The 40bp Indel Polymorphism rs150550023 in the MDM2 Promoter is Associated with Intriguing Shifts in Gene Expression in the p53-MDM2 Regulatory Hub
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
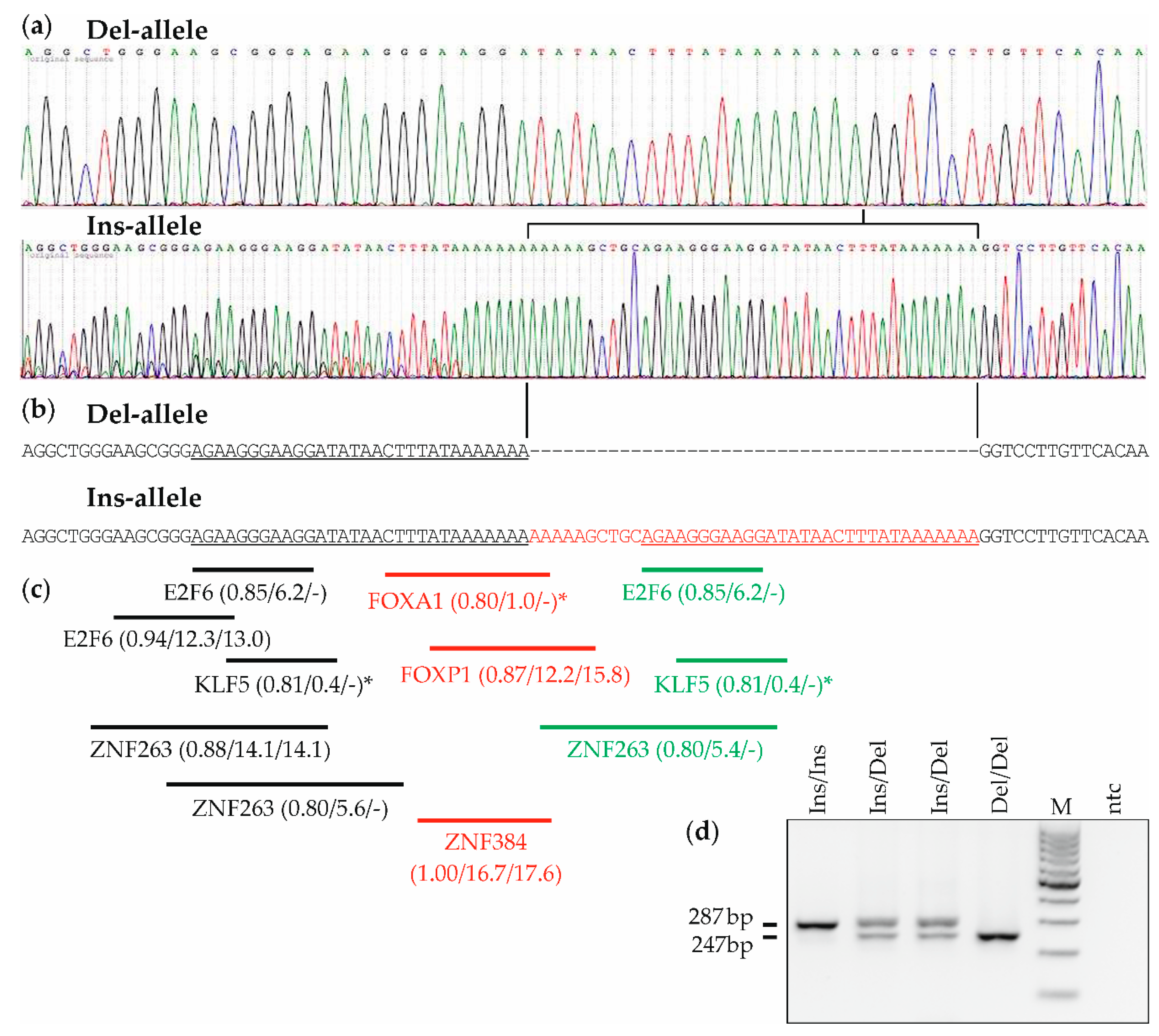
2.1. Sequence Analysis and Genotyping of the MDM2 Indel Variant rs150550023
2.2. Distribution of rs150550023 Genotypes
2.3. MDM2 rs150550023 and Breast Cancer Risk
2.4. Exploratory Analysis of MDM2 rs150550023 and Breast Cancer Risk in Subpopulations
2.5. Association of MDM2 rs150550023 with the Age at Breast Cancer Onset
2.6. MDM2 rs150550023 and mRNA Levels of MDM2, p53, and p53 Target Genes
2.7. Association of MDM2 rs150550023 with Expression of p53 Protein
2.8. Association of MDM2 rs150550023 with Breast Cancer Prognosis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Cell Lines
4.3. In Silico Analyses
4.4. DNA Isolation and Genotyping
4.5. qRT-PCR Analysis of mRNA Expression Levels
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Hainaut, P.; Pfeifer, G.P. Somatic TP53 Mutations in the Era of Genome Sequencing. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Burgess, A.; Chia, K.M.; Haupt, S.; Thomas, D.; Haupt, Y.; Lim, E. Clinical Overview of MDM2/X-Targeted Therapies. Front. Oncol. 2016, 6, 7. [Google Scholar] [CrossRef]
- Oliner, J.D.; Saiki, A.Y.; Caenepeel, S. The Role of MDM2 Amplification and Overexpression in Tumorigenesis. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Pant, V.; Lozano, G. Limiting the power of p53 through the ubiquitin proteasome pathway. Genes Dev. 2014, 28, 1739–1751. [Google Scholar] [CrossRef]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Haronikova, L.; Olivares-Illana, V.; Wang, L.; Karakostis, K.; Chen, S.; Fahraeus, R. The p53 mRNA: An integral part of the cellular stress response. Nucleic Acids Res. 2019, 47, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.N.; Roe, A.E.; Donehower, L.A.; Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995, 378, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca Luna, R.; Wagner, D.S.; Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995, 378, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.E.; Ouimet, M.; Lariviere, M.; Kritikou, E.A.; Sinnett, D. Identification of functional DNA variants in the constitutive promoter region of MDM2. Hum. Genom. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Oliner, J.D.; Pietenpol, J.A.; Thiagalingam, S.; Gyuris, J.; Kinzler, K.W.; Vogelstein, B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 1993, 362, 857–860. [Google Scholar] [CrossRef]
- Acun, T.; Terzioglu-Kara, E.; Konu, O.; Ozturk, M.; Yakicier, M.C. Mdm2 Snp309 G allele displays high frequency and inverse correlation with somatic P53 mutations in hepatocellular carcinoma. Mutat Res. 2010, 684, 106–108. [Google Scholar] [CrossRef]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef]
