Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Molecular Characterization of Bone Afferents
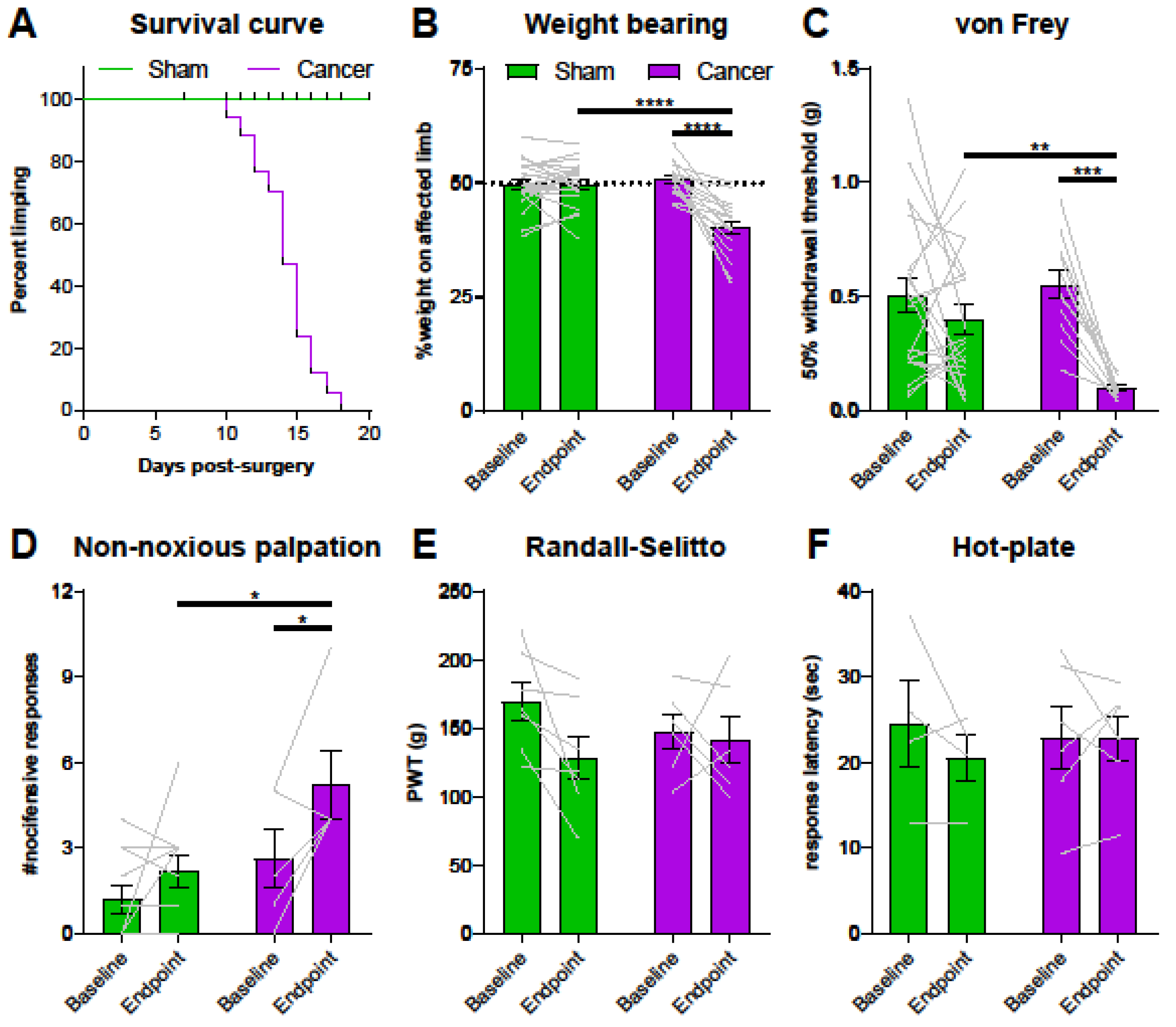
2.2. GCaMP3-Expressing Mice Show a Moderate Pain Phenotype in a Model of CIBP
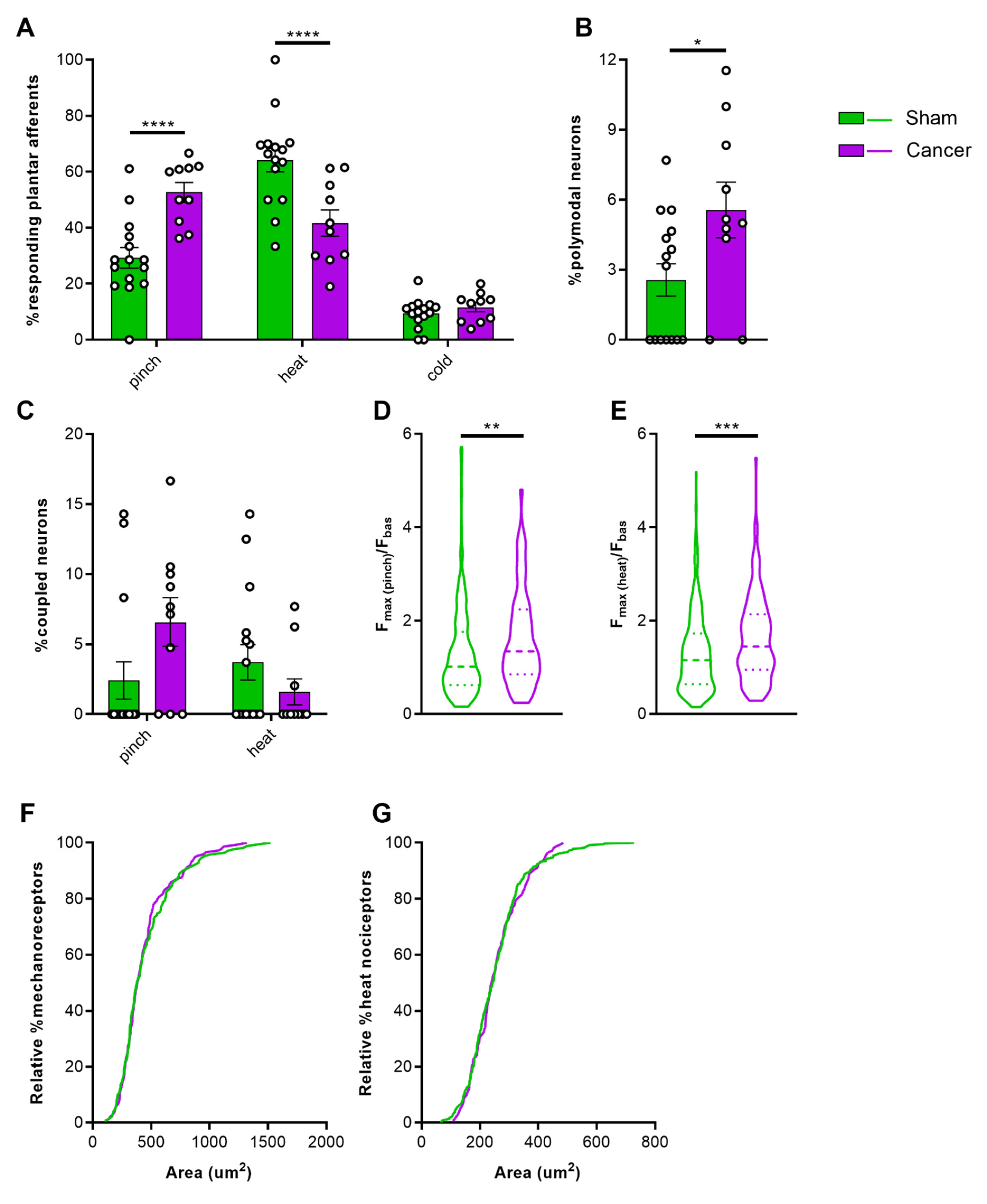
2.3. Properties of Cutaneous Afferents in CIBP Animals
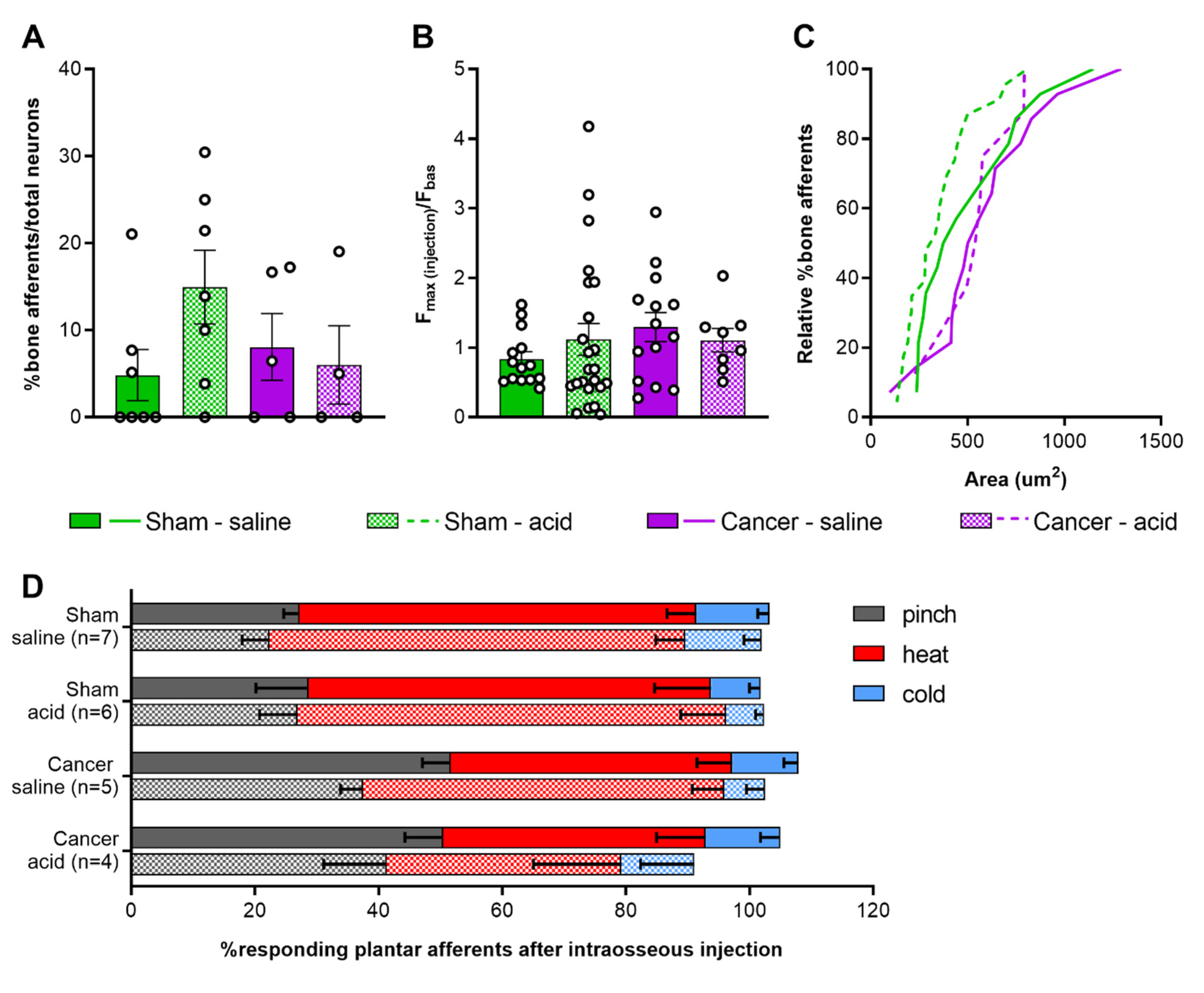
2.4. Activation of Femoral Bone Marrow Afferents
2.5. Response of Plantar Cutaneous Afferents Is Unchanged after Intraosseous Stimulation
3. Discussion
3.1. Femoral Bone Marrow Afferents Are Largely Nociceptive and Not Proprioceptive
3.2. Cancer Bearing Mice with a Moderate Reduction of Limb Use Develop Secondary Mechanical Hypersensitivity
3.3. Increased Sensitivity of Cutaneous Afferents Innervating the Paw in Animals with CIBP
3.4. Response Properties of Bone Afferents Are Unaffected in Mice with Moderate Bone Cancer Pain
4. Materials and Methods
4.1. Cell Culture
4.2. Animals
4.3. Surgery
4.4. Behavioural Tests
4.5. Immunohistochemistry
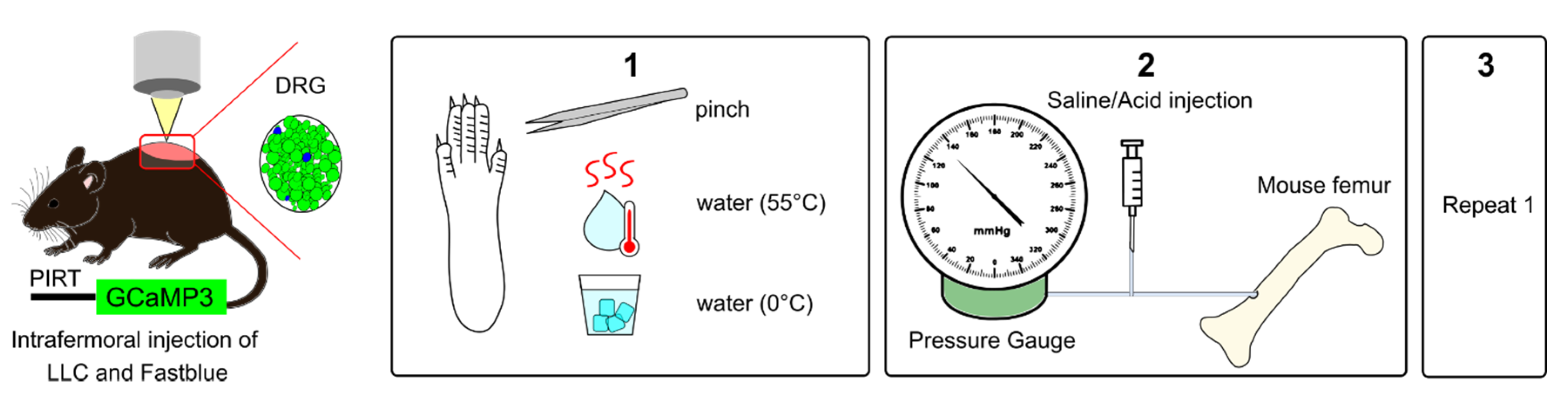
4.6. In Vivo Calcium Imaging
4.6.1. Peripheral Stimulation
4.6.2. Image Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Den Beuken-Van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243–6250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grond, S.; Zech, D.; Diefenbach, C.; Radbruch, L.; Lehmann, K.A. Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain 1996, 64, 107–114. [Google Scholar] [CrossRef]
- Scarpi, E.; Calistri, D.; Klepstad, P.; Kaasa, S.; Skorpen, F.; Habberstad, R.; Nanni, O.; Amadori, D.; Maltoni, M. Clinical and Genetic Factors Related to Cancer-Induced Bone Pain and Bone Pain Relief. Oncologist 2014, 19, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Deandrea, S.; Corli, O.; Consonni, D.; Villani, W.; Greco, M.T.; Apolone, G. Prevalence of breakthrough cancer pain: A systematic review and a pooled analysis of published literature. J. Pain Symptom Manag. 2014, 47, 57–76. [Google Scholar] [CrossRef]
- Mercadante, S.; Villari, P.; Ferrera, P.; Casuccio, A. Optimization of opioid therapy for preventing incident pain associated with bone metastases. J. Pain Symptom Manag. 2004, 28, 505–510. [Google Scholar] [CrossRef]
