymphedema
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Limb Volume Measurement
2.3. Bio-Impedance Measurement
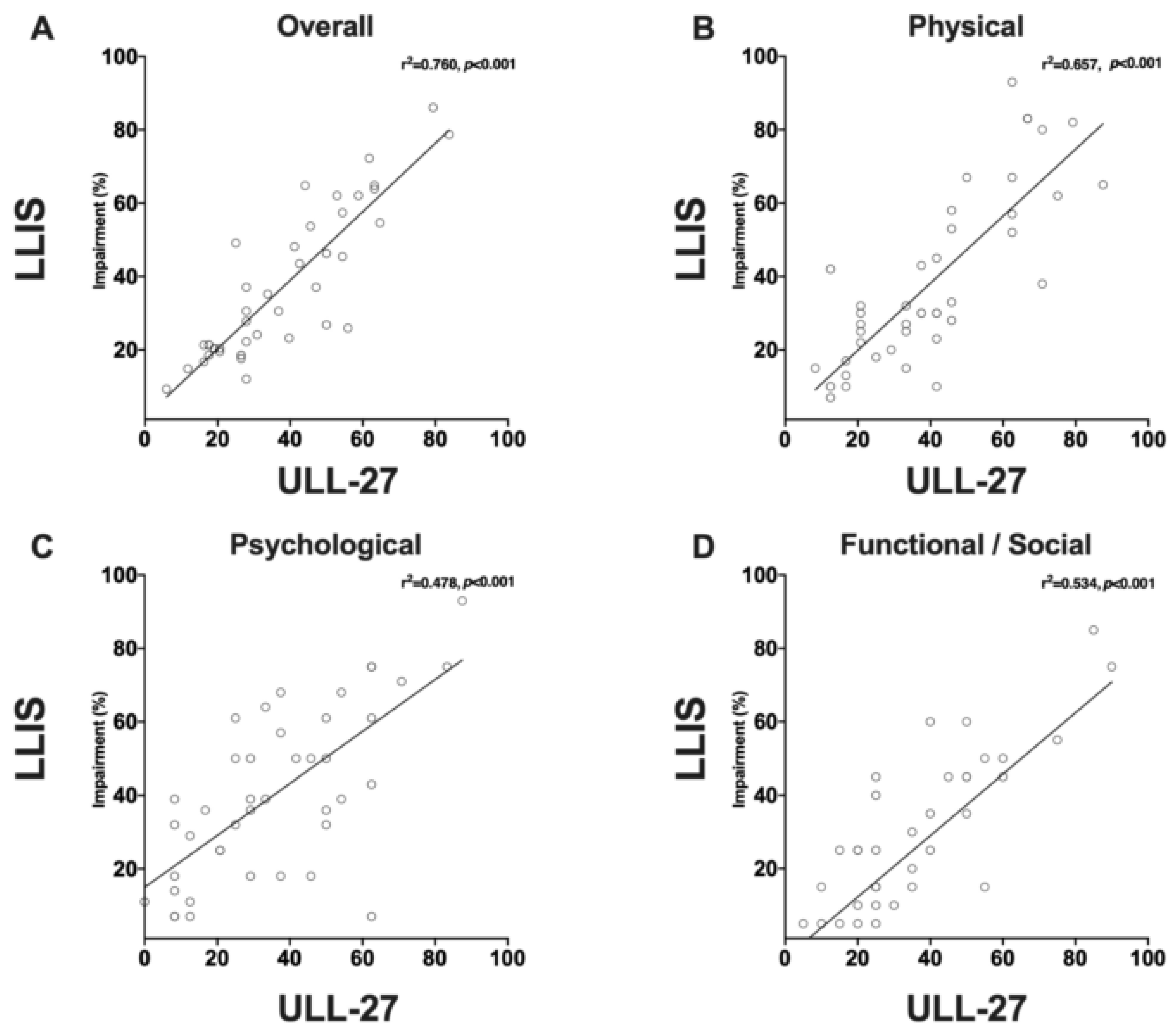
2.4. Quality of Life Measurement
2.5. MRA Imaging
2.6. Lymphoscintigraphy
2.7. ICG Lymphography
2.8. Statistical Methods
3. Results
3.1. Patient Demographics
3.2. Limb Volume Measurements Are More Sensitive and Specific than Circumferential Measurements
3.3. Bio-Impedance Measurements Are a Sensitive Means of Diagnosing Early Stage Lymphedema
3.4. Even Minor Changes in Limb Volume can Have Significant Effects on PRO Measures
3.5. Lymphoscintigraphy Has a Low Specificity and Positive Predictive Value for Lymphedema Diagnosis but May Be Useful for Surgical Planning
3.6. ICG Lymphography Is Highly Sensitive for Detecting Lymphedema
3.7. MRA Imaging Is Useful for Identification of Abnormalities in Venous Outflow and Diagnosis of Lymphedema
4. Discussion
4.1. ISL Staging Is Subjective and Not Useful for Preoperative Classification
4.2. Limb Volume Measurements Are More Sensitive than Circumferential Changes
4.3. Bio-Impedance Is Sensitive and Specific but Requires Optimization for Reproducible Results
4.4. PROMs
4.5. MRA Imaging
4.6. Lymphoscintigraphy
4.7. ICG Lymphography
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dayan, J.H.; Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Lymphedema: Pathogenesis and Novel Therapies. Annu. Rev. Med. 2018, 69, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, E.O.; Nelson, J.A.; Verma, R.; Mbabuike, J.; Mehrara, B.J.; Dayan, J.H. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J. Surg. Oncol. 2018, 117, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.J.; Mehrara, B.J.; Neligan, P.; Cheng, M.H.; Patel, K.M. Lymph node transplantation for the treatment of lymphedema. J. Surg. Oncol. 2018, 118, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Skoracki, R.J.; Chang, D.W. Lymphovenous Anastomosis Bypass Surgery. Semin. Plast. Surg. 2018, 32, 22–27. [Google Scholar] [PubMed]
- Greene, A.K.; Voss, S.D.; Maclellan, R.A. Liposuction for Swelling in Patients with Lymphedema. N. Engl. J. Med. 2017, 377, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hara, H.; Kawakami, Y.; Zhou, H.P.; Tange, S.; Kikuchi, K.; Iida, T. Multi-site lymphatic venous anastomosis using echography to detect suitable subcutaneous vein in severe lymphedema patients. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, e1–e7. [Google Scholar] [CrossRef]
- Cornelissen, A.J.M.; Beugels, J.; Ewalds, L.; Heuts, E.M.; Keuter, X.H.A.; Piatkowski, A.; van der Hulst, R.R.W.J.; Qiu Shao, S.S. The Effect of Lymphaticovenous Anastomosis in Breast Cancer-Related Lymphedema: A Review of the Literature. Lymphat. Res. Biol. 2018, 16, 426–434. [Google Scholar] [CrossRef]
- Giacalone, G.; Yamamoto, T. Supermicrosurgical lymphaticovenous anastomosis for a patient with breast lymphedema secondary to breast cancer treatment. Microsurgery 2017, 37, 680–683. [Google Scholar] [CrossRef]
