Efficacy and Safety of CAP7.1 as Second-Line Treatment for Advanced Biliary Tract Cancers: Data from a Randomised Phase II Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Primary Endpoint
2.3. Secondary Endpoints
2.4. Safety
2.5. Exploratory Efficacy Endpoints
2.5.1. Post-hoc Analysis of Patients Who Crossed Over to CAP7.1
2.5.2. Analysis of Tumour CES2
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Dosing
4.3. Patients
4.4. Study Endpoints
4.5. Measurements of Efficacy
4.6. Measurements of Safety
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v28–v37. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Khan, S.A.; Davidson, B.R.; Goldin, R.D.; Heaton, N.; Karani, J.; Pereira, S.P.; Rosenberg, W.M.; Tait, P.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef]
- Yao, K.J.; Jabbour, S.; Parekh, N.; Lin, Y.; Moss, R.A. Increasing mortality in the United States from cholangiocarcinoma: An analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016, 16, 117. [Google Scholar] [CrossRef]
- Pillai, R.K.; Jayasree, K. Rare cancers: Challenges & issues. Indian J. Med. Res. 2017, 145, 17–27. [Google Scholar]
- Song, W.; Miao, D.-L.; Chen, L. Survival rates are higher in married patients with biliary tract cancer: A population-based study. Oncotarget 2018, 9, 9531–9539. [Google Scholar] [CrossRef][Green Version]
- Lamarca, A.; Hubner, R.A.; David Ryder, W.; Valle, J.W. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef]
- Glimelius, B.; Hoffman, K.; Sjoden, P.O.; Jacobsson, G.; Sellstrom, H.; Enander, L.K.; Linne, T.; Svensson, C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996, 7, 593–600. [Google Scholar] [CrossRef]
- Rao, S.; Cunningham, D.; Hawkins, R.E.; Hill, M.E.; Smith, D.; Daniel, F.; Ross, P.J.; Oates, J.; Norman, A.R. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br. J. Cancer 2005, 92, 1650–1654. [Google Scholar] [CrossRef][Green Version]
- Yuan, P.; Di, L.; Zhang, X.; Yan, M.; Wan, D.; Li, L.; Zhang, Y.; Cai, J.; Dai, H.; Zhu, Q.; et al. Efficacy of Oral Etoposide in Pretreated Metastatic Breast Cancer. Medicine 2015, 94, e774. [Google Scholar] [CrossRef]
- Kim, Y.H.; Seo, J.H.; Kim, B.S.; Shin, S.W.; Shin, J.J.; Kang, K.H.; Choi, Y.H.; Kim, K.T.; Kim, J.S. Clinical Efficacy of Combination Chemotherapy with Cisplatin, Ifosfamide, and Oral Etoposide (PIE) in Advanced Non-Small Cell Lung Cancer. J. Korean Cancer Assoc. 1999, 31, 297–305. [Google Scholar]
- Rath, U.; Flechtner, H.; Selbach, J.; Harjung, H.; Manegold, C.; Kabelitz, K.; Trux, F.A.; Edler, L.; Schlag, P.; Queisser, W. Etoposide, adriamycin, and cisplatinum (EAP) combination chemotherapy for advanced gastric cancer. A phase II trial by the “Chemotherapiegruppe Gastrointestinaler Tumoren (CGT)”. Onkologie 1990, 13, 194–197. [Google Scholar]
- Shigematsu, A.; Ozawa, Y.; Onizuka, M.; Fujisawa, S.; Suzuki, R.; Atsuta, Y.; Hatanaka, K.; Masuko, M.; Ito, T.; Kobayashi, N.; et al. A Safety and Efficacy Study of Medium-Dose Etoposide, Cyclophosphamide and Total Body Irradiation Conditioning Before Allogeneic Stem Cell Transplantation for Acute Lymphoblastic Leukemia. Transpl. Direct 2015, 1, e8. [Google Scholar] [CrossRef]
- Carney, D.N. The pharmacology of intravenous and oral etoposide. Cancer 1991, 67, 299–302. [Google Scholar] [CrossRef]
- Slevin, M.L. The clinical pharmacology of etoposide. Cancer 1991, 67, 319–329. [Google Scholar] [CrossRef]
- Smith, P.J.; Soues, S.; Gottlieb, T.; Falk, S.J.; Watson, J.V.; Osborne, R.J.; Bleehen, N.M. Etoposide-induced cell cycle delay and arrest-dependent modulation of DNA topoisomerase H in small-cell lung cancer cells. Br. J. Cancer 1994, 70, 914–921. [Google Scholar] [CrossRef]
- Accord Healthcare Limited. Etoposide 20 mg/ml Concentrate for Solution for Infusion. Available online: https://www.medicines.org.uk/emc/product/3385/smpc (accessed on 20 October 2020).