- Carlson, C. Effectiveness of the World Health Organization Cancer Pain Relief Guidelines: An integrative review. J. Pain Res. 2016, 9, 515–534. [Google Scholar] [CrossRef] [Green Version]
- Falk, S.; Dickenson, A.H. Pain and nociception: Mechanisms of cancer-induced bone pain. J. Clin. Oncol. 2014, 32, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Mantyh, P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain 2013, 154, S54–S62. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Corral, G.; Jimenez-Andrade, J.M.; Bloom, A.P.; Taylor, R.N.; Mantyh, W.G.; Kaczmarska, M.J.; Ghilardi, J.R.; Mantyh, P.W. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011, 178, 196–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Xu, H.; Ferng, A.S.; Dussor, G.; Vanderah, T.W.; Mantyh, P.W. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: Therapeutic opportunity for treating skeletal pain. Bone 2010, 46, 306–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, A.P.; Jimenez-Andrade, J.M.; Taylor, R.N.; Castañeda-Corral, G.; Kaczmarska, M.J.; Freeman, K.T.; Coughlin, K.A.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain 2011, 12, 698–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantyh, W.G.; Jimenez-Andrade, J.M.; Stake, J.I.; Bloom, A.P.; Kaczmarska, M.J.; Taylor, R.N.; Freeman, K.T.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010, 171, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Andrade, J.M.; Bloom, A.P.; Stake, J.I.; Mantyh, W.G.; Taylor, R.N.; Freeman, K.T.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Pathological Sprouting of Adult Nociceptors in Chronic Prostate Cancer-Induced Bone Pain. J. Neurosci. 2010, 30, 14649–14656. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Dai, J.; Zhang, H.; Campbell, B.; Keller, E.T. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015, 75, 2151–2158. [Google Scholar] [CrossRef] [Green Version]