- Deben, C.; Op de Beeck, K.; Van den Bossche, J.; Jacobs, J.; Lardon, F.; Wouters, A.; Peeters, M.; Van Camp, G.; Rolfo, C.; Deschoolmeester, V.; et al. MDM2 SNP309 and SNP285 Act as Negative Prognostic Markers for Non-small Cell Lung Cancer Adenocarcinoma Patients. J. Cancer 2017, 8, 2154–2162. [Google Scholar] [CrossRef][Green Version]
- Hu, W.; Feng, Z.; Ma, L.; Wagner, J.; Rice, J.J.; Stolovitzky, G.; Levine, A.J. A single nucleotide polymorphism in the MDM2 gene disrupts the oscillation of p53 and MDM2 levels in cells. Cancer Res. 2007, 67, 2757–2765. [Google Scholar] [CrossRef]
- Miedl, H.; Lebhard, J.; Ehart, L.; Schreiber, M. Association of the MDM2 SNP285 and SNP309 Genetic Variants with the Risk, Age at Onset and Prognosis of Breast Cancer in Central European Women: A Hospital-Based Case-Control Study. Int. J. Mol. Sci. 2019, 20, 509. [Google Scholar] [CrossRef]
- Post, S.M.; Quintas-Cardama, A.; Pant, V.; Iwakuma, T.; Hamir, A.; Jackson, J.G.; Maccio, D.R.; Bond, G.L.; Johnson, D.G.; Levine, A.J.; et al. A high-frequency regulatory polymorphism in the p53 pathway accelerates tumor development. Cancer Cell 2010, 18, 220–230. [Google Scholar] [CrossRef]
- Sanchez-Carbayo, M.; Socci, N.D.; Kirchoff, T.; Erill, N.; Offit, K.; Bochner, B.H.; Cordon-Cardo, C. A polymorphism in HDM2 (SNP309) associates with early onset in superficial tumors, TP53 mutations, and poor outcome in invasive bladder cancer. Clin. Cancer Res. 2007, 13, 3215–3220. [Google Scholar] [CrossRef]
- Economopoulos, K.P.; Sergentanis, T.N. Differential effects of MDM2 SNP309 polymorphism on breast cancer risk along with race: A meta-analysis. Breast Cancer Res. Treat. 2010, 120, 211–216. [Google Scholar] [CrossRef]
- Hu, Z.; Jin, G.; Wang, L.; Chen, F.; Wang, X.; Shen, H. MDM2 promoter polymorphism SNP309 contributes to tumor susceptibility: Evidence from 21 case-control studies. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2717–2723. [Google Scholar]
- Zhao, E.; Cui, D.; Yuan, L.; Lu, W. MDM2 SNP309 polymorphism and breast cancer risk: A meta-analysis. Mol. Biol Rep. 2012, 39, 3471–3477. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, A.; Duan, P.; Zhu, J.; Zhao, Y.; He, J.; Zhang, Z. MDM2 promoter del1518 polymorphism and cancer risk: Evidence from 22,931 subjects. Onco. Targets Ther. 2017, 10, 3773–3780. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Zhang, J.; Liu, G. Influence of MDM2 polymorphisms on squamous cell carcinoma susceptibility: A meta-analysis. Onco. Targets Ther. 2016, 9, 6211–6224. [Google Scholar] [CrossRef]
- Moazeni-Roodi, A.; Ghavami, S.; Hashemi, M. The 40bp indel polymorphism of MDM2 increase the risk of cancer: An updated meta-analysis. Mol. Biol. Res. Commun. 2019, 8, 1–8. [Google Scholar]
