Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Measured Outcomes of CT-based Anthropometric Values
4.2. Defining the Cut-Off Values
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. Esmo consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef]
- NCCN Guidelines®. National Comprehensive Cancer Network (nccn) Guidelines for Treatment of Cancer by Site. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 30 September 2019).
- Boyne, D.J.; Cuthbert, C.A.; O’Sullivan, D.E.; Sajobi, T.T.; Hilsden, R.J.; Friedenreich, C.M.; Cheung, W.Y.; Brenner, D.R. Association between adjuvant chemotherapy duration and survival among patients with stage ii and iii colon cancer: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e194154. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.; Kennedy, E.B.; Bergsland, E.; Berlin, J.; George, T.J.; Gill, S.; Gold, P.J.; Hantel, A.; Jones, L.; Mahmoud, N.; et al. Duration of oxaliplatin-containing adjuvant therapy for stage iii colon cancer: Asco clinical practice guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 658–665. [Google Scholar]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Williams, G.R.; Nyrop, K.A.; Popuri, K.; Choi, S.K.; Muss, H.B. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Sandini, M.; Patino, M.; Ferrone, C.R.; Alvarez-Perez, C.A.; Honselmann, K.C.; Paiella, S.; Catania, M.; Riva, L.; Tedesco, G.; Casolino, R.; et al. Association between changes in body composition and neoadjuvant treatment for pancreatic cancer. JAMA Surg. 2018, 153, 809–815. [Google Scholar] [PubMed]
- Lee, J.; Chang, C.L.; Lin, J.B.; Wu, M.H.; Sun, F.J.; Jan, Y.T.; Hsu, S.M.; Chen, Y.J. Skeletal muscle loss is an imaging biomarker of outcome after definitive chemoradiotherapy for locally advanced cervical cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5028–5036. [Google Scholar] [CrossRef]
- Lee, J.; Lin, J.B.; Wu, M.H.; Jan, Y.T.; Chang, C.L.; Huang, C.Y.; Sun, F.J.; Chen, Y.J. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 814–826. [Google Scholar] [CrossRef]
- Chung, E.; Lee, H.S.; Cho, E.S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Prognostic significance of sarcopenia and skeletal muscle mass change during preoperative chemoradiotherapy in locally advanced rectal cancer. Clin. Nutr. (Edinb. Scotl.) 2019. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Petit, J.M.; Bonnetain, F.; Ladoire, S.; Guiu, S.; Cercueil, J.P.; Krause, D.; Hillon, P.; Borg, C.; Chauffert, B.; et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010, 59, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kurk, S.; Peeters, P.; Stellato, R.; Dorresteijn, B.; de Jong, P.; Jourdan, M.; Creemers, G.J.; Erdkamp, F.; de Jongh, F.; Kint, P.; et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Watanabe, M.; et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS ONE 2015, 10, e0129742. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Caan, B.J.; Meyerhardt, J.A.; Weltzien, E.; Xiao, J.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Castillo, A.; Kwan, M.L.; Prado, C.M. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage i-iii colorectal cancer: A population-based cohort study (c-scans). J. Cachexia Sarcopenia Muscle 2018, 9, 664–672. [Google Scholar] [CrossRef]
- Hopkins, J.J.; Reif, R.; Bigam, D.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.M. Change in skeletal muscle following resection of stage I to iii colorectal cancer is predictive of poor survival: A cohort study. World J. Surg. 2019. [Google Scholar] [CrossRef]
- Choe, E.K.; Park, K.J.; Ryoo, S.B.; Moon, S.H.; Oh, H.K.; Han, E.C. Prognostic impact of changes in adipose tissue areas after colectomy in colorectal cancer patients. J. Korean Med. Sci. 2016, 31, 1571–1578. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Yang, L.; Zbuk, K.; Wong, R.K.; Chow, T.; Koff, D.; Moran, G.R.; Mourtzakis, M. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br. J. Nutr. 2013, 109, 302–312. [Google Scholar] [CrossRef]
- Kurk, S.A.; Peeters, P.H.M.; Dorresteijn, B.; de Jong, P.A.; Jourdan, M.; Kuijf, H.J.; Punt, C.J.A.; Koopman, M.; May, A.M. Impact of different palliative systemic treatments on skeletal muscle mass in metastatic colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 909–919. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.G.; Kruitwagen, R.; Kleijnen, J.; Lambrechts, S.; Olde Damink, S.W.M.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.L.; Shen, X.; Huang, Y.Y.; Zhang, F.M.; Chen, X.Y.; Ma, L.L.; Chen, X.L.; Yu, Z.; Wang, S.L. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: A propensity score-matched analysis from a large-scale cohort. Surgery 2019, 166, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Prado, C.M.; Meyerhardt, J.A.; Weltzien, E.K.; Xiao, J.; Cespedes Feliciano, E.M.; Caan, B.J. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018, 124, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.J.; Reif, R.L.; Bigam, D.L.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.B. The impact of muscle and adipose tissue on long-term survival in patients with stage i to iii colorectal cancer. Dis. Colon Rectum 2019, 62, 549–560. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Yin, C.; Narumi, A.; Omura, Y.; Ide, S.; Kitajima, T.; Fujikawa, H.; et al. Clinical impact of muscle quantity and quality in colorectal cancer patients: A propensity score matching analysis. Jpn. J. Parenter. Enter. Nutr. 2018, 42, 1322–1333. [Google Scholar] [CrossRef]
- Dalal, S.; Hui, D.; Bidaut, L.; Lem, K.; Del Fabbro, E.; Crane, C.; Reyes-Gibby, C.C.; Bedi, D.; Bruera, E. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: A pilot study. J. Pain Symptom Manag. 2012, 44, 181–191. [Google Scholar] [CrossRef]
