Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases
Abstract
Simple Summary
Abstract
1. Introduction
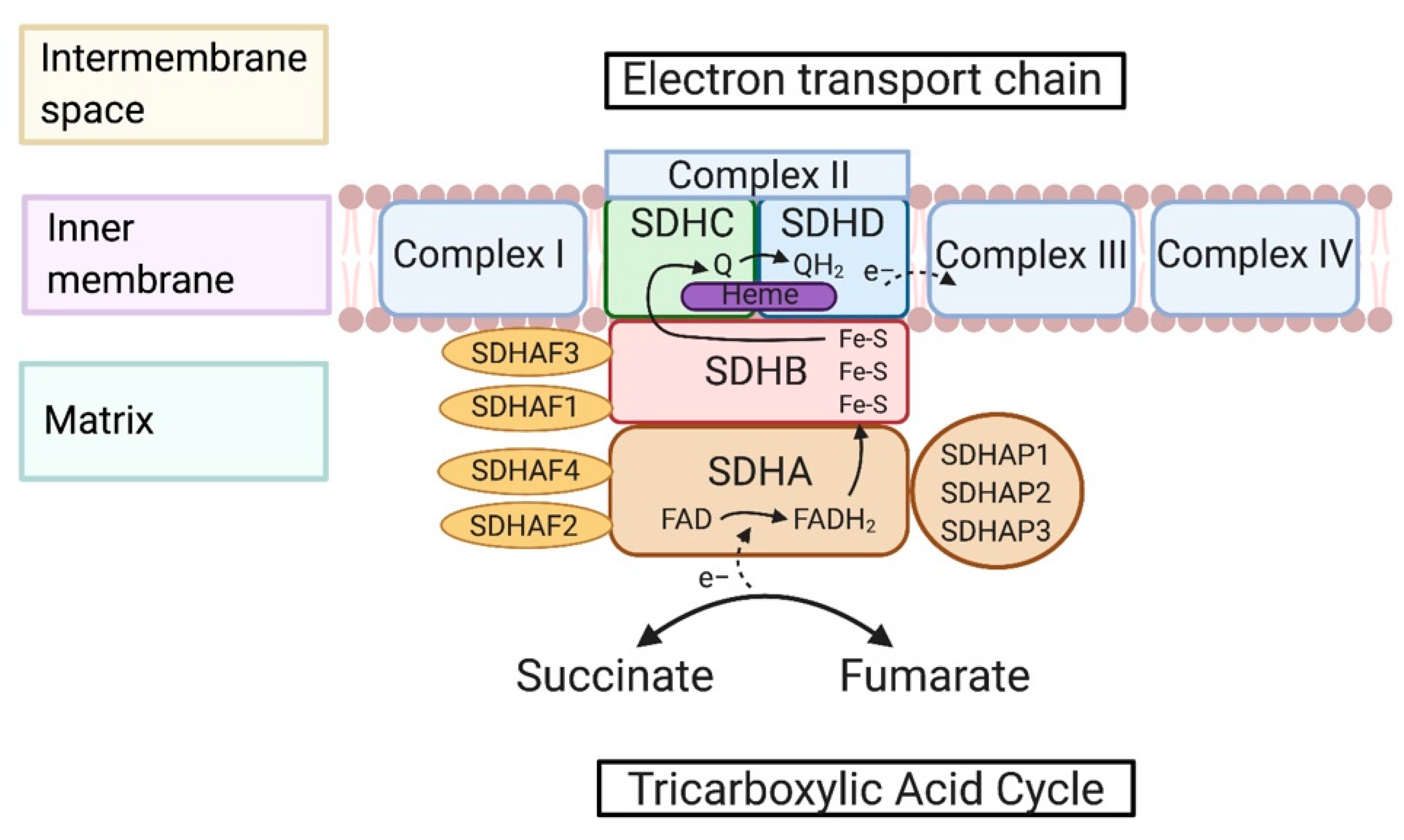
2. Succinate Dehydrogenase-Associated Genes and Protein Structures
2.1. SDH Complex-Associated Genes
2.2. Maturation and Assembly of the SDH Complex
2.3. Metabolic Reactions of the SDH Complex
3. Pathogenesis of Succinate Dehydrogenase-Relevant Diseases and Mechanisms
3.1. Cancers
3.1.1. Paraganglioma and Pheochromocytomas
3.1.2. Other Cancers
3.2. High-Altitude Illness (Acute Mountain Sickness)
3.3. Inflammation
3.4. Neurodegenerative Disease
3.5. Diabetes
3.6. Ischemia-Reperfusion Injury
4. A Network of RNA Regulators Interacting with Succinate Dehydrogenase
4.1. Non-Coding RNAs
4.2. RNA-Editing Enzymes
4.3. RNA-Modification Genes
4.4. Transcription Factors
4.5. Alternative Splicing
5. Treatment against Succinate Dehydrogenase Dysfunction
5.1. Small Molecules Inducing or Blocking SDH Activity
5.1.1. SDH Inhibitors
5.1.2. SDH Activators
5.2. CRISPR
6. Pre-Clinical Models and Clinical Trials for Succinate Dehydrogenase
6.1. Approaches to Measuring SDH Activity
6.2. Pre-Clinical Models
6.3. Clinical Trials
7. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADARs | Adenosine deaminases acting on RNA |
| ALKBH5 | Alpha-ketoglutarate-dependent dioxygenase AlkB homolog 5 |
| APOBEC | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like |
| APOBEC1 | Apolipoprotein B mRNA editing enzyme catalytic subunit 1 |
| APOBEC3A | Apolipoprotein B mRNA editing enzyme catalytic subunit 3A |
| A-to-I | Adenine to inosine |
| ATP | Adenosine triphosphate |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| C-to-U | Cytidine to uracil |
| DCPIP | 2,6-dichlorophenolindophenol |
| DNA | Deoxyribonucleic acid |
| ETC | Electron transport chain |
| FAD | Flavin adenine dinucleotide |
| Fe-S | Iron-Sulfur |
| FTO | Fat mass and obesity-associated protein |
| GBM | Glioblastoma multiforme |
| GISTs | Gastrointestinal tumors |
| GPR91 | G-protein coupled receptor 91 |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HRLCC | Hereditary leiomyomatosis and renal cell carcinoma |
| IL-1β | Interleukin 1 beta |
| lncRNA | Long non-coding ribonucleic acid |
| mTOR | Mammalian target of rapamycin |
| miRNA | Micro-ribonucleic acid |
| m6A | N6-methyladenosine |
| NAD | Nicotinamide adenine dinucleotide |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| NADPH | Nicotinamide adenine dinucleotide hydrogen |
| NBT | Nitro blue tetrazolium |
| NRF1 | Nuclear respiratory factor 1 |
| PCC | Pheochromocytoma |
| PGL | Paraganglioma |
| PD-1 | Programmed cell death-1 |
| PHD | HIF-α prolyl hydroxylase domain |
| PTEN | Protein and tensin homolog |
| QD | Distal binding site in ubiquinone |
| Qp | Proximal binding site in ubiquinone |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SIRT3 | Sirtuin 3 |
| SDH | Succinate dehydrogenase |
| SDHA | Succinate dehydrogenase subunit A |
| SDHAF1 | Succinate dehydrogenase complex assembly factor 1 |
| SDHAP1 | Succinate dehydrogenase complex flavoprotein subunit a pseudogene 1 |
| SDHB | Succinate dehydrogenase subunit B |
| SDHC | Succinate dehydrogenase subunit C |
| SDHD | Succinate dehydrogenase subunit D |
| SGI-110 | Guadecitabine |
| SKP2 | S-phase kinase associated protein 2 |
| sRNA | Small non-coding ribonucleic acid |
| SUCLG1 | Succinate-CoA ligase GDP/ADP-forming subunit alpha |
| SUCLG2 | Succinate-CoA ligase GDP-forming subunit beta |
| SUCLA2 | Succinate-CoA ligase ADP-forming subunit beta |
| SUCNR1 | Succinate receptor 1 |
| T2D | Type 2 diabetes mellitus |
| TCA cycle | Tricarboxylic acid cycle |
| TMZ | Temozolomide |
| TRAP1 | Tumor necrosis factor receptor associated protein 1 |
| TTFA | Thenoyltrifluoroacetone |
References
- Rutter, J.; Winge, D.R.; Schiffman, J.D. Succinate dehydrogenase—Assembly, regulation and role in human disease. Mitochondrion 2010, 10, 393–401. [Google Scholar] [CrossRef]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, I.; Kouzarides, T. Role of RNA modifications in cancer. Nat. Rev. Cancer 2020, 20, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef] [PubMed]