- Ivanusic, J.J. Molecular Mechanisms That Contribute to Bone Marrow Pain. Front. Neurol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mantyh, P.W. The neurobiology of skeletal pain. Eur. J. Neurosci. 2014, 39, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Tanaka, S.; Sekiguchi, T.; Sugiyama, D.; Kawamata, M. Spinal nociceptive transmission by mechanical stimulation of bone marrow. Mol. Pain 2016, 12, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nencini, S.; Ivanusic, J. Mechanically sensitive Aδ nociceptors that innervate bone marrow respond to changes in intra-osseous pressure. J. Physiol. 2017, 595, 4399–4415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nencini, S.; Ringuet, M.; Kim, D.H.; Chen, Y.J.; Greenhill, C.; Ivanusic, J.J. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol. Pain 2017, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nencini, S.; Thai, J.; Ivanusic, J.J. Sequestration of artemin reduces inflammation-induced activation and sensitization of bone marrow nociceptors in a rodent model of carrageenan-induced inflammatory bone pain. Eur. J. Pain 2018, 1–13. [Google Scholar] [CrossRef]
- Nencini, S.; Ringuet, M.; Kim, D.-H.; Greenhill, C.; Ivanusic, J.J. GDNF, Neurturin, and Artemin Activate and Sensitize Bone Afferent Neurons and Contribute to Inflammatory Bone Pain. J. Neurosci. 2018, 38, 4899–4911. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Nagata, Y.; Okui, T.; White, F. Contribution of acidic extracellular microenvironment of cancer-colonized bone to bone pain. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2677–2684. [Google Scholar] [CrossRef] [Green Version]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, T.; Hata, K.; Nakanishi, M.; Nagae, M.; Nagayama, T.; Wakabayashi, H.; Nishisho, T.; Sakurai, T.; Hiraga, T. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone 2011, 48, 100–105. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Wakisaka, S.; Hiraga, T.; Hata, K.; Nishimura, R.; Tominaga, M.; Yoneda, T. Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J. Bone Miner. Metab. 2018, 36, 274–285. [Google Scholar] [CrossRef]