- Gansmo, L.B.; Vatten, L.; Romundstad, P.; Hveem, K.; Ryan, B.M.; Harris, C.C.; Knappskog, S.; Lonning, P.E. Associations between the MDM2 promoter P1 polymorphism del1518 (rs3730485) and incidence of cancer of the breast, lung, colon and prostate. Oncotarget 2016, 7, 28637–28646. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Z.; Zhai, X.; Wang, S.; Wang, X.; Qin, J.; Jin, G.; Liu, J.; Wang, X.; Wei, Q.; et al. Polymorphisms in the MDM2 promoter and risk of breast cancer: A case-control analysis in a Chinese population. Cancer Lett. 2006, 240, 261–267. [Google Scholar] [CrossRef]
- Hashemi, M.; Omrani, M.; Eskandari-Nasab, E.; Hasani, S.S.; Mashhadi, M.A.; Taheri, M. A 40-bp insertion/deletion polymorphism of Murine Double Minute2 (MDM2) increased the risk of breast cancer in Zahedan, Southeast Iran. Iran. Biomed. J. 2014, 18, 245–249. [Google Scholar]
- Gallegos-Arreola, M.P.; Marquez-Rosales, M.G.; Sanchez-Corona, J.; Figuera, L.E.; Zuniga-Gonzalez, G.; Puebla-Perez, A.M.; Delgado-Saucedo, J.I.; Montoya-Fuentes, H. Association of the Del1518 Promoter (rs3730485) Polymorphism in the MDM2 Gene with Breast Cancer in a Mexican Population. Ann. Clin. Lab. Sci. 2017, 47, 291–297. [Google Scholar]
- Wang, M.; Zhang, Z.; Zhu, H.; Fu, G.; Wang, S.; Wu, D.; Zhou, J.; Wei, Q.; Zhang, Z. A novel functional polymorphism C1797G in the MDM2 promoter is associated with risk of bladder cancer in a Chinese population. Clin. Cancer Res. 2008, 14, 3633–3640. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, H.; Lu, D.; Qian, J.; Zhou, J.; Chen, Y.; Xu, L.; Wang, X.; Wei, Q.; Shen, H. Genetic variants in the MDM2 promoter and lung cancer risk in a Chinese population. Int J. Cancer 2006, 118, 1275–1278. [Google Scholar] [CrossRef]
- Tripon, F.; Iancu, M.; Trifa, A.; Crauciuc, G.A.; Boglis, A.; Balla, B.; Cosma, A.; Dima, D.; Candea, M.; Lazar, E.; et al. Association Analysis of TP53 rs1042522, MDM2 rs2279744, rs3730485, MDM4 rs4245739 Variants and Acute Myeloid Leukemia Susceptibility, Risk Stratification Scores, and Clinical Features: An Exploratory Study. J. Clin. Med. 2020, 9, 1672. [Google Scholar] [CrossRef]
- Maruei-Milan, R.; Heidari, Z.; Salimi, S. Role of MDM2 309T>G (rs2279744) and I/D (rs3730485) polymorphisms and haplotypes in risk of papillary thyroid carcinoma, tumor stage, tumor size, and early onset of tumor: A case control study. J. Cell Physiol. 2019, 234, 12934–12940. [Google Scholar] [CrossRef]
- Gansmo, L.B.; Bjornslett, M.; Halle, M.K.; Salvesen, H.B.; Romundstad, P.; Hveem, K.; Vatten, L.; Dorum, A.; Lonning, P.E.; Knappskog, S. MDM2 promoter polymorphism del1518 (rs3730485) and its impact on endometrial and ovarian cancer risk. BMC Cancer 2017, 17, 97. [Google Scholar] [CrossRef]
