Circulating Tumour DNA in Advanced Melanoma Patients Ceasing PD1 Inhibition in the Absence of Disease Progression
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
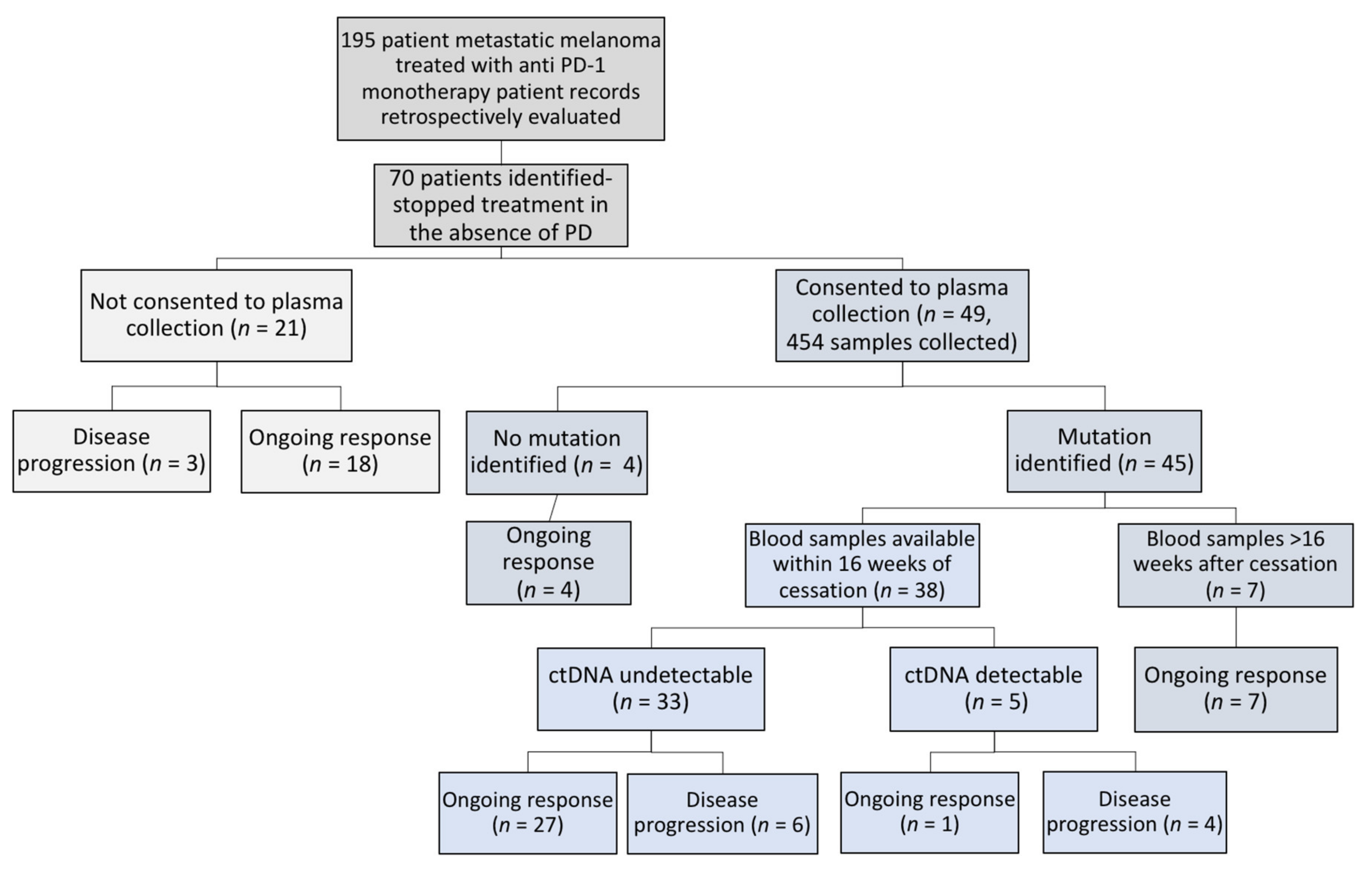
2.1. Patient Description
2.2. Progressive Disease in the Whole Cohort
2.3. ctDNA in Patients Ceasing Anti-PD1 Therapy
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Collection and ctDNA Analysis
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.; Hwu, W.; Kefford, R.; Wolchok, J.; Hersey, P.; Joseph, R.; Weber, J.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.-S.; McNeil, C.-M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.-V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.-S.; McNeil, C.; Lotem, M.; et al. KEYNOTE-006 investigators Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.-J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Fares, C.M.; van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Smith, A.; Shaghayegh, G.; Menzies, A.M.; Roberts-Thomson, R. Duration of immunotherapy-should we continue ad infinitum? Internal Medicine Journal 2020, 50, 865–868. [Google Scholar] [CrossRef]
- Lorigan, P.; Eggermont, A. Anti-PD1 treatment of advanced melanoma: Development of criteria for a safe stop. Ann. Oncol. 2019, 30, 1038–1040. [Google Scholar] [CrossRef]
- Hench, I.B.; Hench, J.; Tolnay, M. Liquid Biopsy in Clinical Management of Breast, Lung, and Colorectal Cancer. Front. Med. 2018, 5, 9. [Google Scholar] [CrossRef]
- Boonstra, P.A.; Wind, T.T.; van Kruchten, M.; Schuuring, E.; Hospers, G.A.P.; van der Wekken, A.J.; de Groot, D.-J.; Schröder, C.P.; Fehrmann, R.S.N.; Reyners, A.K.L. Clinical utility of circulating tumor DNA as a response and follow-up marker in cancer therapy. Cancer Metastasis Rev. 2020, 39, 999–1013. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Gray, E.S.; Rizos, H.; Reid, A.L.; Boyd, S.C.; Pereira, M.R.; Lo, J.; Tembe, V.; Freeman, J.; Lee, J.H.; Scolyer, R.A.; et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 2015, 6, 42008–42018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Long, G.V.; Boyd, S.; Lo, S.; Menzies, A.M.; Tembe, V.; Guminski, A.; Jakrot, V.; Scolyer, R.A.; Mann, G.J.; et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann. Oncol. 2017, 28, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Herbreteau, G.; Vallee, A.; Knol, A.-C.; Théoleyre, S.; Quéreux, G.; Varey, E.; Khammari, A.; Dréno, B.; Denis, M.G. Quantitative monitoring of circulating tumor DNA predicts response of cutaneous metastatic melanoma to anti-PD1 immunotherapy. Oncotarget 2018, 9, 25265–25276. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Velculescu, V.E.; Pritchard, T.S.; Sausen, M.; Pardoll, D.M.; Topalian, S.L.; Diaz, L.A. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J. Immunother. Cancer 2014, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Riva, F.; Servois, V.; Livartowski, A.; Daniel, C.; Rampanou, A.; Lantz, O.; Romano, E.; Milder, M.; Buecher, B.; et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: A proof-of-concept study. Ann. Oncol. 2017, 28, 1996–2001. [Google Scholar] [CrossRef]