- Klepstad, P.; Kurita, G.P.; Mercadante, S.; Sjøgren, P. Evidence of peripheral nerve blocks for cancer-related pain: A systematic review. Minerva Anestesiol. 2015, 81, 789–793. [Google Scholar]
- Remeniuk, B.; Sukhtankar, D.; Okun, A.; Navratilova, E.; Xie, J.Y.; King, T.; Porreca, F. Behavioral and neurochemical analysis of ongoing bone cancer pain in rats. Pain 2015, 156, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Forsthuber, L.; Schoffnegger, D.; Sandkühler, J. Modification of classical neurochemical markers in identified primary afferent neurons with Aβ-, Aδ-, and C-fibers after chronic constriction injury in mice. J. Comp. Neurol. 2007, 502, 325–336. [Google Scholar] [CrossRef]
- Bangash, M.A.; Alles, S.R.A.; Santana-Varela, S.; Millet, Q.; Sikandar, S.; de Clauser, L.; ter Heegde, F.; Habib, A.M.; Pereira, V.; Sexton, J.E.; et al. Distinct transcriptional responses of mouse sensory neurons in models of human chronic pain conditions. Wellcome Open Res. 2018, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Luiz, A.P.; Sikandar, S.; Magnúsdóttir, R.; Dong, X.; Wood, J.N. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Sci. Adv. 2016, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Anderson, M.; Park, K.; Zheng, Q.; Agarwal, A.; Gong, C.; Saijilafu; Young, L.; He, S.; LaVinka, P.C.; et al. Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron 2016, 91, 1085–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigaud, M.; Gemes, G.; Barabas, M.-E.; Chernoff, D.I.; Abram, S.E.; Stucky, C.L.; Hogan, Q.H. Species and strain differences in rodent sciatic nerve anatomy: Implications for studies of neuropathic pain. Pain 2008, 136, 188–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanusic, J.J. Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J. Comp. Neurol. 2009, 517, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Minett, M.S.; Falk, S.; Santana-Varela, S.; Bogdanov, Y.D.; Nassar, M.A.; Heegaard, A.M.; Wood, J.N. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 2014, 6, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Mach, D.B.; Rogers, S.D.; Sabino, M.C.; Luger, N.M.; Schwei, M.J.; Pomonis, J.D.; Keyser, C.P.; Clohisy, D.R.; Adams, D.J.; O’leary, P.; et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience 2002, 113, 155–166. [Google Scholar] [CrossRef]