- Slevin, M.L.; Clark, P.I.; Joel, S.P.; Malik, S.; Osborne, R.J.; Gregory, W.M.; Lowe, D.G.; Reznek, R.H.; Wrigley, P.F. A randomized trial to evaluate the effect of schedule on the activity of etoposide in small-cell lung cancer. J. Clin. Oncol. 1989, 7, 1333–1340. [Google Scholar] [CrossRef]
- Thompson, D.S.; Hainsworth, J.D.; Hande, K.R.; Holzmer, M.C.; Greco, F.A. Prolonged administration of low-dose, infusional etoposide in patients with etoposide-sensitive neoplasms: A phase I/II study. J. Clin. Oncol. 1993, 11, 1322–1328. [Google Scholar] [CrossRef]
- Schroeder, U.; Bernt, K.M.; Lange, B.; Wenkel, J.; Jikai, J.; Shabat, D.; Amir, R.; Huebener, N.; Niethammer, A.G.; Hagemeier, C.; et al. Hydrolytically activated etoposide prodrugs inhibit MDR-1 function and eradicate established MDR-1 multidrug-resistant T-cell leukemia. Blood 2003, 102, 246–253. [Google Scholar] [CrossRef]
- Keilholz, U.; Rohde, L.; Mehlitz, P.; Knoedler, M.; Schmittel, A.; Kümmerlen, V.; Klinghammer, K.; Treasure, P.; Lassus, M.; Steventon, G.; et al. First-in-man dose escalation and pharmacokinetic study of CAP7.1, a novel prodrug of etoposide, in adults with refractory solid tumours. Eur. J. Cancer 2017, 80, 14–25. [Google Scholar] [CrossRef]
- The Human Protein Atlas. CES2. In. 2019. Available online: https://www.proteinatlas.org/ (accessed on 20 October 2020).
- Wrasidlo, W.; Schroder, U.; Bernt, K.; Hübener, N.; Shabat, D.; Gaedicke, G.; Lode, H. Synthesis, hydrolytic activation and cytotoxicity of etoposide prodrugs. Bioorg. Med. Chem. Lett. 2002, 12, 557–560. [Google Scholar] [CrossRef]
- Cereda, S.; Belli, C.; Rognone, A.; Mazza, E.; Reni, M. Second-line therapy in advanced biliary tract cancer: What should be the standard? Crit. Rev. Oncol. Hematol. 2013, 88, 368–374. [Google Scholar] [CrossRef]
- Marcano-Bonilla, L.; Mohamed, E.A.; Mounajjed, T.; Roberts, L.R. Biliary tract cancers: Epidemiology, molecular pathogenesis and genetic risk associations. Chin. Clin. Oncol. 2016, 5, 61. [Google Scholar] [CrossRef]