- Baltzer, H.L.; Winocour, S.; Harless, C.; Saint-Cyr, M. Lymphaticovenous Bypass: Adaptations and Lessons Learned. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1328. [Google Scholar] [CrossRef]
- Ciudad, P.; Mouchammed, A.; Manrique, O.J.; Chang, W.L.; Huang, T.C.T.; Chen, H.C. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center’s approach to the treatment of lymphedema. J. Surg. Oncol. 2018, 117, 1346–1347. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Suami, H.; Hanasono, M.M.; Womack, V.A.; Wong, F.C.; Chang, E.I. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Gratzon, A.; Schultz, J.; Secrest, K.; Lee, K.; Feiner, J.; Klein, R.D. Clinical and Psychosocial Outcomes of Vascularized Lymph Node Transfer for the Treatment of Upper Extremity Lymphedema After Breast Cancer Therapy. Ann. Surg. Oncol. 2017, 24, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Wee, C.; Meyerson, J.; Eiferman, D.; Skoracki, R. Vascularized Jejunal Mesenteric Lymph Node Transfer: A Novel Surgical Treatment for Extremity Lymphedema. J. Am. Coll. Surg. 2017, 225, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.G.; Mayo, W.; Notohamiprodjo, M.; Wallmichrath, J.; Springer, S.; Frick, A. Microsurgical Lymphatic Vessel Transplantation. J. Reconstr. Microsurg. 2016, 32, 34–41. [Google Scholar]
- Dadras, M.; Mallinger, P.J.; Corterier, C.C.; Theodosiadi, S.; Ghods, M. Liposuction in the Treatment of Lipedema: A Longitudinal Study. Arch. Plast. Surg. 2017, 44, 324–331. [Google Scholar] [CrossRef]
- Pappalardo, M.; Patel, K.; Cheng, M.H. Vascularized lymph node transfer for treatment of extremity lymphedema: An overview of current controversies regarding donor sites, recipient sites and outcomes. J. Surg. Oncol. 2018, 117, 1420–1431. [Google Scholar] [CrossRef]
- Agko, M.; Ciudad, P.; Chen, H.C. Staged surgical treatment of extremity lymphedema with dual gastroepiploic vascularized lymph node transfers followed by suction-assisted lipectomy-A prospective study. J. Surg. Oncol. 2018, 117, 1148–1156. [Google Scholar] [CrossRef]
- Mardonado, A.A.; Chen, R.; Chang, D.W. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J. Surg. Oncol. 2017, 115, 68–71. [Google Scholar] [CrossRef]
- Masià, J.; Pons, G.; Rodriguez-Bauza, E. Barcelona Lymphedema Algorithm for Surgical Treatment in Breast Cancer-Related Lymphedema. J. Reconstr. Microsurg. 2016, 32, 329–335. [Google Scholar]
- Granzow, J.W.; Soderberg, J.M.; Kaji, A.H.; Dauphine, C. An effective system of surgical treatment of lymphedema. Ann. Surg. Oncol. 2014, 21, 1189–1194. [Google Scholar] [CrossRef]