- Havelin, J.; Imbert, I.; Sukhtankar, D.; Remeniuk, B.; Pelletier, I.; Gentry, J.; Okun, A.; Tiutan, T.; Porreca, F.; King, T.E. Mediation of Movement-Induced Breakthrough Cancer Pain by IB4-Binding Nociceptors in Rats. J. Neurosci. 2017, 37, 5111–5122. [Google Scholar] [CrossRef] [Green Version]
- Thai, J.; Kyloh, M.; Travis, L.; Spencer, N.J.; Ivanusic, J.J. Identifying spinal afferent (sensory) nerve endings that innervate the marrow cavity and periosteum using anterograde tracing. J. Comp. Neurol. 2020, 528, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.A.C.; Luger, N.M.; Mach, D.B.; Rogers, S.D.; Schwei, M.J.; Mantyh, P.W. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int. J. Cancer 2003, 104, 550–558. [Google Scholar] [CrossRef]
- Luger, N.M.; Sabino, M.A.C.; Schwei, M.J.; Mach, D.B.; Pomonis, J.D.; Keyser, C.P.; Rathbun, M.; Clohisy, D.R.; Honore, P.; Yaksh, T.L.; et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs. inflammatory pain. Pain 2002, 99, 397–406. [Google Scholar] [CrossRef]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef]
- Guedon, J.M.G.; Longo, G.; Majuta, L.A.; Thomspon, M.L.; Fealk, M.N.; Mantyh, P.W. Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain 2016, 157, 1239–1247. [Google Scholar] [CrossRef]
- Scott, A.C.; McConnell, S.; Laird, B.; Colvin, L.; Fallon, M. Quantitative Sensory Testing to assess the sensory characteristics of cancer-induced bone pain after radiotherapy and potential clinical biomarkers of response. Eur. J. Pain 2011. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Ma, Z.; Wang, J.; Zhou, X.; Jin, Y.; Xia, X.; Gao, Q.; Mei, F. The role of N-methyl-d-aspartate receptor subunit NR2B in spinal cord in cancer pain. Eur. J. Pain 2009, 14, 496–502. [Google Scholar] [CrossRef]
- Abdelaziz, D.M.; Stone, L.S.; Komarova, S.V. Localized experimental bone metastasis drives osteolysis and sensory hypersensitivity at distant non-tumor-bearing sites. Breast Cancer Res. Treat. 2015, 153, 9–20. [Google Scholar] [CrossRef]
