Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways
Abstract
1. Introduction
2. Results
2.1. DSF/Cu Irreversibly Reduces the Viability of NPC Cells
2.2. DSF/Cu Induces Both Apoptosis and Necrosis in NPC Cells by an ALDH-Independent Method
2.3. DSF/Cu Promotes NPC Cell Apoptosis Via ROS/MAPK Pathways
2.4. p53-Mediated Ferroptosis Plays an Important Role in DSF/Cu-Induced Cell Death
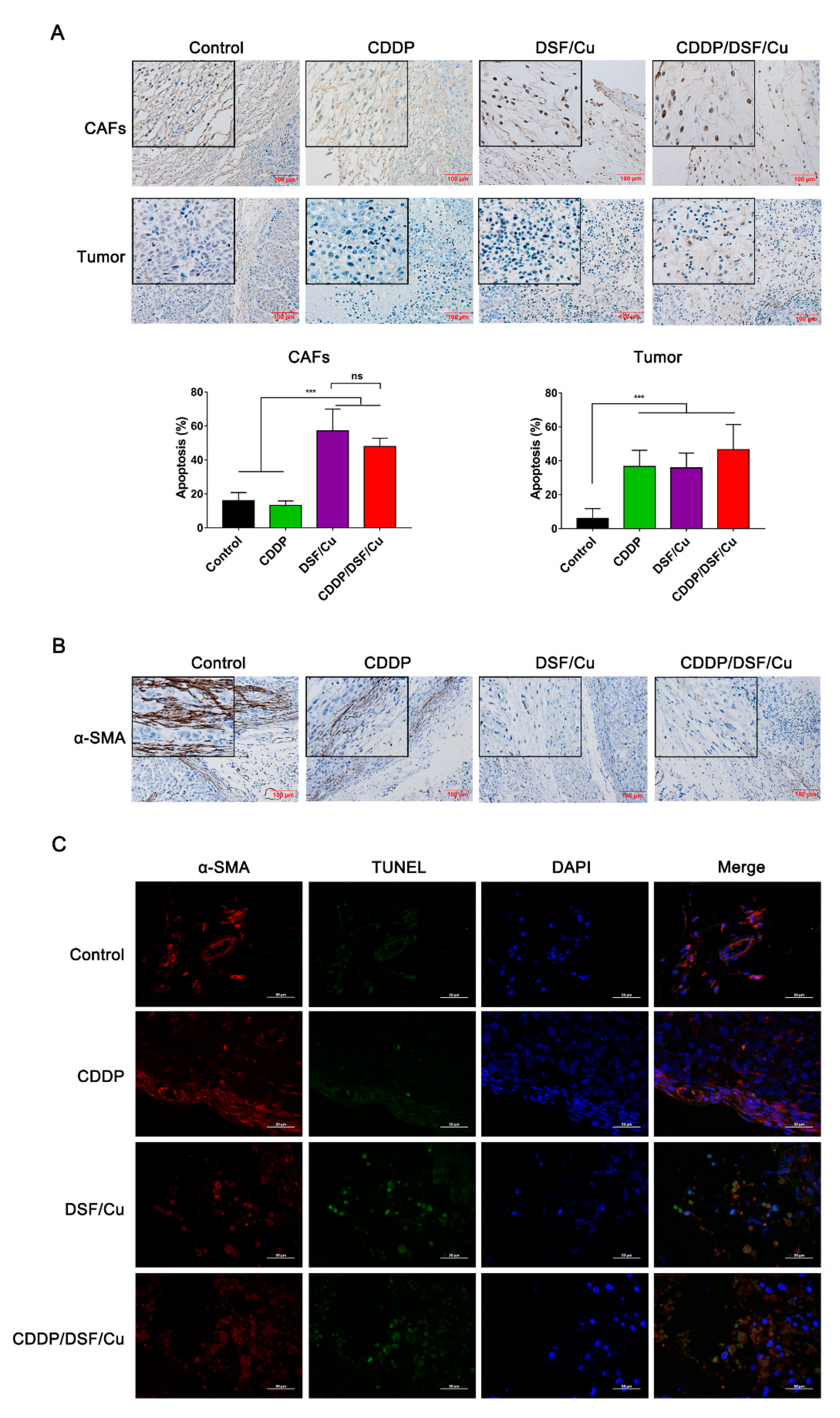
2.5. DSF/Cu Reduces the Viability of Fibroblasts and Attenuates Fibroblast Activation
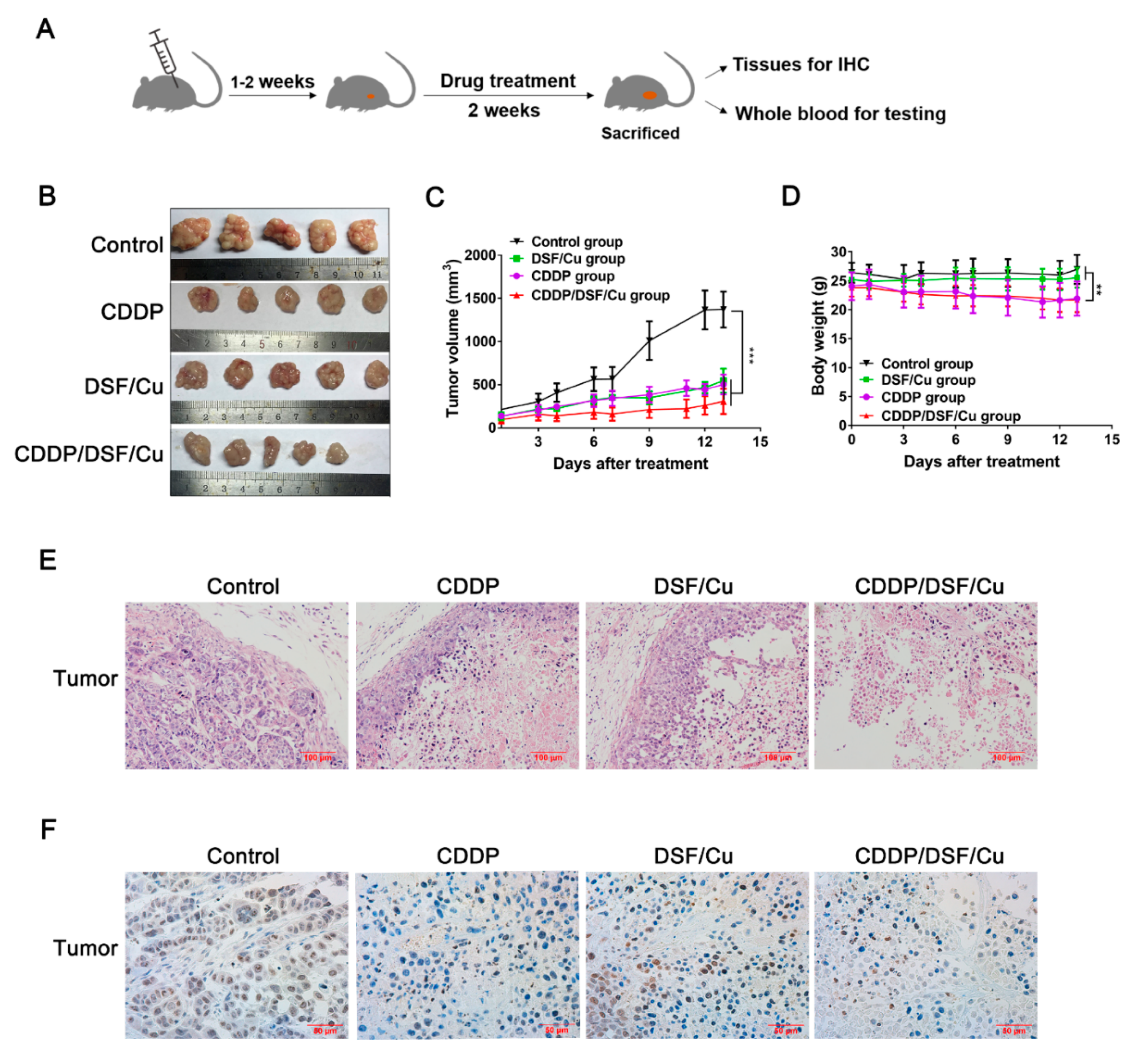
2.6. Antitumor Activity of DSF/Cu against NPC In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Cell Viability Assay
4.3. Colony-Forming Assay
4.4. Apoptosis Assessment
4.5. Analysis of ROS Production
4.6. siRNA
4.7. qRT-PCR and RNA Sequencing
4.8. Western Blot Analysis
4.9. In Vivo Tumor Experiments
4.10. Biochemical Indices, Blood Routine Indices and Determination of Copper Ions
4.11. Histology and Immunofluorescence
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, S.J.; Tang, Y.Y.; Liu, H.M.; Tan, G.X.; Wang, X.; Zhang, H.; Yang, F.; Yang, S. Impact of age on survival of locoregional nasopharyngeal carcinoma: An analysis of the surveillance, epidemiology, and end results program database, 2004–2013. Clin. Otolaryngol. 2018, 43, 1209–1218. [Google Scholar] [CrossRef]
- Zhang, L.-F.; Li, Y.-H.; Xie, S.-H.; Ling, W.; Chen, S.-H.; Liu, Q.; Huang, Q.-H.; Cao, S.-M. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: An age-period-cohort analysis. Chin. J. Cancer 2015, 34, 350–357. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Yu, C. Mortality trend in nasopharynx cancer in Chinese resident from 1987 to 2015. J. Cent. South Univ. Med Sci. 2018, 43, 760–766. [Google Scholar]
- McDowell, L.J.; Rock, K.; Xu, W.; Chan, B.; Waldron, J.; Lu, L.; Ezzat, S.; Pothier, D.; Bernstein, L.J.; So, N.; et al. Long-Term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int. J. Radiat. Oncol. 2018, 102, 340–352. [Google Scholar] [CrossRef]
- Lu, S.; Wei, J.; Sun, F.; Xiao, W.; Cai, R.; Zhen, Z.; Zhu, J.; Wang, J.; Huang, J.; Lu, L.; et al. Late Sequelae of childhood and adolescent nasopharyngeal carcinoma survivors after radiation therapy. Int. J. Radiat. Oncol. 2019, 103, 45–51. [Google Scholar] [CrossRef]