- Dong, D.; Gao, X.; Zhu, Z.; Yu, Q.; Bian, S.; Gao, Y. A 40-bp insertion/deletion polymorphism in the constitutive promoter of MDM2 confers risk for hepatocellular carcinoma in a Chinese population. Gene 2012, 497, 66–70. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Amador, M.A.; Ribeiro Dos Santos, A.M.; Carvalho, D.C.; Andrade, R.B.; Pereira, E.E.; Fernandes, M.R.; Costa, D.F.; Santos, N.P.; Assumpcao, P.P.; et al. Analysis of 12 variants in the development of gastric and colorectal cancers. World J. Gastroenterol. 2017, 23, 8533–8543. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.D.; Wu, H.Y.; Wang, K.J. Association between SNP309 and del 1518 Polymorphism in MDM2 Homologue and Esophageal Squamous Cell Carcinoma Risk in Chinese Population of Shandong Province. Ann. Clin. Lab. Sci. 2015, 45, 433–437. [Google Scholar]
- Hashemi, M.; Amininia, S.; Ebrahimi, M.; Simforoosh, N.; Basiri, A.; Ziaee, S.A.M.; Narouie, B.; Sotoudeh, M.; Mollakouchekian, M.J.; Rezghi Maleki, E.; et al. Association between polymorphisms in TP53 and MDM2 genes and susceptibility to prostate cancer. Oncol. Lett. 2017, 13, 2483–2489. [Google Scholar] [CrossRef]
- Salimi, S.; Hajizadeh, A.; Khodamian, M.; Pejman, A.; Fazeli, K.; Yaghmaei, M. Age-dependent association of MDM2 promoter polymorphisms and uterine leiomyoma in South-East Iran: A preliminary report. J. Obstet. Gynaecol. Res. 2015, 41, 729–734. [Google Scholar] [CrossRef]
- Maleki, Y.; Alahbakhshi, Z.; Heidari, Z.; Moradi, M.T.; Rahimi, Z.; Yari, K.; Rahimi, Z.; Aznab, M.; Ahmadi-Khajevand, M.; Bahremand, F. NOTCH1, SF3B1, MDM2 and MYD88 mutations in patients with chronic lymphocytic leukemia. Oncol. Lett. 2019, 17, 4016–4023. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Ning, T.; Chen, Z.; Xu, C. Association of genetic polymorphisms in MDM2, PTEN and P53 with risk of esophageal squamous cell carcinoma. J. Hum. Genet. 2012, 57, 261–264. [Google Scholar] [CrossRef][Green Version]
- Kang, S.; Wang, D.J.; Li, W.S.; Wang, N.; Zhou, R.M.; Sun, D.L.; Duan, Y.N.; Li, S.Z.; Li, X.F.; Li, Y. Association of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancer in Chinese women. Int. J. Gynecol. Cancer 2009, 19, 572–577. [Google Scholar] [CrossRef]
- Hashemi, M.; Naderi, M.; Eskandari Nasab, E.; Hasani, S.S.; Sadeghi Bojd, S.; Taheri, M. Evaluation of 40-bp Insertion/Deletion Polymorphism of MDM2 and the Risk of Childhood Acute Lymphoblastic Leukemia. Gene. Cell. Tissue 2015, 2, e26974. [Google Scholar] [CrossRef]
- Fornes, O.; Castro-Mondragon, J.A.; Khan, A.; van der Lee, R.; Zhang, X.; Richmond, P.A.; Modi, B.P.; Correard, S.; Gheorghe, M.; Baranasic, D.; et al. JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020, 48, D87–D92. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Weirauch, M.T.; Yang, A.; Albu, M.; Cote, A.G.; Montenegro-Montero, A.; Drewe, P.; Najafabadi, H.S.; Lambert, S.A.; Mann, I.; Cook, K.; et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 2014, 158, 1431–1443. [Google Scholar] [CrossRef]