- Puissegur, M.P.; Mazure, N.M.; Bertero, T.; Pradelli, L.; Grosso, S.; Robbe-Sermesant, K.; Maurin, T.; Lebrigand, K.; Cardinaud, B.; Hofman, V.; et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011, 18, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Wells, J.M.; Chow, K.H.; Huang, H.; Yuan, M.; Saxena, T.; Melnick, M.A.; Politi, K.; Asara, J.M.; Costa, D.B.; et al. miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nat. Metab. 2019, 1, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; Buchumenski, I.; Levanon, E.Y.; Eisenberg, E. Human cancer tissues exhibit reduced A-to-I editing of miRNAs coupled with elevated editing of their targets. Nucleic Acids Res. 2018, 46, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Sharma, P.; Maklashina, E.; Cecchini, G.; Iverson, T.M. The roles of SDHAF2 and dicarboxylate in covalent flavinylation of SDHA, the human complex II flavoprotein. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Ghezzi, D.; Verrigni, D.; Rizza, T.; Bertini, E.; Martinelli, D.; Zeviani, M.; Singh, A.; Carrozzo, R.; Rouault, T.A. Disease-Causing SDHAF1 Mutations Impair Transfer of Fe-S Clusters to SDHB. Cell Metab. 2016, 23, 292–302. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, A.; Zhang, Z. LncRNA SDHAP1 confers paclitaxel resistance of ovarian cancer by regulating EIF4G2 expression via miR-4465. J. Biochem. 2020, 168, 171–181. [Google Scholar] [CrossRef]
- Hu, X.; Yang, L.; Mo, Y.-Y. Role of Pseudogenes in Tumorigenesis. Cancers 2018, 10, 256. [Google Scholar] [CrossRef]
- Kim, H.J.; Winge, D.R. Emerging concepts in the flavinylation of succinate dehydrogenase. Biochim. Biophys. Acta 2013, 1827, 627–636. [Google Scholar] [CrossRef]
- Van Vranken, J.G.; Bricker, D.K.; Dephoure, N.; Gygi, S.P.; Cox, J.E.; Thummel, C.S.; Rutter, J. SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration. Cell Metab. 2014, 20, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Delatycki, M.B.; Williamson, R.; Forrest, S.M. Friedreich ataxia: An overview. J. Med. Genet. 2000, 37, 1–8. [Google Scholar] [CrossRef]
- Stehling, O.; Elsässer, H.-P.; Brückel, B.; Mühlenhoff, U.; Lill, R. Iron–sulfur protein maturation in human cells: Evidence for a function of frataxin. Hum. Mol. Genet. 2004, 13, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Na, U.; Yu, W.; Cox, J.; Bricker, D.K.; Brockmann, K.; Rutter, J.; Thummel, C.S.; Winge, D.R. The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase. Cell Metab. 2014, 20, 253–266. [Google Scholar] [CrossRef]
- Oyedotun, K.S.; Sit, C.S.; Lemire, B.D. The Saccharomyces cerevisiae succinate dehydrogenase does not require heme for ubiquinone reduction. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1436–1445. [Google Scholar] [CrossRef]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal Structure of Mitochondrial Respiratory Membrane Protein Complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef]
- Enriquez, J.A.; Lenaz, G. Coenzyme q and the respiratory chain: Coenzyme q pool and mitochondrial supercomplexes. Mol. Syndromol. 2014, 5, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Van Vranken, J.G.; Na, U.; Winge, D.R.; Rutter, J. Protein-mediated assembly of succinate dehydrogenase and its cofactors. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 168–180. [Google Scholar] [CrossRef]
- Guzy, R.D.; Sharma, B.; Bell, E.; Chandel, N.S.; Schumacker, P.T. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell. Biol. 2008, 28, 718–731. [Google Scholar] [CrossRef]
- Ishii, T.; Yasuda, K.; Akatsuka, A.; Hino, O.; Hartman, P.S.; Ishii, N. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. 2005, 65, 203–209. [Google Scholar]
- Slane, B.G.; Aykin-Burns, N.; Smith, B.J.; Kalen, A.L.; Goswami, P.C.; Domann, F.E.; Spitz, D.R. Mutation of succinate dehydrogenase subunit C results in increased O2.-, oxidative stress, and genomic instability. Cancer Res. 2006, 66, 7615–7620. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Smith, E.H.; Janknecht, R.; Maher, L.J., III. Succinate inhibition of α-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Hum. Mol. Genet. 2007, 16, 3136–3148. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhu, W.; Yu, J.; Wang, Q.; Zhang, J.; Cui, Y.; Pan, X.; Gao, X.; Sun, H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 2020, 28, 101365. [Google Scholar] [CrossRef] [PubMed]
- Letouzé, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Benit, P.; et al. SDH Mutations Establish a Hypermethylator Phenotype in Paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef]
- Astuti, D.; Latif, F.; Dallol, A.; Dahia, P.L.M.; Douglas, F.; George, E.; Sköldberg, F.; Husebye, E.S.; Eng, C.; Maher, E.R. Gene Mutations in the Succinate Dehydrogenase Subunit SDHB Cause Susceptibility to Familial Pheochromocytoma and to Familial Paraganglioma. Am. J. Hum. Genet. 2001, 69, 49–54. [Google Scholar] [CrossRef]