- Suh, J.J.; Pettinati, H.M.; Kampman, K.M.; O’Brien, C.P. The status of disulfiram. J. Clin. Psychopharmacol. 2006, 26, 290–302. [Google Scholar] [CrossRef]
- Jiao, Y.; Hannafon, B.N.; Ding, W.-Q. Disulfiram’s anticancer activity: Evidences and mechanisms. Anti-Cancer Agents Med. Chem. 2016, 16, 1378–1384. [Google Scholar] [CrossRef]
- Irving, C.C.; Tice, A.J.; Murphy, W.M. Inhibition of N-n-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder cancer in rats by administration of disulfiram in the diet. Cancer Res. 1979, 39, 3040. [Google Scholar]
- Chen, D.; Dou, Q.P. New uses for old copper-binding drugs: Converting the pro-angiogenic copper to a specific cancer cell death inducer. Expert Opin. Ther. Targets 2008, 12, 739–748. [Google Scholar] [CrossRef]
- Han, J.; Liu, L.; Yue, X.; Chang, J.; Shi, W.; Hua, Y. A binuclear complex constituted by diethyldithiocarbamate and copper(I) functions as a proteasome activity inhibitor in pancreatic cancer cultures and xenografts. Toxicol. Appl. Pharmacol. 2013, 273, 477–483. [Google Scholar] [CrossRef]
- Skrott, Z.; Mistrik, M.; Andersen, K.K.; Friis, S.; Majera, D.; Gursky, J.; Ozdian, T.; Bartkova, J.; Turi, Z.; Moudry, P.; et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017, 552, 194–199. [Google Scholar] [CrossRef]
- Cen, D.; Brayton, D.; Shahandeh, B.; Meyskens, F.L.; Farmer, P.J. Disulfiram facilitates intracellular cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem. 2004, 47, 6914–6920. [Google Scholar] [CrossRef]
- Hassani, S.; Ghaffari, P.; Chahardouli, B.; Alimoghaddam, K.; Ghavamzadeh, A.; Alizadeh, S.; Ghaffari, S.H. Disulfiram/copper causes ROS levels alteration, cell cycle inhibition, and apoptosis in acute myeloid leukaemia cell lines with modulation in the expression of related genes. Biomed. Pharmacother. 2018, 99, 561–569. [Google Scholar] [CrossRef]
- Xu, B.; Wang, S.Y.; Li, R.W.; Chen, K.; He, L.L.; Deng, M.M.; Kannappan, V.; Zha, J.; Dong, H.J.; Wang, W.G. Disulfiram/copper selectively eradicates AML leukemia stem cells in vitro and in vivo by simultaneous induction of ROS-JNK and inhibition of NF-КB and Nrf2. Cell Death Dis. 2017, 8, e2797. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, V.; Lal, N.; Yadav, S.K.; Sarkar, S.; Mandalapu, D.; Porwal, K.; Rawat, T.; Maikhuri, J.P.; Rajender, S.; et al. Disulfiram and its novel derivative sensitize prostate cancer cells to the growth regulatory mechanisms of the cell by re-expressing the epigenetically repressed tumor suppressor-estrogen receptor β. Mol. Carcinog. 2016, 55, 1843–1857. [Google Scholar] [CrossRef]