- Bernardo, G.M.; Keri, R.A. FOXA1: A transcription factor with parallel functions in development and cancer. Biosci. Rep. 2012, 32, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Gleiberman, A.S.; Shi, C.; Simon, D.I.; Rosenfeld, M.G. Cooperative regulation in development by SMRT and FOXP1. Genes Dev. 2008, 22, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, M.; Nakamoto, T.; Hino, K.; Tsuji, K.; Shen, Z.J.; Nakashima, K.; Nifuji, A.; Yamamoto, H.; Hirai, H.; Noda, M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J. Exp. Med. 2005, 201, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, J.M.; Fairchild, B.; Verona, R.; Moberg, K.; Andon, N.; Lees, J.A. E2F-6, a member of the E2F family that can behave as a transcriptional repressor. Proc. Natl. Acad. Sci. USA 1998, 95, 2850–2855. [Google Scholar] [CrossRef]
- Liu, R.; Dong, J.T.; Chen, C. Role of KLF5 in hormonal signaling and breast cancer development. Vitam. Horm. 2013, 93, 213–225. [Google Scholar]
- Frietze, S.; Lan, X.; Jin, V.X.; Farnham, P.J. Genomic targets of the KRAB and SCAN domain-containing zinc finger protein 263. J. Biol. Chem. 2010, 285, 1393–1403. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Bond, G.L.; Hirshfield, K.M.; Kirchhoff, T.; Alexe, G.; Bond, E.E.; Robins, H.; Bartel, F.; Taubert, H.; Wuerl, P.; Hait, W.; et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006, 66, 5104–5110. [Google Scholar] [CrossRef]
- Bond, G.L.; Levine, A.J. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene 2007, 26, 1317–1323. [Google Scholar] [CrossRef]
- Lum, S.S.; Chua, H.W.; Li, H.; Li, W.F.; Rao, N.; Wei, J.; Shao, Z.; Sabapathy, K. MDM2 SNP309 G allele increases risk but the T allele is associated with earlier onset age of sporadic breast cancers in the Chinese population. Carcinogenesis 2008, 29, 754–761. [Google Scholar] [CrossRef]
- Lacroix, M.; Toillon, R.A.; Leclercq, G. p53 and breast cancer, an update. Endocr. Relat. Cancer 2006, 13, 293–325. [Google Scholar] [CrossRef] [PubMed]
- Dusek, R.L.; Bascom, J.L.; Vogel, H.; Baron, S.; Borowsky, A.D.; Bissell, M.J.; Attardi, L.D. Deficiency of the p53/p63 target Perp alters mammary gland homeostasis and promotes cancer. Breast Cancer Res. 2012, 14, R65. [Google Scholar] [CrossRef]