- Niemann, S.; Müller, U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat. Genet. 2000, 26, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Baysal, B.E.; Ferrell, R.E.; Willett-Brozick, J.E.; Lawrence, E.C.; Myssiorek, D.; Bosch, A.; van der Mey, A.; Taschner, P.E.; Rubinstein, W.S.; Myers, E.N.; et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000, 287, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Bardella, C.; Pollard, P.J.; Tomlinson, I. SDH mutations in cancer. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 1432–1443. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Foster, T.H. Optogenetic control of ROS production. Redox Biol. 2014, 2, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Roqueplo, A.-P.; Favier, J.; Rustin, P.; Mourad, J.-J.; Plouin, P.-F.; Corvol, P.; Rötig, A.; Jeunemaitre, X. The R22X Mutation of the SDHD Gene in Hereditary Paraganglioma Abolishes the Enzymatic Activity of Complex II in the Mitochondrial Respiratory Chain and Activates the Hypoxia Pathway. Am. J. Hum. Genet. 2001, 69, 1186–1197. [Google Scholar] [CrossRef]
- Pasini, B.; Stratakis, C.A. SDH mutations in tumorigenesis and inherited endocrine tumours: Lesson from the phaeochromocytoma–paraganglioma syndromes. J. Int. Med. 2009, 266, 19–42. [Google Scholar] [CrossRef]
- Bayley, J.P.; Kunst, H.P.; Cascon, A.; Sampietro, M.L.; Gaal, J.; Korpershoek, E.; Hinojar-Gutierrez, A.; Timmers, H.J.; Hoefsloot, L.H.; Hermsen, M.A.; et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010, 11, 366–372. [Google Scholar] [CrossRef]
- Fishbein, L.; Nathanson, K.L. Pheochromocytoma and paraganglioma: Understanding the complexities of the genetic background. Cancer Genet. 2012, 205, 1–11. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, S.; Zhou, J.; Wang, Y.; Di, W. Succinate dehydrogenase subunit B inhibits the AMPK-HIF-1α pathway in human ovarian cancer in vitro. J. Ovarian Res. 2014, 7, 115. [Google Scholar] [CrossRef]
- Tseng, P.-L.; Wu, W.-H.; Hu, T.-H.; Chen, C.-W.; Cheng, H.-C.; Li, C.-F.; Tsai, W.-H.; Tsai, H.-J.; Hsieh, M.-C.; Chuang, J.-H.; et al. Decreased succinate dehydrogenase B in human hepatocellular carcinoma accelerates tumor malignancy by inducing the Warburg effect. Sci. Rep. 2018, 8, 3081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, W.; Xiang, B.; Li, N.; Huang, S.; Zhou, W.; Sun, Y.; Wang, X.; Ma, J.; Li, G.; et al. Reduced succinate dehydrogenase B expression is associated with growth and de-differentiation of colorectal cancer cells. Tumor Biol. 2013, 34, 2337–2347. [Google Scholar] [CrossRef]
- Janeway, K.A.; Kim, S.Y.; Lodish, M.; Nosé, V.; Rustin, P.; Gaal, J.; Dahia, P.L.M.; Liegl, B.; Ball, E.R.; Raygada, M.; et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA 2011, 108, 314–318. [Google Scholar] [CrossRef]
- Nannini, M.; Urbini, M.; Indio, V.; Schipani, A.; Vincenzi, B.; Silletta, M.; Grignani, G.; Tolomeo, F.; Rizzo, A.; Fumagalli, E.; et al. Identification of SDHA germline mutations in sporadic SDHA mutant gastrointestinal stromal tumors (GIST): The need of a genetic counselling. J. Clin. Oncol. 2020, 38, 11537. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Drier, Y.; Johnstone, S.E.; Hemming, M.L.; Tarjan, D.R.; Hegazi, E.; Shareef, S.J.; Javed, N.M.; Raut, C.P.; Eschle, B.K.; et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature 2019, 575, 229–233. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Forman, J.R.; Rattenberry, E.; Bradshaw, N.; Lalloo, F.; Izatt, L.; Cole, T.R.; Armstrong, R.; Kumar, V.K.; Morrison, P.J.; et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum. Mutat. 2010, 31, 41–51. [Google Scholar] [CrossRef]
- Xekouki, P.; Szarek, E.; Bullova, P.; Giubellino, A.; Quezado, M.; Mastroyannis, S.A.; Mastorakos, P.; Wassif, C.A.; Raygada, M.; Rentia, N.; et al. Pituitary Adenoma With Paraganglioma/Pheochromocytoma (3PAs) and Succinate Dehydrogenase Defects in Humans and Mice. J. Clin. Endocrinol. Metab. 2015, 100, E710–E719. [Google Scholar] [CrossRef]
- Roh, T.H.; Yim, H.; Roh, J.; Lee, K.B.; Park, S.H.; Jeong, S.-Y.; Kim, S.-H.; Kim, J.-H. The loss of succinate dehydrogenase B expression is frequently identified in hemangioblastoma of the central nervous system. Sci. Rep. 2019, 9, 5873. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R.; Bärtsch, P. Acute high-altitude sickness. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef]
- Murray, A.J.; Horscroft, J.A. Mitochondrial function at extreme high altitude. J. Physiol. 2016, 594, 1137–1149. [Google Scholar] [CrossRef]
- Bakonyi, T.; Radak, Z. High altitude and free radicals. J. Sports Sci. Med. 2004, 3, 64–69. [Google Scholar]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef]
- Lu, H.; Wang, R.; Li, W.; Xie, H.; Wang, C.; Hao, Y.; Sun, Y.; Jia, Z. Plasma proteomic study of acute mountain sickness susceptible and resistant individuals. Sci. Rep. 2018, 8, 1265. [Google Scholar] [CrossRef]
- Landsverk, M.L.; Zhang, V.W.; Wong, L.-J.C.; Andersson, H.C. A SUCLG1 mutation in a patient with mitochondrial DNA depletion and congenital anomalies. Mol. Genet. Metab. Rep. 2014, 1, 451–454. [Google Scholar] [CrossRef]
