MR Imaging Features to Differentiate Retinoblastoma from Coats’ Disease and Persistent Fetal Vasculature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Populations and MR Images
2.2. Imaging Features’ Associations with Diagnosis
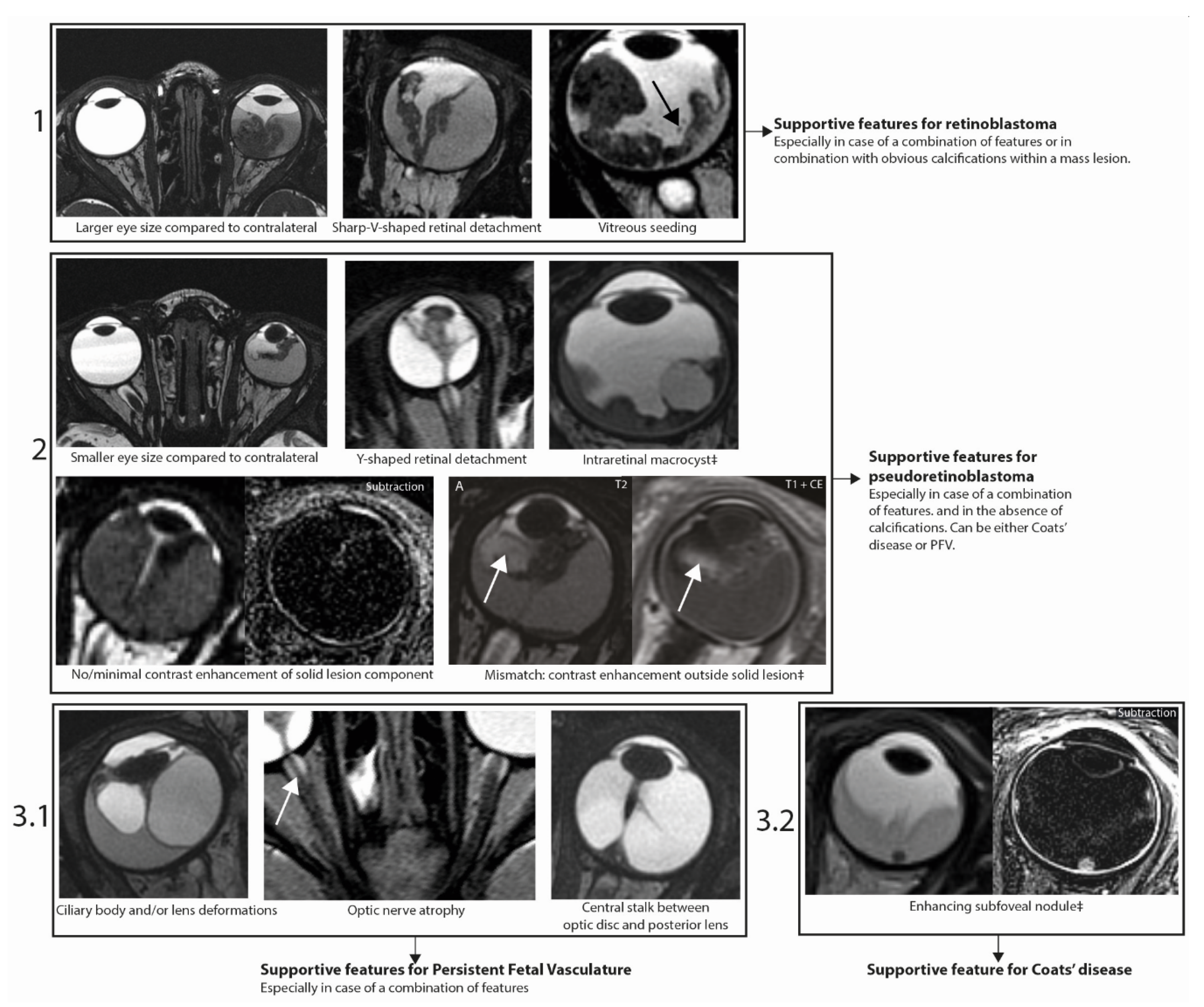
2.3. Imaging Features Predicting Retinoblastoma or Pseudoretinoblastoma
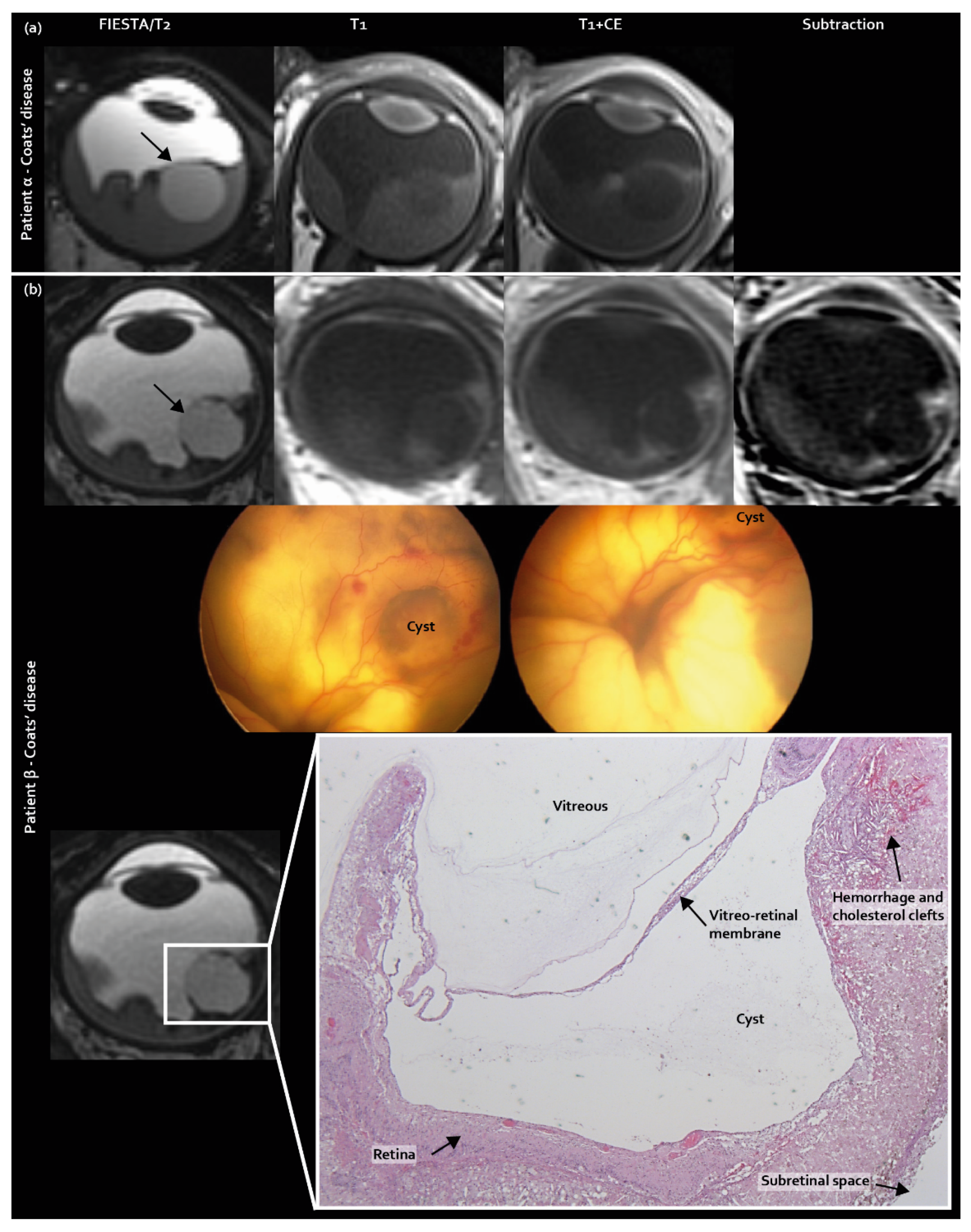
2.4. Three Newly Identified MR Imaging Features in Coats’ Disease and PFV/Retinal Dysplasia
2.5. Assessment Strategy for Differentiating Retinoblastoma, Coats’ Disease and PFV/Retinal Dysplasia
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. MR Imaging Assessment
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vahedi, A.; Lumbroso-Le Rouic, L.; Levy Gabriel, C.; Doz, F.; Aerts, I.; Brisse, H.; Berges, O.; Iba Zizen, M.T.; Desjardins, L. Differential diagnosis of retinoblastoma: A retrospective study of 486 cases. J. Fr. Ophtalmol. 2008, 31, 165–172. [Google Scholar] [CrossRef]
- Shields, C.L.; Schoenberg, E.; Kocher, K.; Shukla, S.Y.; Kaliki, S.; Shields, J.A. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases: Results based on age at presentation. Ophthalmology 2013, 120, 311–316. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, P.; van der Valk, P.; Moll, A.C.; Imhof, S.M.; Schouten-van Meeteren, A.Y.; Castelijns, J.A. Retinal dysplasia mimicking intraocular tumor: MR imaging findings with histopathologic correlation. Ajnr Am. J. Neuroradiol 2007, 28, 1731–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, n.G.; Mamalis, n.; Patel, B.C.K.; Ramasubramanian, A. Unilateral Retinal Dysplasia Mimicking Retinoblastoma. J. Pediatr 2015, 167, 1449. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.E.; Wan, M.J.; Heon, E.; Hazrati, L.N.; Gallie, B. Retinoblastoma versus advanced Coats’ disease: Is enucleation the answer? Ophthalmic Genet. 2017, 38, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.; Calvo, J.P.; Fernandez-Zubillaga, A.; Rios, J.C.; Gomez, J.A. A magnetic resonance imaging diagnostic dilemma: Diffuse infiltrating retinoblastoma versus Coats’ disease. J. Pediatr. Ophthalmol. Strabismus 2010, 47, e1–e3. [Google Scholar] [CrossRef]