- Miller, R.E.; Kim, Y.S.; Tran, P.B.; Ishihara, S.; Dong, X.; Miller, R.J.; Malfait, A.M. Visualization of Peripheral Neuron Sensitization in a Surgical Mouse Model of Osteoarthritis by In Vivo Calcium Imaging. Arthritis Rheumatol. 2018, 70, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.F.; Ungard, R.; Seidlitz, E.; Zacal, N.; Huizinga, J.; Henry, J.L.; Singh, G. Differences in electrophysiological properties of functionally identified nociceptive sensory neurons in an animal model of cancer-induced bone pain. Mol. Pain 2016, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, M.W.; Chisholm, K.I.; Denk, F.; Dickenson, A.H.; Bannister, K.; McMahon, S.B. The impact of bone cancer on the peripheral encoding of mechanical pressure stimuli. Pain 2020, 161, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Smith-Edwards, K.M.; DeBerry, J.J.; Saloman, J.L.; Davis, B.M.; Woodbury, C.J. Profound alteration in cutaneous primary afferent activity produced by inflammatory mediators. Elife 2016, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- da Silva Serra, I.; Husson, Z.; Bartlett, J.D.; Smith, E.S.J. Characterization of cutaneous and articular sensory neurons. Mol. Pain 2016, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Middlemiss, T.; Laird, B.J.A.; Fallon, M.T. Mechanisms of Cancer-induced Bone Pain. Clin. Oncol. 2011, 23, 387–392. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Stucky, C.L.; Harding-Rose, C.; Eikmeier, L.; Beitz, A.J.; Coicou, L.G.; Hanson, A.E.; Simone, D.A.; Seybold, V.S. Chemical Interactions between Fibrosarcoma Cancer Cells and Sensory Neurons Contribute to Cancer Pain. J. Neurosci. 2007, 27, 10289–10298. [Google Scholar] [CrossRef] [Green Version]