- Jansen, H.; Pape, U.-F.; Uktu, N. A review of systemic therapy in biliary tract cancer. J. Gastrointest. Oncol. 2020, 11, 770. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. ABC-06|A randomised phase III, multi-centre, open-label study of Active Symptom Control (ASC) alone or ASC with oxaliplatin/5-FUchemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019, 37, 4003. [Google Scholar]
- O’Brien, P.C.; Fleming, T.R. A multiple testing procedure for clinical trials. Biometrics 1979, 35, 549–556. [Google Scholar] [CrossRef]
- Goeppert, B.; Renner, M.; Singer, S.; Albrecht, T.; Zhang, Q.; Mehrabi, A.; Pathil, A.; Springfeld, C.; Köhler, B.; Rupp, C.; et al. Prognostic impact of carboxylesterase 2 in cholangiocarcinoma. Sci. Rep. 2019, 9, 4338. [Google Scholar] [CrossRef]
- Moriwaki, T.; Yamamoto, Y.; Gosho, M.; Kobayashi, M.; Sugaya, A.; Yamada, T.; Endo, S.; Hyodo, I. Correlations of survival with progression-free survival, response rate, and disease control rate in advanced biliary tract cancer: A meta-analysis of randomised trials of first-line chemotherapy. Br. J. Cancer 2016, 114, 881–888. [Google Scholar] [CrossRef]
- Clopper, C.J.; Pearson, E.S. The use of confidence of fiducial limits illustrated in the case of the binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
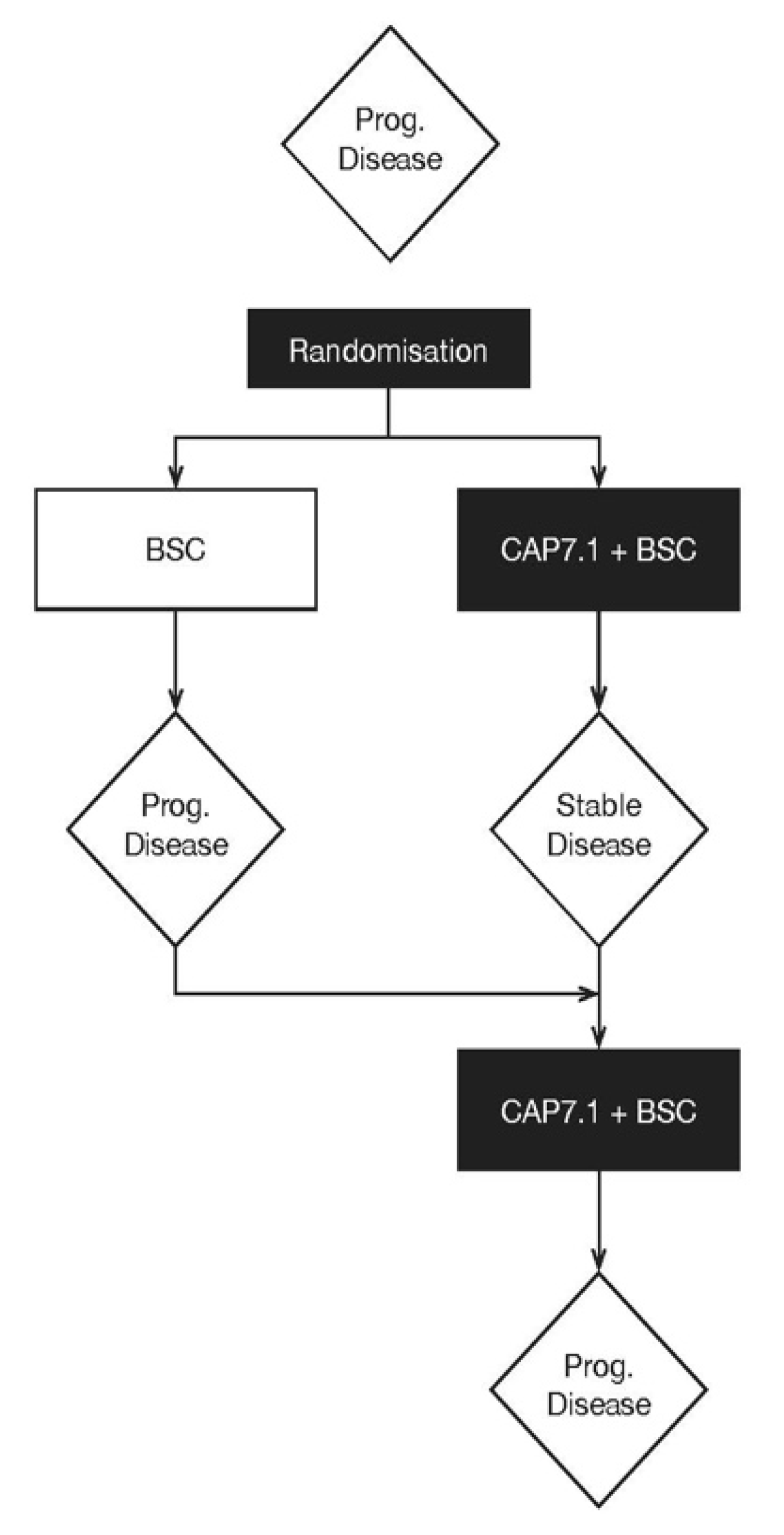








| Disposition | CAP7.1 | BSC | Not Assigned Treatment | Total |
|---|---|---|---|---|
| All patients enrolled | 14 | 13 | 1 | 28 |
| Withdrawals before randomisation | NA | NA | 1 * | 1 |
| Randomised | 14 | 13 | NA | 27 |
| Premature discontinuations | 5 | 8 | NA | 13 |
| Late screen failures/withdrawal of consent (i.e., randomised but not treated) | 1 * | 1 * | NA | 2 |
| Patients treated with BSC | NA | 12 | NA | 12 |
| Patients who crossed over from BSC to CAP7.1 | NA | 10 | NA | 10 |
| All patients treated with CAP7.1 | 13 | 10 | NA | 23 |
| Safety analysis set (SAS) | 13 | 10 | NA | 23 |
| Patients considered inappropriately randomised/eligibility violation | 3 † | 1 * | NA | 4 |
| Lost-to-follow up/lack of post-randomisation data | 0 | 1 * | NA | 1 |
| Full analysis set (FAS) | 10 | 10 | NA | 20 |
| Other significant protocol violations/lack of assessments | 1 ‡ | 0 | NA | 1 |
| Per-protocol analysis set (PAS) | 9 | 10 | NA | 19 |