- Shen, T.; Liu, R.; Lin, J.; Huang, H.; Li, X.; Yan, J. Pars Plana Vitrectomy and Evisceration Resulting in Death Due to Misdiagnosis of Retinoblastoma in Children: A Review of 3 Cases. Medicine 2015, 94, e1338. [Google Scholar] [CrossRef]
- Silva, R.A.; Dubovy, S.R.; Fernandes, C.E.; Hess, D.J.; Murray, T.G. Retinoblastoma with Coats’ response. Ophthalmic. Surg. Lasers Imaging 2011, 42, e139–e143. [Google Scholar] [CrossRef]
- Shields, C.L.; Uysal, Y.; Benevides, R.; Eagle, R.C., Jr.; Malloy, B.; Shields, J.A. Retinoblastoma in an eye with features of Coats’ disease. J.Pediatr. Ophthalmol. Strabismus 2006, 43, 313–315. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, J.-T.; Wang, N.-L.; Ma, J.-M. Retinoblastoma in a young adult mimicking Coats’ disease. Int. J. Ophthalmol. 2012, 5, 625–629. [Google Scholar]
- Nishina, S.; Katagiri, S.; Nakazawa, A.; Kiyotani, C.; Yokoi, T.; Azuma, N. Atypical intravitreal growth of retinoblastoma with a multi-branching configuration. Am. J. Ophthalmol Case Rep. 2017, 7, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.D.; Samuel, M.A.; Rao, N.A.; Murphree, A.L. Retinoblastoma presenting as Coats’ disease. Eye 2008, 22, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, T.; Cao, B.; Shen, H. Pseudoretinoblastoma of 9 enucleated eyes simulating retinoblastoma in 70 enucleated eyes. Int. J. Clin. Exp. Pathol. 2017, 10, 9475–9481. [Google Scholar] [PubMed]
- Huang, S.; Rutar, T.; Bloomer, M.; Crawford, J.B. Analysis of clinical misdiagnoses in children treated with enucleation. Arch. Ophthalmol. 2010, 128, 1009–1013. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, P.; Venturi, C.; Cerase, A.; Vallone, I.M.; Bracco, S.; Bardelli, A.M.; Hadjistilianou, T.; Gennari, P.; Monti, L.; Filosomi, G. Coats disease: Smaller volume of the affected globe. Radiology 2001, 221, 64–69. [Google Scholar] [CrossRef]