- Ostrow, K.L.; Donaldson, K.J.; Caterina, M.J.; Belzberg, A.; Hoke, A. The Secretomes of Painful Versus Nonpainful Human Schwannomatosis Tumor Cells Differentially Influence Sensory Neuron Gene Expression and Sensitivity. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Janal, M.N.; Veeramachaneni, R.; Dolgalev, I.; Dubeykovskaya, Z.; Tu, N.H.; Kim, H.; Zhang, S.; Wu, A.K.; Hagiwara, M.; et al. Oncogenes overexpressed in metastatic oral cancers from patients with pain: Potential pain mediators released in exosomes. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Nyangoga, H.; Mercier, P.; Libouban, H.; Baslé, M.F.; Chappard, D. Three-dimensional characterization of the vascular bed in bone metastasis of the rat by microcomputed tomography (MicroCT). PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zilber, S.; Epstein, N.J.; Lee, S.W.; Larsen, M.; Ma, T.; Smith, R.L.; Biswal, S.; Goodman, S.B. Mouse femoral intramedullary injection model: Technique and microCT scan validation. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.M.; Wacnik, P.W.; Turner, M.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Wilcox, G.L.; Simone, D.A. Functional Interactions between Tumor and Peripheral Nerve: Changes in Excitability and Morphology of Primary Afferent Fibers in a Murine Model of Cancer Pain. J. Neurosci. 2001, 21, 9367–9376. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Andrade, J.M.; Ghilardi, J.R.; Castañeda-Corral, G.; Kuskowski, M.A.; Mantyh, P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152, 2564–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, C.M.; Ghilardi, J.R.; Keyser, C.P.; Kubota, K.; Lindsay, T.H.; Luger, N.M.; Mach, D.B.; Schwei, M.J.; Sevcik, M.A.; Mantyh, P.W. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp. Neurol. 2005, 193, 85–100. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [Green Version]
- Hippenmeyer, S.; Vrieseling, E.; Sigrist, M.; Portmann, T.; Laengle, C.; Ladle, D.R.; Arber, S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005, 3, 0878–0890. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Chu, Y.; Han, L.; Li, M.; Li, Z.; LaVinka, P.C.; Sun, S.; Tang, Z.; Park, K.; Caterina, M.J.; et al. Central Terminal Sensitization of TRPV1 by Descending Serotonergic Facilitation Modulates Chronic Pain. Neuron 2014, 81, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Nigro, M.J.; Hashikawa-Yamasaki, Y.; Rudy, B. Diversity and connectivity of layer 5 somatostatin-expressing interneurons in the mouse barrel cortex. J. Neurosci. 2018, 38, 1622–1633. [Google Scholar] [CrossRef]