| Characteristic | CAP7.1 | BSC | |
|---|---|---|---|
| Number of patients | 14 | 13 | |
| Mean age ± SD | 60.3 ± 10.5 | 65.8 ± 8.70 | |
| Male, n (%) | 8 (57) | 6 (46) | |
| Race Caucasian, n (%) | 14 (100) | 13 (100) | |
| Histology, n (%) | |||
| Differentiation | |||
| Well differentiated | 1 (7) | 2 (15) | |
| Moderately differentiated | 7 (50) | 5 (38) | |
| Poorly differentiated | 2 (14) | 5 (38) | |
| Undifferentiated | 0 | 0 | |
| Unknown | 4 (29) | 1 (8) | |
| Type, n (%) | |||
| Distant metastatic | 11 (79) | 8 (62) | |
| Locally recurrent | 4 (29) | 6 (46) | |
| TNM stage at diagnosis, n (%) | |||
| I | 0 | 2 (15) | |
| II | 2 (14) | 4 (31) | |
| III | 3 (21) | 2 (15) | |
| IVA–IVB | 9 (64) | 5 (38) | |
| IVC | 0 | 0 | |
| I | 0 | 2 (15) | |
| Primary tumour site, n (%) | |||
| Intrahepatic | |||
| Extrahepatic (not further specified) | 3 (21) | 4 (31) | |
| Extrahepatic (Klatskin) | 2 (14) | 0 | |
| Extrahepatic (Distal/ampulla of Vater) | 4 (29) | 1 (8) | |
| Gallbladder | 2 (14) | 2 (15) | |
| Multiple locations | 2 (14) | 1 (8) | |
| Cholangiocarcinoma not further specified | 0 | 1 (8) | |
| ECOG PS | |||
| 0 | 4 (29) | 9 (63) | |
| 1 | 10 (71) | 4 (31) | |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| 4 | 0 | 0 | |
| Bilirubin (mg/dl) | |||
| mean ± SD | 0.63 ± 0.71 | 0.58 ± 0.49 | |
| median | 0.4 | 0.4 | |
| Number of prior lines of chemotherapy, n (%) | |||
| 1 | 9 (100) | 6 (60) | |
| 2 | 0 | 3 (30) | |
| 3 | 0 | 0 | |
| 4 | 0 | 1 (10) | |
| Previous surgery, n (%) | |||
| Resection | 3 (21) | 7 (50) | |
| Laparotomy | 1 (7) | 1 (8) | |
| Patients | FAS | PAS | ||
|---|---|---|---|---|
| CAP7.1 (n = 10) | BSC (n = 10) | CAP7.1 (n = 9) | BSC (n = 10) | |
| Total number of patients with disease control | 5 | 2 | 5 | 2 |
| % of patients with disease control (95% CI) * | 50.0 (18.7, 81.3) | 20.0 (2.5, 55.6) | 55.6 (21.2, 86.3) | 20.0 (2.5, 55.6) |
| Treatment difference (95% CI) | 30.0 (−18.44, 69.22) | 35.56 (−12.80, 72.39) | ||
| p-value for treatment difference † | 0.175 | 0.130 | ||
| Adverse Events | CAP7.1 Randomised n = 13 | BSC Before Crossover n = 10 | BSC After Crossover n = 10 | Total on CAP7.1 n = 23 | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | E | n (%) | E | n (%) | E | n (%) | E | |