- Chinopoulos, C.; Batzios, S.; van den Heuvel, L.P.; Rodenburg, R.; Smeets, R.; Waterham, H.R.; Turkenburg, M.; Ruiter, J.P.; Wanders, R.J.A.; Doczi, J.; et al. Mutated SUCLG1 causes mislocalization of SUCLG2 protein, morphological alterations of mitochondria and an early-onset severe neurometabolic disorder. Mol. Genet. Metab. 2019, 126, 43–52. [Google Scholar] [CrossRef]
- Cerecer-Gil, N.Y.; Figuera, L.E.; Llamas, F.J.; Lara, M.; Escamilla, J.G.; Ramos, R.; Estrada, G.; Hussain, A.K.; Gaal, J.; Korpershoek, E.; et al. Mutation of SDHB is a Cause of Hypoxia-Related High-Altitude Paraganglioma. Clin. Cancer Res. 2010, 16, 4148–4154. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e413. [Google Scholar] [CrossRef]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Vicario, N.; Costa, A.S.H.; Kwok, C.K.; Leonardi, T.; Booty, L.M.; Bicci, I.; Balzarotti, B.; Volpe, G.; et al. Macrophage-Derived Extracellular Succinate Licenses Neural Stem Cells to Suppress Chronic Neuroinflammation. Cell Stem Cell 2018, 22, 355–368.e313. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chen, X.Q.; Oliver Chen, C.Y. Ubiquinol is superior to ubiquinone to enhance Coenzyme Q10 status in older men. Food Funct. 2018, 9, 5653–5659. [Google Scholar] [CrossRef]
- Rustin, P.; Munnich, A.; Rötig, A. Succinate dehydrogenase and human diseases: New insights into a well-known enzyme. Eur. J. Hum. Genet. 2002, 10, 289–291. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, J.; Johns, T.; Fu, K.; Bie, I.D.; Macmillan, C.; Cuthbert, A.P.; Newbold, R.F.; Wang, J.-C.; Chevrette, M.; et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998, 20, 337–343. [Google Scholar] [CrossRef]
- Parfait, B.; Chretien, D.; Rötig, A.; Marsac, C.; Munnich, A.; Rustin, P. Compound heterozygous mutations in the flavoprotein gene of the respiratory chain complex II in a patient with Leigh syndrome. Hum. Genet. 2000, 106, 236–243. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Taylor, R.W.; Cochran, B.; Ackrell, B.A.; Turnbull, D.M. Late-onset optic atrophy, ataxia, and myopathy associated with a mutation of a complex II gene. Ann. Neurol. 2000, 48, 330–335. [Google Scholar] [CrossRef]
- Alston, C.L.; Davison, J.E.; Meloni, F.; van der Westhuizen, F.H.; He, L.; Hornig-Do, H.T.; Peet, A.C.; Gissen, P.; Goffrini, P.; Ferrero, I.; et al. Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J. Med. Genet. 2012, 49, 569–577. [Google Scholar] [CrossRef]
- Jackson, C.B.; Nuoffer, J.-M.; Hahn, D.; Prokisch, H.; Haberberger, B.; Gautschi, M.; Häberli, A.; Gallati, S.; Schaller, A. Mutations in SDHD lead to autosomal recessive encephalomyopathy and isolated mitochondrial complex II deficiency. J. Med. Genet. 2014, 51, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Goffrini, P.; Uziel, G.; Horvath, R.; Klopstock, T.; Lochmüller, H.; D’Adamo, P.; Gasparini, P.; Strom, T.M.; Prokisch, H.; et al. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat. Genet. 2009, 41, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.J.; Kiani-Esfahani, A. Succinate dehydrogenase: Prospect for neurodegenerative diseases. Mitochondrion 2018, 42, 77–83. [Google Scholar] [CrossRef]
- Villa-Cuesta, E.; Holmbeck, M.A.; Rand, D.M. Rapamycin increases mitochondrial efficiency by mtDNA-dependent reprogramming of mitochondrial metabolism in Drosophila. J. Cell Sci. 2014, 127, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, J.B.; Laberge, R.-M. Age-Related Neurodegeneration Prevention Through mTOR Inhibition: Potential Mechanisms and Remaining Questions. Curr. Top. Med. Chem. 2015, 15, 2139–2151. [Google Scholar] [CrossRef]
- Fahien, L.A.; MacDonald, M.J. The succinate mechanism of insulin release. Diabetes 2002, 51, 2669–2676. [Google Scholar] [CrossRef]
- Haythorne, E.; Rohm, M.; van de Bunt, M.; Brereton, M.F.; Tarasov, A.I.; Blacker, T.S.; Sachse, G.; dos Santos, M.S.; Exposito, R.T.; Davis, S.; et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat. Commun. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Sadagopan, N.; Li, W.; Roberds, S.L.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar] [CrossRef]
- Toma, I.; Kang, J.J.; Sipos, A.; Vargas, S.; Bansal, E.; Hanner, F.; Meer, E.; Peti-Peterdi, J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Investig. 2008, 118, 2526–2534. [Google Scholar] [CrossRef]
- Gurley, S.B.; Coffman, T.M. The Renin-Angiotensin System and Diabetic Nephropathy. Semin. Nephrol. 2007, 27, 144–152. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef]