- McEvoy, A.C.; Warburton, L.; Al-Ogaili, Z.; Celliers, L.; Calapre, L.; Pereira, M.R.; Khattak, M.A.; Meniawy, T.M.; Millward, M.; Ziman, M.; et al. Correlation between circulating tumour DNA and metabolic tumour burden in metastatic melanoma patients. BMC Cancer 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Jansen, Y.; Rozeman, E.; Mason, R.; Goldinger, S.; Foppen, M.G.; Hoejberg, L.; Schmidt, H.; Van Thienen, J.; Haanen, J.; Tiainen, L.; et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann. Oncol. 2019, 30, 1154–1161. [Google Scholar] [CrossRef]
- Warner, A.B.; Shoushtari, A.N.; Houghton, S.; Panageas, K.; Chapman, P.B.; Wolchok, J.; Postow, M. Characterization of complete responders to combination nivolumab (nivo) and ipilimumab (ipi) in patients (pts) with advanced, unresectable melanoma. J. Clin. Oncol. 2018, 36, 9552. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Hamid, O.; Daud, A.; Wolchok, J.D.; Joshua, A.M.; Hwu, W.-J.; Weber, J.S.; Gangadhar, T.C.; Joseph, R.W.; et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J. Clin. Oncol. 2018, 36, 1668–1674. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Ladwa, R.; Atkinson, V. The cessation of anti-PD-1 antibodies of complete responders in metastatic melanoma. Melanoma Res. 2017, 27, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Schachter, J.; Ribas, A.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.N.A.; Carlino, M.; McNeil, C.M.; Lotem, M.; et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J. Clin. Oncol. 2018, 36, 9503. [Google Scholar] [CrossRef]
- Fiala, C.; Diamandis, E.P. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 2018, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, C.C.S.P.R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Lee, R.J.; Gremel, G.; Marshall, A.; A Myers, K.; Fisher, N.; Corrie, P.; Dunn, J.; Dhomen, N.; Middleton, M.R.; Marais, R.; et al. Use of circulating tumor DNA to predict survival in patients with resected high-risk stage II/III melanoma. J. Clin. Oncol. 2017, 35, 9583. [Google Scholar] [CrossRef]
- Lee, J.; Saw, R.; Thompson, J.; Lo, S.; Spillane, A.; Shannon, K.; Stretch, J.; Howle, J.; Menzies, A.; Carlino, M.; et al. Pre-operative ctDNA predicts survival in high-risk stage III cutaneous melanoma patients. Ann. Oncol. 2019, 30, 815–822. [Google Scholar] [CrossRef]
- Calapre, L.; Giardina, T.; Robinson, C.; Reid, A.L.; Al-Ogaili, Z.; Pereira, M.R.; McEvoy, A.C.; Warburton, L.; Hayward, N.K.; Khattak, M.A.; et al. Locus-specific concordance of genomic alterations between tissue and plasma circulating tumor DNA in metastatic melanoma. Mol. Oncol. 2019, 13, 171–184. [Google Scholar] [CrossRef]
- Gray, E.; Witkowski, T.; Pereira, M.; Calapre, L.; Herron, K.; Irwin, D.; Chapman, B.; Khattak, M.A.; Raleigh, J.; Hatzimihalis, A.; et al. Genomic Analysis of Circulating Tumor DNA Using a Melanoma-Specific UltraSEEK Oncogene Panel. J. Mol. Diagn. 2019, 21, 418–426. [Google Scholar] [CrossRef]
- Marsavela, G.; Lee, J.; Calapre, L.; Wong, S.Q.; Pereira, M.R.; McEvoy, A.C.; Reid, A.L.; Robinson, C.; Warburton, L.; Abed, A.; et al. Circulating Tumor DNA Predicts Outcome from First-, but not Second-line Treatment and Identifies Melanoma Patients Who May Benefit from Combination Immunotherapy. Clin. Cancer Res. 2020, 26, 5926–5933. [Google Scholar] [CrossRef] [PubMed]