- Tisdale, M.J. Zinc-alpha2-glycoprotein in cachexia and obesity. Curr. Opin. Support. Palliat. Care 2009, 3, 288–293. [Google Scholar] [CrossRef]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.M.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three cachexia phenotypes and the impact of fat-only loss on survival in folfirinox therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern cooperative oncology group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Johns, N.; Fearon, K.C.; Darzi, A.; Kennedy, R.H.; Athanasiou, T.; Jenkins, J.T. Skeletal muscle changes after elective colorectal cancer resection: A longitudinal study. Ann. Surg. Oncol. 2016, 23, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.S.; Chen, T.-Y.; Giovannucci, E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef]
- Walter, V.; Jansen, L.; Hoffmeister, M.; Brenner, H. Smoking and survival of colorectal cancer patients: Systematic review and meta-analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1517–1525. [Google Scholar] [CrossRef]
- Kim, C.-W.; Go, R.-E.; Lee, H.-M.; Hwang, K.-A.; Lee, K.; Kim, B.; Lee, M.-Y.; Choi, K.-C. Cigarette smoke extracts induced the colon cancer migration via regulating epithelial mesenchymal transition and metastatic genes in human colon cancer cells. Environ. Toxicol. 2017, 32, 690–704. [Google Scholar] [CrossRef]
- Condoluci, A.; Mazzara, C.; Zoccoli, A.; Pezzuto, A.; Tonini, G. Impact of smoking on lung cancer treatment effectiveness: A review. Future Oncol. 2016, 12, 2149–2161. [Google Scholar] [CrossRef]
- Hermosillo-Rodriguez, J.; Anaya, D.A.; Sada, Y.; Walder, A.; Amspoker, A.B.; Berger, D.H.; Naik, A.D. The effect of age and comorbidity on patient-centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. J. Geriatr. Oncol. 2013, 4, 99–106. [Google Scholar] [CrossRef]
- Pezzuto, A.; Stellato, M.; Catania, G.; Mazzara, C.; Tonini, S.; Caricato, M.; Crucitti, P.; Tonini, G. Short-term benefit of smoking cessation along with glycopirronium on lung function and respiratory symptoms in mild copd patients: A retrospective study. J. Breath Res. 2018, 12, 046007. [Google Scholar] [CrossRef]
- Nogueira, L.; Trisko, B.M.; Lima-Rosa, F.L.; Jackson, J.; Lund-Palau, H.; Yamaguchi, M.; Breen, E.C. Cigarette smoke directly impairs skeletal muscle function through capillary regression and altered myofibre calcium kinetics in mice. J. Physiol. 2018, 596, 2901–2916. [Google Scholar] [CrossRef]