- Dudek, J.; Cheng, I.F.; Chowdhury, A.; Wozny, K.; Balleininger, M.; Reinhold, R.; Grunau, S.; Callegari, S.; Toischer, K.; Wanders, R.J.; et al. Cardiac-specific succinate dehydrogenase deficiency in Barth syndrome. EMBO Mol. Med. 2016, 8, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Lam, M.T.Y.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Santos, R.M.; Moreno, C.; Zhang, W.C. Non-Coding RNAs in Lung Tumor Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2774. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.T.; Hu, N.; Tan, L. Non-coding RNAs in Alzheimer’s disease. Mol. Neurobiol. 2013, 47, 382–393. [Google Scholar] [CrossRef]
- Lee, M.R.; Mantel, C.; Lee, S.A.; Moon, S.-H.; Broxmeyer, H.E. MiR-31/SDHA Axis Regulates Reprogramming Efficiency through Mitochondrial Metabolism. Stem Cell Rep. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X. Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Res. 2006, 34, 1646–1652. [Google Scholar] [CrossRef]
- Metruccio, M.M.E.; Fantappiè, L.; Serruto, D.; Muzzi, A.; Roncarati, D.; Donati, C.; Scarlato, V.; Delany, I. The Hfq-Dependent Small Noncoding RNA NrrF Directly Mediates Fur-Dependent Positive Regulation of Succinate Dehydrogenase in Neisseria meningitidis. J. Bacteriol. 2009, 191, 1330–1342. [Google Scholar] [CrossRef]
- Sirey, T.M.; Roberts, K.; Haerty, W.; Bedoya-Reina, O.; Rogatti-Granados, S.; Tan, J.Y.; Li, N.; Heather, L.C.; Carter, R.N.; Cooper, S.; et al. The long non-coding RNA Cerox1 is a post transcriptional regulator of mitochondrial complex I catalytic activity. eLife 2019, 8. [Google Scholar] [CrossRef]
- Simpson, L.; Emeson, R.B. RNA editing. Annu. Rev. Neurosci. 1996, 19, 27–52. [Google Scholar] [CrossRef]
- Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010, 79, 321–349. [Google Scholar] [CrossRef]
- Zhang, W.C.; Slack, F.J. ADARs Edit MicroRNAs to Promote Leukemic Stem Cell Activity. Cell Stem Cell 2016, 19, 141–142. [Google Scholar] [CrossRef][Green Version]
- Salter, J.D.; Bennett, R.P.; Smith, H.C. The APOBEC Protein Family: United by Structure, Divergent in Function. Trends Biochem. Sci. 2016, 41, 578–594. [Google Scholar] [CrossRef]
- Baysal, B.E.; De Jong, K.; Liu, B.; Wang, J.; Patnaik, S.K.; Wallace, P.K.; Taggart, R.T. Hypoxia-inducible C-to-U coding RNA editing downregulates SDHB in monocytes. PeerJ 2013, 1, e152. [Google Scholar] [CrossRef]
- Sharma, S.; Patnaik, S.K.; Taggart, R.T.; Kannisto, E.D.; Enriquez, S.M.; Gollnick, P.; Baysal, B.E. APOBEC3A cytidine deaminase induces RNA editing in monocytes and macrophages. Nat. Commun. 2015, 6, 6881. [Google Scholar] [CrossRef]
- Kawahara, Y.; Zinshteyn, B.; Sethupathy, P.; Iizasa, H.; Hatzigeorgiou, A.G.; Nishikura, K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science 2007, 315, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lin, S.; Zhang, W.; Liu, Q.; Wang, L.; Ramirez-Moya, J.; Du, P.; Kim, W.; Tang, S.; Sliz, P.; et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 2018, 561, 556–560. [Google Scholar] [CrossRef]
- Shi, H.; Wei, J.; He, C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell. 2019, 74, 640–650. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr. Cancer and altered metabolism: Potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 335–345. [Google Scholar] [CrossRef]
- Ma, S.; Chen, C.; Ji, X.; Liu, J.; Zhou, Q.; Wang, G.; Yuan, W.; Kan, Q.; Sun, Z. The interplay between m6A RNA methylation and noncoding RNA in cancer. J. Hematol. Oncol. 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Latchman, D.S. Transcription factors: An overview. Int. J. Exp. Pathol. 1993, 74, 417–422. [Google Scholar] [CrossRef]
- Lee, T.I.; Young, R.A. Transcriptional regulation and its misregulation in disease. Cell 2013, 152, 1237–1251. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Transcriptional Regulation of SDHa flavoprotein by nuclear respiratory factor-1 prevents pseudo-hypoxia in aerobic cardiac cells. J. Biol. Chem. 2008, 283, 10967–10977. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-T.; Huang, D.; Shen, S.; Cai, Y.; Xing, S.; Wu, G.; Jiang, Z.; Hao, Y.; Yuan, M.; Wang, N.; et al. Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis. Nat. Metab. 2020, 2, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Huang, B.O.; Xu, Y.-M.; Li, J.; Huang, L.-F.; Lin, J.; Zhang, J.; Min, Q.-H.; Yang, W.-M.; et al. Mechanism of alternative splicing and its regulation. Biomed. Rep. 2015, 3, 152–158. [Google Scholar] [CrossRef]
- Satoh, N.; Yokoyama, C.; Itamura, N.; Miyajima-Nakano, Y.; Hisatomi, H. Alternative splicing isoform in succinate dehydrogenase complex, subunit C causes downregulation of succinate-coenzyme Q oxidoreductase activity in mitochondria. Oncol. Lett. 2015, 9, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Maklashina, E.; Cecchini, G. The quinone-binding and catalytic site of complex II. Biochim. Biophys. Acta 2010, 1797, 1877–1882. [Google Scholar] [CrossRef]
- Hagerhall, C. Succinate: Quinone oxidoreductases. Variations on a conserved theme. Biochim. Biophys. Acta 1997, 1320, 107–141. [Google Scholar] [CrossRef]