- Ryan, M.; Campisi, C.C.; Boccardo, F.; Campisi, C. Surgical treatment for lymphedema: Optimal timing and optimal techniques. J. Am. Coll. Surg. 2013, 216, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.M.; Casabona, F.; Friedman, D.; Puglisi, M.; De Cian, F.; Ansaldi, F.; Campisi, C. Surgical prevention of arm lymphedema after breast cancer treatment. Ann. Surg. Oncol. 2011, 18, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.J.M.; Kool, M.; Keuter, X.H.A.; Heuts, E.M.; Piatkowski de Grzymala, A.A.; van der Hulst, R.; Qiu, S.S. Quality of Life Questionnaires in Breast Cancer-Related Lymphedema Patients: Review of the Literature. Lymphat. Res. Biol. 2018, 16, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Daniel, T. VALIDATION OF THE LYMPHEDEMA LIFE IMPACT SCALE (LLIS): A CONDITION-SPECIFIC MEASUREMENT TOOL FOR PERSONS WITH LYMPHEDEMA. Lymphology 2015, 48, 128–138. [Google Scholar] [PubMed]
- Viehoff, P.B.; van Genderen, F.R.; Wittink, H. Upper limb lymphedema 27 (ULL27): Dutch translation and validation of an illness-specific health-related quality of life questionnaire for patients with upper limb lymphedema. Lymphology 2008, 41, 131–138. [Google Scholar]
- Alliot, F.; Launois, R. Lymphedema and quality of life. Rev. Med. Interne. 2002, 23, 431s–435s. [Google Scholar] [CrossRef]
- Brorson, H.; Höijer, P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J. Plast. Surg. Hand. Surg. 2012, 46, 410–415. [Google Scholar] [CrossRef]
- Armer, J.M.; Stewart, B.R. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat. Res. Biol. 2005, 3, 208–217. [Google Scholar] [CrossRef]
- Fu, M.R.; Cleland, C.M.; Guth, A.A.; Kayal, M.; Haber, J.; Cartwright, F.; Kleinman, R.; Kang, Y.; Scagliola, J.; Axelrod, D. L-dex ratio in detecting breast cancer-related lymphedema: Reliability, sensitivity, and specificity. Lymphology 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Seward, C.; Skolny, M.; Brunelle, C.; Asdourian, M.; Salama, L.; Taghian, A.G. A comprehensive review of bioimpedance spectroscopy as a diagnostic tool for the detection and measurement of breast cancer-related lymphedema. J. Surg. Oncol. 2016, 114, 537–542. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Hara, H.; Mihara, M.; Narushima, M.; Koshima, I. Upper extremity lymphedema index: A simple method for severity evaluation of upper extremity lymphedema. Ann. Plast. Surg. 2013, 70, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Garza, R.M.; Ooi, A.S.H.; Falk, J.; Chang, D.W. The Relationship Between Clinical and Indocyanine Green Staging in Lymphedema. Lymphat. Res. Biol. 2019, 17, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Morris, C.M.; Czerniec, S.A.; Mangion, A.J. Does Lymphedema Severity Affect Quality of Life? Simple Question. Challenging Answers. Lymphat. Res. Biol. 2018, 16, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Laidley, A.; Anglin, B. The Impact of L-Dex(®) Measurements in Assessing Breast Cancer-Related Lymphedema as Part of Routine Clinical Practice. Front. Oncol. 2016, 6, 192. [Google Scholar] [CrossRef]
- Vicini, F.; Shah, C.; Lyden, M.; Whitworth, P. Bioelectrical impedance for detecting and monitoring patients for the development of upper limb lymphedema in the clinic. Clin. Breast Cancer 2012, 12, 133–137. [Google Scholar] [CrossRef]