- Gosker, H.R.; Clarke, G.; de Theije, C.C.; Cryan, J.F.; Schols, A. Impaired skeletal muscle kynurenine metabolism in patients with chronic obstructive pulmonary disease. J. Clin. Med. 2019, 8, 915. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-B.; Park, J.-S.; Kim, D.-W.; Lee, T.-G. Intraoperative technical difficulty during laparoscopy-assisted surgery as a prognostic factor for colorectal cancer. Dis. Colon Rectum 2010, 53, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.D.; Korfiatis, P.; Kline, T.L.; Philbrick, K.A.; Kostandy, P.; Sakinis, T.; Sugimoto, M.; Takahashi, N.; Erickson, B.J. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 2019, 290, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Innominato, P.F.; Giacchetti, S.; Moreau, T.; Bjarnason, G.A.; Smaaland, R.; Focan, C.; Garufi, C.; Iacobelli, S.; Tampellini, M.; Tumolo, S.; et al. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer 2013, 119, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, J.H.; Jeong, W.K.; Lee, J.; Kim, Y.K.; Choi, D.; Lee, W.J. Semiautomatic software for measurement of abdominal muscle and adipose areas using computed tomography: A strobe-compliant article. Medicine 2019, 98, e15867. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, W.K.; Baik, S.K.; Cha, S.H.; Kim, M.Y. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and meld score. J. Cachexia Sarcopenia Muscle 2018, 9, 860–870. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Ebadi, M.; Martin, L.; Ghosh, S.; Field, C.J.; Lehner, R.; Baracos, V.E.; Mazurak, V.C. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br. J. Cancer 2017, 117, 148–155. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef]

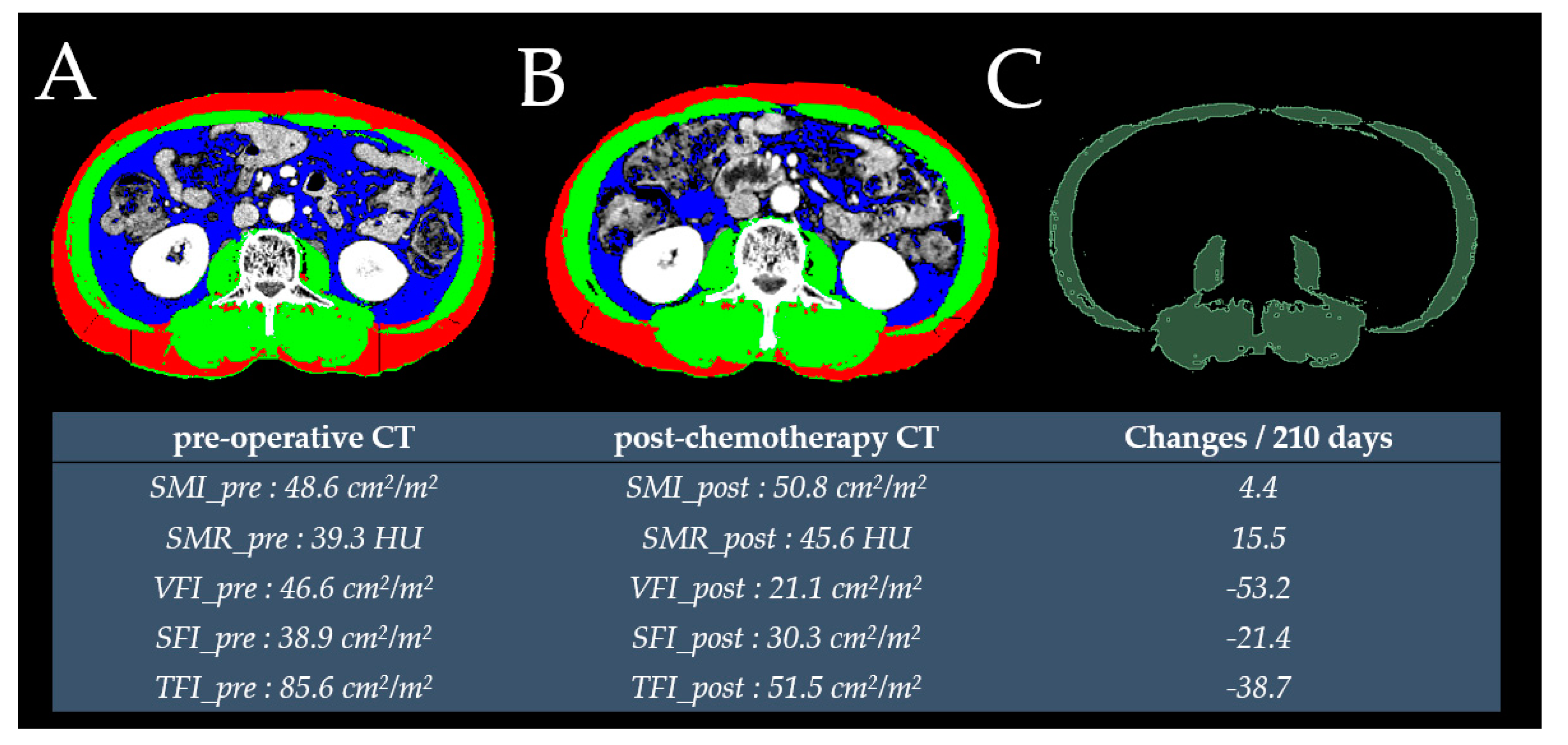
| Variables | Female (n = 67) (%) | Male (n = 100) (%) | p | |
|---|---|---|---|---|
| Age (years) | <65 | 51 (76.1) | 63 (63) | 0.106 |
| ≥65 | 16 (23.9) | 37 (37) | ||
| ASA grade | I | 32 (47.8) | 43 (43) | 0.928 |
| II | 25 (37.3) | 39 (39) | ||
| III | 5 (7.5) | 9 (9) | ||
| No data | 5 (7.5) | 9 (9) | ||
| BMI (kg/m2) | <25 | 55 (82.1) | 66 (66) | 0.035 |
| ≥25 | 12 (17.9) | 34 (34) | ||
| Smoking | Yes | 0 | 47 (47) | <0.001 a |
| No | 67 (100) | 53 (53) | ||
| CEA (ng/mL) | <5 | 47 (70.1) | 63 (63) | 0.430 |
| ≥5 | 20 (29.9) | 37 (37) | ||
| Tumor location b | proximal | 28 (41.8) | 31 (31) | 0.206 |
| distal | 39 (58.2) | 69 (69) | ||
| Operation time (min) | <300 | 50 (74.6) | 84 (84) | 0.196 |
| ≥300 | 17 (25.4) | 16 (16) | ||
| Complications | No | 59 (88.1) | 82 (82) | 0.4 |
| Yes | 8 (11.9) | 18 (18) | ||
| Tumor size (cm) | <5 | 39 (58.2) | 63 (63) | 0.645 |
| ≥5 | 28 (41.8) | 37 (37) | ||
| LVI | Absent | 37 (55.2) | 60 (60) | 0.753 a |
| Present | 26 (38.8) | 36 (36) | ||
| No data | 4 (6) | 4 (4) | ||
| Stage | II | 10 (14.9) | 16 (16) | >0.99 |
| III | 57 (85.1) | 84 (84) | ||
| Chemotherapy cycles | <10 | 8 (11.9) | 14 (14) | 0.879 |
| ≥10 | 59 (88.1) | 86 (86) |
| Female (n = 67) (Mean ± SD) | Male (n = 100) (Mean ± SD) | pa | |
|---|---|---|---|
| Values at preoperative CT | |||
| SMI_pre (cm2/m2) | 38.9 ± 5.7 | 47.9 ± 7.5 | <0.001 |
| SMR_pre (HU) | 41.2 ± 8.8 | 44.4 ± 7.4 | 0.012 |