- El-Deiry, W.S. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016, 76, 5189–5191. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Tan, B.X.; Lane, D. How the Other Half Lives: What p53 Does When It Is Not Being a Transcription Factor. Int. J. Mol. Sci. 2019, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.S.; Hu, W.; Belyi, V.; Rabadan, R.; Levine, A.J. Differential levels of transcription of p53-regulated genes by the arginine/proline polymorphism: p53 with arginine at codon 72 favors apoptosis. Faseb. J. 2010, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Proestling, K.; Hebar, A.; Pruckner, N.; Marton, E.; Vinatzer, U.; Schreiber, M. The Pro Allele of the p53 Codon 72 Polymorphism Is Associated with Decreased Intratumoral Expression of BAX and p21, and Increased Breast Cancer Risk. PLoS ONE 2012, 7, e47325. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Deiry, W.S. p73 or p53 directly regulates human p53 transcription to maintain cell cycle checkpoints. Cancer Res. 2006, 66, 6982–6989. [Google Scholar] [CrossRef]
- Brekman, A.; Singh, K.E.; Polotskaia, A.; Kundu, N.; Bargonetti, J. A p53-independent role of Mdm2 in estrogen-mediated activation of breast cancer cell proliferation. Breast Cancer Res. 2011, 13, R3. [Google Scholar] [CrossRef]
- Swetzig, W.M.; Wang, J.; Das, G.M. Estrogen receptor alpha (ERalpha/ESR1) mediates the p53-independent overexpression of MDM4/MDMX and MDM2 in human breast cancer. Oncotarget 2016, 7, 16049–16069. [Google Scholar] [CrossRef]
- Shen, Z.J.; Nakamoto, T.; Tsuji, K.; Nifuji, A.; Miyazono, K.; Komori, T.; Hirai, H.; Noda, M. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J. Biol. Chem. 2002, 277, 29840–29846. [Google Scholar] [CrossRef]
- Jen, J.; Lin, L.L.; Chen, H.T.; Liao, S.Y.; Lo, F.Y.; Tang, Y.A.; Su, W.C.; Salgia, R.; Hsu, C.L.; Huang, H.C.; et al. Oncoprotein ZNF322A transcriptionally deregulates alpha-adducin, cyclin D1 and p53 to promote tumor growth and metastasis in lung cancer. Oncogene 2016, 35, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.M.; Lin, Y.; Hatoum, D. Good Guy or Bad Guy? The Duality of Wild-Type p53 in Hormone-Dependent Breast Cancer Origin, Treatment, and Recurrence. Cancers 2018, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.R.; Bartley, J.C. Human mammary epithelial cells in culture: Differentiation and transformation. Cancer Treat. Res. 1988, 40, 1–24. [Google Scholar] [PubMed]
- Pacher, M.; Seewald, M.J.; Mikula, M.; Oehler, S.; Mogg, M.; Vinatzer, U.; Eger, A.; Schweifer, N.; Varecka, R.; Sommergruber, W.; et al. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis 2007, 28, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Friesenhengst, A.; Pribitzer-Winner, T.; Schreiber, M. Association of the G473A Polymorphism and Expression of Lysyl Oxidase with Breast Cancer Risk and Survival in European Women: A Hospital-Based Case-Control Study. PLoS ONE 2014, 9, e105579. [Google Scholar] [CrossRef]
- Taubenschuß, E.; Marton, E.; Mogg, M.; Frech, B.; Ehart, L.; Muin, D.; Schreiber, M. The L10P Polymorphism and Serum Levels of Transforming Growth Factor β1 in Human Breast Cancer. Int. J. Mol. Sci. 2013, 14, 15376–15385. [Google Scholar] [CrossRef]