- Tappel, A.L. Inhibition of electron transport by antimycin A, alkyl hydroxy naphthoquinones and metal coordination compounds. Biochem. Pharmacol. 1960, 3, 289–296. [Google Scholar] [CrossRef]
- Yao, T.T.; Fang, S.W.; Li, Z.S.; Xiao, D.X.; Cheng, J.L.; Ying, H.Z.; Du, Y.J.; Zhao, J.H.; Dong, X.W. Discovery of Novel Succinate Dehydrogenase Inhibitors by the Integration of in Silico Library Design and Pharmacophore Mapping. J. Agric. Food Chem. 2017, 65, 3204–3211. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Karabutova, L.A.; Pokusina, T.A. Light regulation of succinate dehydrogenase subunit B gene SDH2-3 expression in maize leaves. Russ. J. Plant Physiol. 2016, 63, 505–510. [Google Scholar] [CrossRef]
- Kruspig, B.; Valter, K.; Skender, B.; Zhivotovsky, B.; Gogvadze, V. Targeting succinate:ubiquinone reductase potentiates the efficacy of anticancer therapy. Biochim. Biophys. Acta 2016, 1863, 2065–2071. [Google Scholar] [CrossRef]
- Dong, L.F.; Low, P.; Dyason, J.C.; Wang, X.F.; Prochazka, L.; Witting, P.K.; Freeman, R.; Swettenham, E.; Valis, K.; Liu, J.; et al. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene 2008, 27, 4324–4335. [Google Scholar] [CrossRef]
- Valls-Lacalle, L.; Barba, I.; Miró-Casas, E.; Ruiz-Meana, M.; Rodríguez-Sinovas, A.; García-Dorado, D. Selective Inhibition of Succinate Dehydrogenase in Reperfused Myocardium with Intracoronary Malonate Reduces Infarct Size. Sci. Rep. 2018, 8, 2442. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.; Shurubor, Y.; Valsecchi, F.; Manfredi, G.; Galkin, A. Differential susceptibility of mitochondrial complex II to inhibition by oxaloacetate in brain and heart. Biochim. Biophys. Acta 2016, 1857, 1561–1568. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The Mitochondrial Chaperone TRAP1 Promotes Neoplastic Growth by Inhibiting Succinate Dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, G.; Sciacovelli, M.; Bernardi, P.; Rasola, A. Inhibition of succinate dehydrogenase by the mitochondrial chaperone TRAP1 has anti-oxidant and anti-apoptotic effects on tumor cells. Oncotarget 2014, 5, 11897–11908. [Google Scholar] [CrossRef]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 2016, 1857, 1086–1101. [Google Scholar] [CrossRef]
- Fan, F.; Sam, R.; Ryan, E.; Alvarado, K.; Villa-Cuesta, E. Rapamycin as a potential treatment for succinate dehydrogenase deficiency. Heliyon 2019, 5, e01217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prakash, A.; Dogra, S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food Chem. Toxicol. 2010, 48, 626–632. [Google Scholar] [CrossRef]
- Cimen, H.; Han, M.J.; Yang, Y.; Tong, Q.; Koc, H.; Koc, E.C. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 2010, 49, 304–311. [Google Scholar] [CrossRef]
- Finley, L.W.; Haigis, M.C. Metabolic regulation by SIRT3: Implications for tumorigenesis. Trends Mol. Med. 2012, 18, 516–523. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Dobychina, M.A.; Igamberdiev, A.U. Expression and promoter methylation of succinate dehydrogenase and fumarase genes in maize under anoxic conditions. J. Plant Physiol. 2017, 216, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.J.; Martin-Urdiroz, M.; Yan, X.; Wright, H.S.; Soanes, D.M.; Talbot, N.J. CRISPR-Cas9 ribonucleoprotein-mediated co-editing and counterselection in the rice blast fungus. Sci. Rep. 2018, 8, 14355. [Google Scholar] [CrossRef]
- Rosland, G.V.; Dyrstad, S.E.; Tusubira, D.; Helwa, R.; Tan, T.Z.; Lotsberg, M.L.; Pettersen, I.K.N.; Berg, A.; Kindt, C.; Hoel, F.; et al. Epithelial to mesenchymal transition (EMT) is associated with attenuation of succinate dehydrogenase (SDH) in breast cancer through reduced expression of SDHC. Cancer Metab. 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Munujos, P.; Collcanti, J.; Gonzalezsastre, F.; Gella, F.J. Assay of Succinate Dehydrogenase Activity by a Colorimetric-Continuous Method Using Iodonitrotetrazolium Chloride as Electron Acceptor. Anal. Biochem. 1993, 212, 506–509. [Google Scholar] [CrossRef]
- Hollywood, K.A.; Shadi, I.T.; Goodacre, R. Monitoring the Succinate Dehydrogenase Activity Isolated from Mitochondria by Surface Enhanced Raman Scattering. J. Phys. Chem. C 2010, 114, 7308–7313. [Google Scholar] [CrossRef]
- Old, S.L.; Johnson, M.A. Methods of microphotometric assay of succinate dehydrogenase and cytochromec oxidase activities for use on human skeletal muscle. Histochem. J. 1989, 21, 545–555. [Google Scholar] [CrossRef]
- Škorjanc, D.; Heine, G.; Pette, D. Time-dependent increase of succinate dehydrogenase activity in low-frequency stimulated rabbit muscle: A comparison between microphotometric and biochemical methods. Histochem. Cell Biol. 1997, 107, 47–55. [Google Scholar] [CrossRef]