- Gaw, R.; Box, R.; Cornish, B. Bioimpedance in the assessment of unilateral lymphedema of a limb: The optimal frequency. Lymphat. Res. Biol. 2011, 9, 93–99. [Google Scholar] [CrossRef]
- Coroneos, C.J.; Wong, F.C.; DeSnyder, S.M.; Shaitelman, S.F.; Schaverien, M.V. Correlation of L-Dex Bioimpedance Spectroscopy with Limb Volume and Lymphatic Function in Lymphedema. Lymphat. Res. Biol. 2019, 17, 301–307. [Google Scholar] [CrossRef]
- Imamoğlu, N.; Karadibak, D.; Ergin, G.; Yavuzşen, T. The Effect of Education on Upper Extremity Function in Patients with Lymphedema after Breast Cancer Treatments. Lymphat. Res. Biol. 2016, 14, 142–147. [Google Scholar] [CrossRef]
- Pinto, M.; Gimigliano, F.; Tatangelo, F.; Megna, M.; Izzo, F.; Gimigliano, R.; Iolascon, G. Upper limb function and quality of life in breast cancer related lymphedema: A cross-sectional study. Eur. J. Phys Rehabil. Med. 2013, 49, 665–673. [Google Scholar]
- van de Pas, C.B.; Biemans, A.A.; Boonen, R.S.; Viehoff, P.B.; Neumann, H.A. Validation of the Lymphoedema Quality-of-Life Questionnaire (LYMQOL) in Dutch Patients Diagnosed with Lymphoedema of the Lower Limbs. Phlebology 2016, 31, 257–263. [Google Scholar] [CrossRef]
- Tsauo, J.Y.; Hung, H.C.; Tsai, H.J.; Huang, C.S. Can ICF model for patients with breast-cancer-related lymphedema predict quality of life? Support. Care Cancer 2011, 19, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Yoo, R.E.; Choi, S.H.; Park, S.O.; Chang, H.; Suh, M.; Cheon, G.J. Evaluation of lymphedema in upper extremities by MR lymphangiography: Comparison with lymphoscintigraphy. Magn. Reson. Imaging 2018, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Neligan, P.C.; Kung, T.A.; Maki, J.H. MR lymphangiography in the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Maclellan, R.A.; Zurakowski, D.; Voss, S.; Greene, A.K. Correlation between Lymphedema Disease Severity and Lymphoscintigraphic Findings: A Clinical-Radiologic Study. J. Am. Coll. Surg. 2017, 225, 366–370. [Google Scholar] [CrossRef]
- Hassanein, A.H.; Maclellan, R.A.; Greene, A.K. Abstract: Diagnostic Accuracy of Lymphoscintigraphy for Lymphedema. Plast. Reconstr. Surg. Glob. Open 2016, 4, 154–155. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: A novel severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, N.; Kikuchi, K.; Todokoro, T.; Iida, T.; Koshima, I. Dynamic Indocyanine Green (ICG) lymphography for breast cancer-related arm lymphedema. Ann. Plast. Surg. 2014, 73, 706–709. [Google Scholar] [CrossRef]
- Mihara, M.; Hayashi, Y.; Hara, H.; Iida, T.; Narushima, M.; Yamamoto, T.; Todokoro, T.; Murai, N.; Koshima, I. High-accuracy diagnosis and regional classification of lymphedema using indocyanine green fluorescent lymphography after gynecologic cancer treatment. Ann. Plast. Surg. 2014, 72, 204–208. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Araki, J.; Kikuchi, K.; Narushima, M.; Yamamoto, T.; Iida, T.; Yoshimatsu, H.; Murai, N.; Mitsui, K.; et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS ONE 2012, 7, e38182. [Google Scholar] [CrossRef]