| VFI_pre (cm2/m2) | 30.1 ± 22.6 | 41.1 ± 23.8 | 0.003 |
| SFI_pre (cm2/m2) | 56.0 ± 23.9 | 36.2 ± 15.6 | <0.001 |
| TFI_pre (cm2/m2) | 86.1 ± 41 | 77.3 ± 37.2 | 0.153 |
| Values at postchemotherapy CT | |||
| SMI_post (cm2/m2) | 43.5 ± 5.4 | 51.6 ± 8.1 | <0.001 |
| SMR_post (HU) | 42.1 ± 7.5 | 46.5 ± 6.7 | <0.001 |
| VFI_post (cm2/m2) | 24.8 ± 17.1 | 30.3 ± 17.6 | 0.048 |
| SFI_post (cm2/m2) | 56.2 ± 22.5 | 36.2 ± 15.0 | <0.001 |
| TFI_post (cm2/m2) | 81.0 ± 35.1 | 66.5 ± 30.7 | 0.005 |
| Percentage changes over 210 days between preoperative and postchemotherapy CTs | |||
| SMIC (%/210 days) | 12.8 ± 12.7 | 8.6 ± 12.0 | 0.030 |
| SMRC (HU/210 days) | 4.1 ± 18.1 | 5.7 ± 14.5 | 0.560 |
| VFIC (%/210 days) | 12.6 ± 80.9 | 5.6 ± 124.5 | 0.660 |
| SFIC (%/210 days) | 7.6 ± 33.2 | 7.3 ± 44.9 | 0.955 |
| TFIC (%/210 days) | 3.6 ± 36.9 | −1.0 ± 58.4 | 0.541 |
| Value Distribution | SMIC | SMRC | VFIC | SFIC | TFIC |
|---|---|---|---|---|---|
| Minimum | −21.3 | −34.29 | −95.33 | −68.94 | −76.33 |
| 25th percentile | 2.75 | −4.4 | −40.68 | −16.04 | −24.66 |
| Median | 8.7 | 3.42 | −19.04 | −3.46 | −11.93 |
| 75th percentile | 17.05 | 13.40 | 8.67 | 19.78 | 10.33 |
| Maximum | 59.8 | 56.43 | 648.39 | 172.59 | 260.48 |
| Preoperative CT | Postchemotherapy CT | Changes/210 Days between Two CTs | |||
|---|---|---|---|---|---|
| SMI_pre low vs. SMI_pre high | p = 0.8 | SMI_post low vs. SMI_post high | p = 0.4 | SMIC: Loss of 2% or more vs. Loss of less than 2% | p = 0.16 |
| SMR_pre low vs. SMR_pre high | p = 0.066 | SMR_post low vs. SMR_post high | p = 0.05 | SMRC: Loss of 2% or more vs. Loss of less than 2% | p = 0.94 |
| VFI_pre low vs. VFI_pre high | p = 0.28 | VFI_post low vs. VFI_post high | p = 0.89 | VFIC: Loss of 46.57% or more vs. Loss of less than 46.57% | p = 0.00078 |
| SFI_pre low vs. SFI_pre high | p = 0.45 | SFI_post low vs. SFI_post high | p = 0.37 | SFIC: Loss of 17.03% or more vs. Loss of less than 17.03% | p = 0.091 |
| TFI_pre low vs. TFI_pre high | p = 0.74 | TFI_post low vs. TFI_post high | p = 0.22 | TFIC: Loss of 42.61% or more vs. Loss of less than 42.61% | p = 0.0033 |
| Univariable Analysis | |||
|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p | |
| Gender | Female | 1 | |
| Male | 1.79 (0.83–3.88) | 0.137 | |
| Age (years) | <65 | 1 | |
| ≥65 | 0.76 (0.35–1.65) | 0.492 | |
| ASA grade | 1 | 1 | |
| 2 | 0.77 (0.35–1.70) | 0.524 | |
| 3 | 0.75 (0.17–3.31) | 0.715 | |
| No data | 1.02 (0.33–3.11) | 0.965 | |
| BMI (kg/m2) | <25 | 1 | |
| ≥25 | 0.59 (0.24–1.44) | 0.253 | |
| Smoking | No | 1 | |
| Yes | 1.83 (0.90–3.72) | 0.091 | |
| CEA (ng/mL) | <5 | 1 | |
| ≥5 | 1.12 (0.55–2.3) | 0.743 | |
| Tumor location a | proximal | 1 | |
| distal | 0.64 (0.32–1.29) | 0.219 | |
| Operation time (min) | <300 | 1 | |
| ≥300 | 2.59 (1.25–5.38) | 0.01 | |
| Complications | No | 1 | |
| Yes | 1.75 (0.78–3.9) | 0.169 | |