- Huang, S.; Braun, H.-P.; Gawryluk, R.M.R.; Millar, A.H. Mitochondrial complex II of plants: Subunit composition, assembly, and function in respiration and signaling. Plant J. 2019, 98, 405–417. [Google Scholar] [CrossRef]
- Schikowsky, C.; Senkler, J.; Braun, H.P. SDH6 and SDH7 Contribute to Anchoring Succinate Dehydrogenase to the Inner Mitochondrial Membrane in Arabidopsis thaliana. Plant Physiol. 2017, 173, 1094–1108. [Google Scholar] [CrossRef]
- Panizza, E.; Ercolino, T.; Mori, L.; Rapizzi, E.; Castellano, M.; Opocher, G.; Ferrero, I.; Neumann, H.P.H.; Mannelli, M.; Goffrini, P. Yeast model for evaluating the pathogenic significance of SDHB, SDHC and SDHD mutations in PHEO–PGL syndrome. Hum. Mol. Genet. 2012, 22, 804–815. [Google Scholar] [CrossRef]
- Park, S.J.; Chao, G.; Gunsalus, R.P. Aerobic regulation of the sucABCD genes of Escherichia coli, which encode alpha-ketoglutarate dehydrogenase and succinyl coenzyme A synthetase: Roles of ArcA, Fnr, and the upstream sdhCDAB promoter. J. Bacteriol. 1997, 179, 4138–4142. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 106, 5–28. [Google Scholar] [CrossRef]
- Huang, J.; Lemire, B.D. Mutations in the C. elegans Succinate Dehydrogenase Iron–Sulfur Subunit Promote Superoxide Generation and Premature Aging. J. Mol. Biol. 2009, 387, 559–569. [Google Scholar] [CrossRef]
- Piruat, J.I.; Millán-Uclés, A. Genetically modeled mice with mutations in mitochondrial metabolic enzymes for the study of cancer. Front. Oncol. 2014, 4, 200. [Google Scholar] [CrossRef]
- Aspuria, P.-J.P.; Lunt, S.Y.; Väremo, L.; Vergnes, L.; Gozo, M.; Beach, J.A.; Salumbides, B.; Reue, K.; Wiedemeyer, W.R.; Nielsen, J.; et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Ricci, R. Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumors: Small Steps Toward Personalized Medicine? Epigenet Insights 2019, 12, 2516865719842534. [Google Scholar] [CrossRef]
- Sarkadi, B.; Meszaros, K.; Krencz, I.; Canu, L.; Krokker, L.; Zakarias, S.; Barna, G.; Sebestyen, A.; Papay, J.; Hujber, Z.; et al. Glutaminases as a Novel Target for SDHB-Associated Pheochromocytomas/Paragangliomas. Cancers (Basel) 2020, 12, 599. [Google Scholar] [CrossRef]
- Lussey-Lepoutre, C.; Hollinshead, K.E.; Ludwig, C.; Menara, M.; Morin, A.; Castro-Vega, L.J.; Parker, S.J.; Janin, M.; Martinelli, C.; Ottolenghi, C.; et al. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat. Commun. 2015, 6, 8784. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.; Jimenez, C. Recent advances in the management of malignant pheochromocytoma and paraganglioma: Focus on tyrosine kinase and hypoxia-inducible factor inhibitors. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Milne, J.L.S.; Borgnia, M.J.; Bartesaghi, A.; Tran, E.E.H.; Earl, L.A.; Schauder, D.M.; Lengyel, J.; Pierson, J.; Patwardhan, A.; Subramaniam, S. Cryo-electron microscopy--a primer for the non-microscopist. FEBS J. 2013, 280, 28–45. [Google Scholar] [CrossRef]
- Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016, 17, 83–96. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Bisbach, C.M.; Hass, D.T.; Robbings, B.M.; Rountree, A.M.; Sadilek, M.; Sweet, I.R.; Hurley, J.B. Succinate Can Shuttle Reducing Power from the Hypoxic Retina to the O2-Rich Pigment Epithelium. Cell Rep. 2020, 31, 107606. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Duvenage, L.; Munro, C.A.; Gourlay, C.W. The potential of respiration inhibition as a new approach to combat human fungal pathogens. Curr. Genet. 2019, 65, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mu, X.; You, Q. Succinate: An initiator in tumorigenesis and progression. Oncotarget 2017, 8, 53819–53828. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zuroske, T.; Watts, J.K. RNA therapeutics on the rise. Nat. Rev. Drug Discov. 2020, 19, 441–442. [Google Scholar] [CrossRef]
- Nogrady, B. The challenge of delivering RNA-interference therapeutics to their target cells. Nature 2019, 574, S8–S9. [Google Scholar] [CrossRef]
- Guo, P.; Haque, F.; Hallahan, B.; Reif, R.; Li, H. Uniqueness, advantages, challenges, solutions, and perspectives in therapeutics applying RNA nanotechnology. Nucleic Acid Ther. 2012, 22, 226–245. [Google Scholar] [CrossRef] [PubMed]



| Study Title | Description | Dates (Start–Completion) and Status | Identifier and Study Type (Enrollment) |
|---|---|---|---|
| Cancers | |||
| An Open-Label, Phase 2 Efficacy Study of Temozolomide (TMZ) In Advanced Succinate Dehydrogenase (SDH)-Mutant/Deficient Gastrointestinal Stromal Tumor (GIST) | Therapies already exist for advanced GIST, but they are not effective against SDH mutant subtypes of GIST. TMZ is already approved for the treatment of other glioblastoma tumors, but its effect on SDH mutant GIST has not been previously studied. | September 2018-September 2024 (Recruiting) | NCT03556384 Interventional (N.A.) |