- de Clauser, L.; Santana-Varela, S.; Wood, J.; Sikandar, S. Physiologic osteoclasts are not sufficient to induce skeletal pain in mice. Eur. J. Pain 2020, 1662. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Takesue, E.I.; Schaefer, W.; Jukniewicz, E. Modification of the Randall-Selitto analgesic apparatus. J. Pharm. Pharmacol. 1969, 21, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Thévenaz, P.; Ruttimann, U.E.; Unser, M. A Pyramid Approach to Sub-Pixel Registration Based on Intensity Mailing address. IEEE Trans. Image Process. 1998, 7, 27–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Proportion of Bone Marrow Afferents That Are | Number of Animals | Number of Retrogradely Labelled Bone Afferent Neurons | Percentage on Total |
|---|---|---|---|
| Nav1.8 cre+ | 5 | 89 | 73.80 ± 5.81% |
| and express TrkA | 2 | 38 | 94.05 ± 1.68% |
| Tmem233 cre+ | 3 | 23 | 22.88 ± 4.55% |
| and express TrkA | 3 | 22 | 93.75 ± 3.61% |
| Pvalb cre+ | 5 | 1 | 0.71 ± 0.64% |
| and express TrkA | 3 | 0 | 0.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Clauser, L.; Luiz, A.P.; Santana-Varela, S.; Wood, J.N.; Sikandar, S. Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging. Cancers 2020, 12, 3491. https://doi.org/10.3390/cancers12123491
de Clauser L, Luiz AP, Santana-Varela S, Wood JN, Sikandar S. Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging. Cancers. 2020; 12(12):3491. https://doi.org/10.3390/cancers12123491
Chicago/Turabian Stylede Clauser, Larissa, Ana P. Luiz, Sonia Santana-Varela, John N. Wood, and Shafaq Sikandar. 2020. "Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging" Cancers 12, no. 12: 3491. https://doi.org/10.3390/cancers12123491
APA Stylede Clauser, L., Luiz, A. P., Santana-Varela, S., Wood, J. N., & Sikandar, S. (2020). Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging. Cancers, 12(12), 3491. https://doi.org/10.3390/cancers12123491