| Tumor size (cm) | <5 | 1 | |
| ≥5 | 1.04 (0.51–2.11) | 0.912 | |
| LVI | Absent | 1 | |
| Present | 2.21 (1.07–4.57) | 0.03 | |
| No data | 1.58 (0.35–7.07) | 0.545 | |
| Stage | II | 1 | |
| III | 1.54 (0.53–4.40) | 0.419 | |
| Chemotherapy cycles | <10 | 1 | |
| ≥10 | 0.47 (0.20–1.09) | 0.08 | |
| SMI_pre | Low | 1 | |
| High | 0.91 (0.45–1.82) | 0.799 | |
| SMR_pre | Low | 1 | |
| High | 0.52 (0.25–1.05) | 0.070 | |
| VFI_pre | Low | 1 | |
| High | 1.5 (0.71–3.18) | 0.284 | |
| SFI_pre | Low | 1 | |
| High | 0.75 (0.36–1.57) | 0.457 | |
| TFI_pre | Low | 1 | |
| High | 1.13 (0.53–2.39) | 0.746 | |
| SMI_post | Low | 1 | |
| High | 0.73 (0.35–1.51) | 0.401 | |
| SMR_post | Low | 1 | |
| High | 0.48 (0.23–1.01) | 0.055 | |
| VFI_post | Low | 1 | |
| High | 0.91 (0.27–3.01) | 0.887 | |
| SFI_post | Low | 1 | |
| High | 0.71 (0.34–1.48) | 0.372 | |
| TFI_post | Low | 1 | |
| High | 0.48 (0.14–1.58) | 0.229 | |
| SMIC | Loss of 2% or more | 1 | |
| Loss of less than 2% | 0.54 (0.23–1.27) | 0.162 | |
| SMRC | Loss of 2% or more | 1 | |
| Loss of less than 2% | 0.97 (0.46–2.01) | 0.939 | |
| VFIC | Loss of 46.57% or more | 1 | |
| Loss of less than 46.57% | 0.31 (0.15–0.64) | 0.001 | |
| SFIC | Loss of 17.03% or more | 1 | |
| Loss of less than 17.03% | 0.53 (0.25–1.17) | 0.096 | |
| TFIC | Loss of 42.61% or more | 1 | |
| Loss of less than 42.61% | 0.31 (0.14–0.71) | 0.005 | |
| Multivariable Analysis | |||
|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p | |
| Smoking | No | 1 | |
| Yes | 2.25 (1.06–4.77) | 0.034 | |
| Operation time (min) | <300 | 1 | |
| ≥300 | 3.87 (1.80–8.33) | 0.0005 | |
| LVI | Absent | 1 | |
| Present | 2.46 (1.16–5.18) | 0.017 | |
| No data | 1.19 (0.26–5.39) | 0.818 | |
| SMR_post | Low | 1 | |
| High | 0.32 (0.15–0.70) | 0.004 | |
| VFIC | Loss of 46.57% or more | 1 | |
| Loss of less than 46.57% | 0.31 (0.14–0.69) | 0.004 | |
| SFIC | Loss of 17.03% or more | 1 | |
| Loss of less than 17.03% | 0.53 (0.23–1.19) | 0.128 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, E.; Lee, H.S.; Cho, E.-S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer. Cancers 2020, 12, 60. https://doi.org/10.3390/cancers12010060
Chung E, Lee HS, Cho E-S, Park EJ, Baik SH, Lee KY, Kang J. Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer. Cancers. 2020; 12(1):60. https://doi.org/10.3390/cancers12010060
Chicago/Turabian StyleChung, Eric, Hye Sun Lee, Eun-Suk Cho, Eun Jung Park, Seung Hyuk Baik, Kang Young Lee, and Jeonghyun Kang. 2020. "Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer" Cancers 12, no. 1: 60. https://doi.org/10.3390/cancers12010060
APA StyleChung, E., Lee, H. S., Cho, E.-S., Park, E. J., Baik, S. H., Lee, K. Y., & Kang, J. (2020). Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer. Cancers, 12(1), 60. https://doi.org/10.3390/cancers12010060