- Friesenhengst, A.; Pribitzer-Winner, T.; Miedl, H.; Pröstling, K.; Schreiber, M. Elevated Aromatase (CYP19A1) Expression Is Associated with a Poor Survival of Patients with Estrogen Receptor Positive Breast Cancer. Horm. Cancer 2018, 9, 128–138. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Bender, R.; Lange, S. Adjusting for multiple testing--when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Harrington, D.P.; Fleming, T.R. A Class of Rank Test Procedures for Censored Survival-Data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Vilborg, A.; Wilhelm, M.T.; Wiman, K.G. Regulation of tumor suppressor p53 at the RNA level. J. Mol. Med. 2010, 88, 645–652. [Google Scholar] [CrossRef]
- Borresen-Dale, A.L. TP53 and breast cancer. Hum. Mutat. 2003, 21, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kaserer, K.; Schmaus, J.; Bethge, U.; Migschitz, B.; Fasching, S.; Walch, A.; Herbst, F.; Teleky, B.; Wrba, F. Staining patterns of p53 immunohistochemistry and their biological significance in colorectal cancer. J. Pathol. 2000, 190, 450–456. [Google Scholar] [CrossRef]
- Midgley, C.A.; Lane, D.P. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene 1997, 15, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Norberg, T.; Lennerstrand, J.; Inganas, M.; Bergh, J. Comparison between p53 protein measurements using the luminometric immunoassay and immunohistochemistry with detection of p53 gene mutations using cDNA sequencing in human breast tumors. Int. J. Cancer 1998, 79, 376–383. [Google Scholar] [CrossRef]
- Vinatzer, U.; Dampier, B.; Streubel, B.; Pacher, M.; Seewald, M.J.; Stratowa, C.; Kaserer, K.; Schreiber, M. Expression of HER2 and the coamplified genes GRB7 and MLN64 in human breast cancer: Quantitative real-time reverse transcription-PCR as a diagnostic alternative to immunohistochemistry and fluorescence in situ hybridization. Clin. Cancer Res. 2005, 11, 8348–8357. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M. More is not always better: Clinical genetics of familial breast cancer in the era of massively enhanced sequencing capacities. Transl. Cancer Res. 2016, 2016, S387–S391. [Google Scholar] [CrossRef]





| Genotypes/Alleles | Unadjusted | Adjusted for SNP309 and Age | ||||
|---|---|---|---|---|---|---|
| OR | 95% c.i. | p-Value | OR | 95% c.i. | p-Value | |
| Del/Del vs. Ins/Ins | 0.78 | 0.48–1.24 | 0.289 | 0.68 | 0.36–1.27 | 0.198 |
| Del/Del vs. Ins/Del | 0.88 | 0.56–1.37 | 0.564 | 0.91 | 0.51–1.61 | 0.732 |
| Del/Del vs. Ins/Del + Ins/Ins | 0.83 | 0.55–1.27 | 0.401 | 0.78 | 0.46–1.34 | 0.371 |
| Ins/Del vs. Ins/Ins | 0.88 | 0.62–1.25 | 0.488 | 0.82 | 0.52–1.29 | 0.353 |
| Ins/Del + Del/Del vs. Ins/Ins | 0.86 | 0.61–1.19 | 0.356 | 0.80 | 0.51–1.23 | 0.304 |