- Duan, L.; Shen, H.; Zhao, G.; Yang, R.; Cai, X.; Zhang, L.; Jin, C.; Huang, Y. Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 1010–1016. [Google Scholar] [CrossRef]
- Wang, W.G.; McLeod, H.L.; Cassidy, J. Disulfiram-mediated inhibition of NF-КB activity enhances cytotoxicity of 5-fluorouracil in human colorectal cancer cell lines. Int. J. Cancer 2003, 104, 504–511. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free. Radic. Boil. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.J.; Li, D.W.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, D.H.; Jeong, S.Y.; Park, S.H.; Oh, S.C.; Park, Y.S.; Yu, J.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-inducing agents enhance TRAIL-induced apoptosis through upregulation of death receptor 5. J. Cell. Biochem. 2019, 120, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.A.; Xia, H.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Yip, N.C.; Fombon, I.S.; Liu, P.; Brown, S.; Kannappan, V.; Armesilla, A.L.; Xu, B.; Cassidy, J.; Darling, J.L.; Wang, W. Disulfiram modulated ROS–MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer 2011, 104, 1564–1574. [Google Scholar] [CrossRef]
- Jin, N.; Zhu, X.; Cheng, F.; Zhang, L. Disulfiram/copper targets stem cell-like ALDH(+) population of multiple myeloma by inhibition of ALDH1A1 and Hedgehog pathway. J. Cell. Biochem. 2018, 119, 6882–6893. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Cui, W.; Yuan, X.; Lin, L.; Cao, Q.; Wang, N.; Li, Y.; Guo, W.; Zhang, X.; et al. Targeting ALDH1A1 by disulfiram/copper complex inhibits non-small cell lung cancer recurrence driven by ALDH-positive cancer stem cells. Oncotarget 2016, 7, 58516–58530. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T Cells to protect tumour cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.M.; Tsao, S.W.; Tsang, C.M. Interplay of viral infection, host cell factors and tumor microenvironment in the pathogenesis of nasopharyngeal carcinoma. Cancers 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Michael, F.B.N.; Michael, B.M. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J. Gastroenterol. 2016, 22, 2678–2700. [Google Scholar]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Chen, J.; Yang, P.; Xiao, Y.; Zhang, Y.; Liu, J.; Xie, D.; Cai, M.; Zhang, X. Overexpression of α-sma-positive fibroblasts (CAFs) in nasopharyngeal carcinoma predicts poor prognosis. J. Cancer 2017, 8, 3897–3902. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J.; Zhao, C.; Hou, X.; Li, M.; Wang, L.; Chen, L.; Chen, Y.; Zhu, L.; Yang, H. Antitumor effects of disulfiram/copper complex in the poorly-differentiated nasopharyngeal carcinoma cells via activating ClC-3 chloride channel. Biomed. Pharmacother. 2019, 120, 109529. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Sun, X.; Zhou, C.; Wang, Y.; Wang, L.; Chen, L.; Liang, Z.; Zhu, L.; Yang, H. The selective cytotoxicity of DSF-Cu attributes to the biomechanical properties and cytoskeleton rearrangements in the normal and cancerous nasopharyngeal epithelial cells. Int. J. Biochem. Cell Boil. 2017, 84, 96–108. [Google Scholar] [CrossRef]
- Chakravarty, P.K.; Ghosh, A.; Chowdhury, J.R. Effect of tumour regression on serum and tissue copper concentration in mice bearing induced fibrosarcoma. Z. Nat. C 1986, 41, 956–958. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharm. Pharm. 2010, 1, 87–93. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, F.; Chen, J.; Chan, S.; He, Y.; Liu, W.; Zhang, G. Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways. Cancers 2020, 12, 138. https://doi.org/10.3390/cancers12010138
Li Y, Chen F, Chen J, Chan S, He Y, Liu W, Zhang G. Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways. Cancers. 2020; 12(1):138. https://doi.org/10.3390/cancers12010138
Chicago/Turabian StyleLi, Yiqiu, Fangfang Chen, Jun Chen, Siocheong Chan, Yi He, Wanli Liu, and Ge Zhang. 2020. "Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways" Cancers 12, no. 1: 138. https://doi.org/10.3390/cancers12010138
APA StyleLi, Y., Chen, F., Chen, J., Chan, S., He, Y., Liu, W., & Zhang, G. (2020). Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways. Cancers, 12(1), 138. https://doi.org/10.3390/cancers12010138