| Any AE | 13 (100) | 219 | 10 (100) | 23 | 10 (100) | 157 | 23 (100) | 376 |
| Any AE leading to discontinuation of study treatment | 4 (31) | 6 | 0 | 0 | 3 (30) | 5 | 7 (30) | 11 |
| Any drug-related AE * | 12 (92) | 140 | 1 (10) | 1 | 10 (100) | 94 | 22 (96) | 234 |
| Any SAE | 7 (54) | 16 | 2 (20) | 3 | 8 (80) | 27 | 15 (65) | 43 |
| Any drug-related SAE * | 6 (46) | 8 | 0 | 0 | 5 (50) | 13 | 11 (48) | 21 |
| Any fatal AE | 4 (31) | 6 | 0 | 0 | 4 (40) | 5 | 8 (35) | 11 |
| Any drug-related fatal AE * | 1 (8) | 1 | 0 | 0 | 1 (10) | 2 | 2 (9) | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pape, U.-F.; Kasper, S.; Meiler, J.; Sinn, M.; Vogel, A.; Müller, L.; Burkhard, O.; Caca, K.; Heeg, S.; Büchner-Steudel, P.; et al. Efficacy and Safety of CAP7.1 as Second-Line Treatment for Advanced Biliary Tract Cancers: Data from a Randomised Phase II Study. Cancers 2020, 12, 3149. https://doi.org/10.3390/cancers12113149
Pape U-F, Kasper S, Meiler J, Sinn M, Vogel A, Müller L, Burkhard O, Caca K, Heeg S, Büchner-Steudel P, et al. Efficacy and Safety of CAP7.1 as Second-Line Treatment for Advanced Biliary Tract Cancers: Data from a Randomised Phase II Study. Cancers. 2020; 12(11):3149. https://doi.org/10.3390/cancers12113149
Chicago/Turabian StylePape, Ulrich-Frank, Stefan Kasper, Johannes Meiler, Marianne Sinn, Arndt Vogel, Lothar Müller, Oswald Burkhard, Karel Caca, Steffen Heeg, Petra Büchner-Steudel, and et al. 2020. "Efficacy and Safety of CAP7.1 as Second-Line Treatment for Advanced Biliary Tract Cancers: Data from a Randomised Phase II Study" Cancers 12, no. 11: 3149. https://doi.org/10.3390/cancers12113149
APA StylePape, U.-F., Kasper, S., Meiler, J., Sinn, M., Vogel, A., Müller, L., Burkhard, O., Caca, K., Heeg, S., Büchner-Steudel, P., Rodriguez-Laval, V., Kühl, A. A., Arsenic, R., Jansen, H., Treasure, P., & Utku, N. (2020). Efficacy and Safety of CAP7.1 as Second-Line Treatment for Advanced Biliary Tract Cancers: Data from a Randomised Phase II Study. Cancers, 12(11), 3149. https://doi.org/10.3390/cancers12113149