| Del vs. Ins | 0.88 | 0.70–1.11 | 0.276 | 0.82 | 0.60–1.12 | 0.215 |
| Genotypes/Alleles | in SNP309TT Subjects | in SNP309TG Subjects | ||||
|---|---|---|---|---|---|---|
| OR | 95% c.i. | p-Value | OR | 95% c.i. | p-Value | |
| Del/Del vs. Ins/Ins | 0.56 | 0.26–1.23 | 0.145 | 2.11 1 | 0.21–21.0 | 0.501 |
| Del/Del vs. Ins/Del | 0.79 | 0.47–1.33 | 0.376 | 2.04 1 | 0.21–20.0 | 0.524 |
| Del/Del vs. Ins/Del + Ins/Ins | 0.73 | 0.44–1.20 | 0.221 | 2.06 1 | 0.21–20.0 | 0.513 |
| Ins/Del vs. Ins/Ins | 0.72 | 0.33–1.54 | 0.388 | 1.04 | 0.64–1.70 | 0.863 |
| Ins/Del + Del/Del vs. Ins/Ins | 0.65 | 0.31–1.33 | 0.227 | 1.06 | 0.65–1.72 | 0.821 |
| Del vs. Ins | 0.76 | 0.53–1.10 | 0.137 | 1.09 | 0.69–1.73 | 0.718 |
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% c.i. | p-Value | HR | 95% c.i. | p-Value | |
| rs150550023 | 0.96 | 0.68–1.36 | 0.821 | 0.92 | 0.64–1.33 | 0.668 |
| SNP309 | 1.05 | 0.76–1.46 | 0.768 | 1.03 | 0.71–1.49 | 0.874 |
| p53 status | 1.36 | 0.84–2.19 | 0.204 | 1.08 | 0.64–1.84 | 0.768 |
| ER status | 1.32 | 0.84–2.06 | 0.228 | 1.17 | 0.71–1.95 | 0.532 |
| PR status | 1.45 | 0.91–2.30 | 0.117 | 1.45 | 0.86–2.45 | 0.163 |
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% c.i. | p-Value | HR | 95% c.i. | p-Value | |
| rs150550023 | 1.10 | 0.75–1.62 | 0.624 | 0.96 | 0.64–1.45 | 0.859 |
| SNP309 | 0.80 | 0.54–1.18 | 0.266 | 0.77 | 0.50–1.18 | 0.231 |
| p53 status | 1.55 | 0.93–2.60 | 0.094 | 1.12 | 0.64–1.98 | 0.689 |
| ER status | 1.41 | 0.87–2.30 | 0.167 | 1.17 | 0.68–2.02 | 0.565 |
| PR status | 1.65 | 0.98–2.77 | 0.057 | 1.65 | 0.91–3.00 | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedl, H.; Dietrich, B.; Kaserer, K.; Schreiber, M. The 40bp Indel Polymorphism rs150550023 in the MDM2 Promoter is Associated with Intriguing Shifts in Gene Expression in the p53-MDM2 Regulatory Hub. Cancers 2020, 12, 3363. https://doi.org/10.3390/cancers12113363
Miedl H, Dietrich B, Kaserer K, Schreiber M. The 40bp Indel Polymorphism rs150550023 in the MDM2 Promoter is Associated with Intriguing Shifts in Gene Expression in the p53-MDM2 Regulatory Hub. Cancers. 2020; 12(11):3363. https://doi.org/10.3390/cancers12113363
Chicago/Turabian StyleMiedl, Heidi, Bianca Dietrich, Klaus Kaserer, and Martin Schreiber. 2020. "The 40bp Indel Polymorphism rs150550023 in the MDM2 Promoter is Associated with Intriguing Shifts in Gene Expression in the p53-MDM2 Regulatory Hub" Cancers 12, no. 11: 3363. https://doi.org/10.3390/cancers12113363
APA StyleMiedl, H., Dietrich, B., Kaserer, K., & Schreiber, M. (2020). The 40bp Indel Polymorphism rs150550023 in the MDM2 Promoter is Associated with Intriguing Shifts in Gene Expression in the p53-MDM2 Regulatory Hub. Cancers, 12(11), 3363. https://doi.org/10.3390/cancers12113363