| Characteristic | Average ± SD or No. () | |
|---|---|---|
| N | 118 | |
| Right Upper Extremity | 61 (51.7) | |
| Age (Years) | 54 ± 11 | |
| Female Sex | 116 (98.3) | |
| BMI | 26.1 ± 3.9 | |
| Cancer Site | Breast | 96 (81.3) |
| Other | 22 (18.7) | |
| Lymphedema Duration (Months) | 41 ± 54 | |
| Chemotherapy | 112 (95) | |
| Radiotherapy | Axilla | 69 (59) |
| Chest | 101 (85.6) | |
| Axillary Lymph Node Dissection | Complete | 101 (85.6) |
| Sentinel | 14 (12) | |
| None | 3 (2.4) | |
| ISL Stage | 0 | 2 (1.7) |
| 1 | 28 (23.8) | |
| 2 | 85 (72) | |
| 3 | 3 (2.5) | |
| Referral Source | Self | 42 (35.6) |
| Breast Surgeon | 25 (21.2) | |
| Plastic Surgeon | 23 (19.5) | |
| Other | 28 (23.7) | |
| Compression Therapy | Around the Clock | 55 (47) |
| Day or Night | 26 (22.2) | |
| Occasionally | 24 (20.5) | |
| Never | 12 (10.3) |
| Test | Sensitivity | Specificity | Negative | Positive |
|---|---|---|---|---|
| Circumference Diff. >2 cm | 82.8 | 85.3 | 90.6 | 74.4 |
| L-Dex >10 | 91.2 | 77.5 | 83.8 | 87.3 |
| Pathological changes on LS | 88.0 | 41.4 | 66.7 | 72.1 |
| MRA Fluid Accumulation | 94.2 | 44.0 | 78.6 | 77.8 |
| MRA Fat Hypertrophy | 96.2 | 64.0 | 84.8 | 88.9 |
| Test | Physical | Domains Psychological | Functional (LLIS)/Social (ULL-27) | Overall |
|---|---|---|---|---|
| LLIS | 40.7 ± 20.7% | 35.5 ± 22.2% | 34.8 ± 19.5% | 37.2 ± 18.8% |
| ULL-27 | 38.8 ± 23.6% | 40.4 ± 22.7% | 25.6 ± 22.5% | 36.8 ± 20.3% |
| Imaging Modality | Characteristic | No. (%) |
|---|---|---|
| Lymphoscintigraphy | Axillary Uptake at 3 h | 26 (33) |
| Dermal Backflow | 24 (30.4) | |
| ICG Lymphography | Stage 0 (“Linear”) | 0 (0) |
| Stage 1 (“Splash”) | 20 (20.6) | |
| Stage 2 (“Stardust”) | 62 (64) | |
| Stage 3 (“Diffuse”) | 15 (15.4) | |
| MRA | Fluid Accumulation | 64 (82) |
| Fat Accumulation | 60 (77) | |
| Recurrence | 1 (1.3) | |
| Axillary Vascular Abnormality | 12 (15.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiser, I.; Mehrara, B.J.; Coriddi, M.; Kenworthy, E.; Cavalli, M.; Encarnacion, E.; Dayan, J.H. Preoperative Assessment of Upper Extremity Secondary Lymphedema. Cancers 2020, 12, 135. https://doi.org/10.3390/cancers12010135
Wiser I, Mehrara BJ, Coriddi M, Kenworthy E, Cavalli M, Encarnacion E, Dayan JH. Preoperative Assessment of Upper Extremity Secondary Lymphedema. Cancers. 2020; 12(1):135. https://doi.org/10.3390/cancers12010135
Chicago/Turabian StyleWiser, Itay, Babak J. Mehrara, Michelle Coriddi, Elizabeth Kenworthy, Michele Cavalli, Elizabeth Encarnacion, and Joseph H. Dayan. 2020. "Preoperative Assessment of Upper Extremity Secondary Lymphedema" Cancers 12, no. 1: 135. https://doi.org/10.3390/cancers12010135
APA StyleWiser, I., Mehrara, B. J., Coriddi, M., Kenworthy, E., Cavalli, M., Encarnacion, E., & Dayan, J. H. (2020). Preoperative Assessment of Upper Extremity Secondary Lymphedema. Cancers, 12(1), 135. https://doi.org/10.3390/cancers12010135