| Demographic Variables | Number of Patients (%) |
|---|---|
| Age (years) | |
| Median | 71 |
| Range | 29–91 |
| Gender | |
| Male | 49 (70) |
| Female | 21 (30) |
| ECOG | |
| 0 | 53 (75) |
| 1 | 11 (16) |
| ≥2 | 6 (9) |
| Stage at commencement of anti-PD1 | |
| M1a | 20 (29) |
| M1b | 12 (17) |
| M1c | 31 (44) |
| M1d | 7 (10) |
| Number of metastatic sites | |
| <3 | 54 (77) |
| >3 | 16 (23) |
| Lines of prior therapy | |
| 0 | 36 (51) |
| ≥1 | 34 (49) |
| Prior systemic therapy | |
| Chemotherapy (DTIC/Fotemustine) | 5 (7) |
| BRAFi or BRAF/MEKi | 13 (19) |
| Ipilimumab | 15 (21) |
| BRAF V600 mutation | |
| Yes | 14 (20) |
| No | 56 (80) |
| LDH prior to anti-PD1 therapy | |
| <ULN | 49 (70) |
| >1×ULN | 13 (19) |
| >2×ULN | 3 (4) |
| Unknown | 5 (7) |
| Brain metastases | |
| Absent | 63 (90) |
| Present | 7 (10) |
| Variable | N (%) |
|---|---|
| Treatment duration (months) | |
| Median | 11.8 |
| Range | 3–33 |
| Response at cessation | |
| Complete Response | 61 (87) |
| Partial Response | 6 (8.6) |
| Stable Disease | 3 (4.3) |
| Time to BOR (months) | |
| Median | 5 |
| Range | 1–29 |
| Time to CR (n= 61) (months) | |
| Median | 5 |
| Range | 1–29 |
| PD post cessation | |
| No | 57 (81) |
| Yes | 13 (19) |
| Follow up post cessation (months) | |
| Median | 34.2 |
| Range | 2–70.8 |
| Treatment free survival (months) | |
| Median | Not reached |
| Range | 2–70.2 |
| Time to PD after cessation (n=13) (months) | |
| Median | 11.1 |
| Range | 2.2–27.8 |
| PFS (months) | |
| Median | Not reached |
| Range | 6–81.8 |
| OS (months) | |
| Median | Not reached |
| Range | 6.3–81.8 |
| Ongoing Response (n) | Disease Progression (n) | Total (n) | |
|---|---|---|---|
| ctDNA detectable | 1 | 4 | 5 |
| ctDNA undetectable | 27 | 6 | 33 |
| Total | 28 | 10 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warburton, L.; Calapre, L.; Pereira, M.R.; Reid, A.; Robinson, C.; Amanuel, B.; Ziman, M.; Millward, M.; Gray, E. Circulating Tumour DNA in Advanced Melanoma Patients Ceasing PD1 Inhibition in the Absence of Disease Progression. Cancers 2020, 12, 3486. https://doi.org/10.3390/cancers12113486
Warburton L, Calapre L, Pereira MR, Reid A, Robinson C, Amanuel B, Ziman M, Millward M, Gray E. Circulating Tumour DNA in Advanced Melanoma Patients Ceasing PD1 Inhibition in the Absence of Disease Progression. Cancers. 2020; 12(11):3486. https://doi.org/10.3390/cancers12113486
Chicago/Turabian StyleWarburton, Lydia, Leslie Calapre, Michelle R. Pereira, Anna Reid, Cleo Robinson, Benhur Amanuel, Mel Ziman, Michael Millward, and Elin Gray. 2020. "Circulating Tumour DNA in Advanced Melanoma Patients Ceasing PD1 Inhibition in the Absence of Disease Progression" Cancers 12, no. 11: 3486. https://doi.org/10.3390/cancers12113486
APA StyleWarburton, L., Calapre, L., Pereira, M. R., Reid, A., Robinson, C., Amanuel, B., Ziman, M., Millward, M., & Gray, E. (2020). Circulating Tumour DNA in Advanced Melanoma Patients Ceasing PD1 Inhibition in the Absence of Disease Progression. Cancers, 12(11), 3486. https://doi.org/10.3390/cancers12113486