| A Phase II Trial of the DNA Methyl Transferase Inhibitor, Guadecitabine (SGI-110), in Children and Adults with Wild Type GIST, Pheochromocytoma and Paraganglioma Associated with Succinate Dehydrogenase Deficiency and HLRCC-associated Kidney Cancer | To determine the overall response to SGI-110 in tumor growth and effects on the body. GIST is resistant to conventional radiation or chemotherapy treatments. Imatinib is the current standard of care but tumor-developed resistance and mutations are becoming more prevalent. SGI-110 is targeting these tumors by preventing DNA methylation and has shown to be effective against imatinib resistant GIST. | May 2017- February 2020 (Completed) | NCT03165721 Interventional (9) |
| Ph1 Study of the Safety, PK, and PDn of Escalating Oral Doses of the Glutaminase Inhibitor CB-839, as a Single Agent and in Combination with Standard Chemotherapy in Patients with Advanced and/or Treatment-Refractory Solid Tumors | Tumor cells have been shown to be dependent on glutamine for cellular respiration. Because this is a unique trait to tumors, this dependence serves as a potential therapeutic target. CB-839 is a highly specific inhibitor targeting glutaminase, the first enzyme involved in glutamine utilization. This study looks at the potency of this inhibitor across a wide range of tumors. | February 2015- March 2019 (Completed) | NCT02071862 Interventional (210) |
| A Phase 2 Open-Label Study of Nivolumab Combined with Cabozantinib in Subjects with Advanced or Metastatic Non-Clear Cell Renal Cell Carcinoma (CA209-9KU) | Nivolumab is a programmed cell death protein 1 inhibitor, and cabozantinib is a tyrosine kinase inhibitor. Both drugs are approved treatments against several types of metastatic kidney cancers, but there are limited data on combination treatments using these two drugs. | August 2018-August 2021 (Recruiting) | NCT03635892 Interventional (N.A.) |
| Impact of Environmental Exposures on Tumor Risk in Subjects at Risk of Hereditary SDHx Paraganglioma (PGL-EXPO-1) | By studying environmental and professional factors of patients with an SDHx mutation and comparing it to a patient with the same sex, age, and type of gene affected but no tumor progression, researchers hope to identify novel contributors to SDHx genetic mediated tumor progression. | January 2021-December 2022 (Not yet recruiting) | NCT04481152 Observational (N.A.) |
| Non-cancer | |||
| Relationship Between Succinate Dehydrogenase Mutations and High-Altitude Illness Associated with Chemoreflex Failure | SDH dysfunction is known to cause hypoxia. At high altitudes where oxygen is limited, a cell already coping with SDH dysfunction would be overwhelmed. The chemoreflex causes hyperventilation when the pressure of oxygen falls in the blood. A dysfunctional chemoreflex can lead to pulmonary and cerebral edema at high altitudes. | March 2005-December 2006 (Completed) | NCT00202683 Observational (83) |
| North American Mitochondrial Disease Consortium Patient Registry and Biorepository (NAMDC) | The NAMDC is building an international network of researchers, patients, and data to help both the researcher and patient connect with the proper clinical trials and potential treatments. | December 2010-December 2025 (Recruiting) | NCT01694940 Observational (N.A.) |
| Targeting Glutamine Metabolism to Prevent Diabetic Cardiovascular Complications (GLUTADIAB) | This study has broad metabolic implications and serves as the starting point for several secondary studies. After glutamine metabolism is better understood in its role in the inflammatory response, several other factors will be analyzed including SDH-controlled intermediates. RNA modification will also be utilized to target monocytes. | June 2020- June 2022 (Not yet recruiting) | NCT04353869 Observational (N.A.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, C.; Santos, R.M.; Burns, R.; Zhang, W.C. Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases. Cancers 2020, 12, 3237. https://doi.org/10.3390/cancers12113237
Moreno C, Santos RM, Burns R, Zhang WC. Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases. Cancers. 2020; 12(11):3237. https://doi.org/10.3390/cancers12113237
Chicago/Turabian StyleMoreno, Cerena, Ruben Mercado Santos, Robert Burns, and Wen Cai Zhang. 2020. "Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases" Cancers 12, no. 11: 3237. https://doi.org/10.3390/cancers12113237
APA StyleMoreno, C., Santos, R. M., Burns, R., & Zhang, W. C. (2020). Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases. Cancers, 12(11), 3237. https://doi.org/10.3390/cancers12113237