- de Graaf, P.; Knol, D.L.; Moll, A.C.; Imhof, S.M.; Schouten-van Meeteren, A.Y.; Castelijns, J.A. Eye size in retinoblastoma: MR imaging measurements in normal and affected eyes. Radiology 2007, 244, 273–280. [Google Scholar] [CrossRef]
- Edward, D.P.; Mafee, M.F.; Garcia-Valenzuela, E.; Weiss, R.A. Coats’ disease and persistent hyperplastic primary vitreous. Role of MR imaging and CT. Radiol Clin. n. Am. 1998, 36, 1119–1131. [Google Scholar] [CrossRef]
- Galluzzi, P. Leukocoria: Clinical and Neuroradiological Findings. Riv. Di Neuroradiol. 2004, 17, 61–78. [Google Scholar] [CrossRef]
- Chung, E.M.; Specht, C.S.; Schroeder, J.W. From the archives of the AFIP: Pediatric orbit tumors and tumorlike lesions: Neuroepithelial lesions of the ocular globe and optic nerve. Radiographics 2007, 27, 1159–1186. [Google Scholar] [CrossRef]
- Kuker, W.; Ramaekers, V. Persistent hyperplastic primary vitreous: MRI. Neuroradiology 1999, 41, 520–522. [Google Scholar] [CrossRef]
- Sun, M.H.; Kao, L.Y.; Kuo, Y.H. Persistent hyperplastic primary vitreous: Magnetic resonance imaging and clinical findings. Chang. Gung Med. J. 2003, 26, 269–276. [Google Scholar] [PubMed]
- Daruich, A.L.; Moulin, A.P.; Tran, H.V.; Matet, A.; Munier, F.L. Subfoveal nodule in Coats’ disease: Toward an Updated Classification Predicting Visual Prognosis. Retina 2017, 37, 1591–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, R.C.; Wilson, M.W.; Kaste, S.; Helton, K.J.; McCarville, M.B. US and MRI of pediatric ocular masses with histopathological correlation. Pediatric Radiol. 2012, 42, 738–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisse, H.J.; Lumbroso, L.; Freneaux, P.C.; Validire, P.; Doz, F.P.; Quintana, E.J.; Berges, O.; Desjardins, L.C.; Neuenschwander, S.G. Sonographic, CT, and MR imaging findings in diffuse infiltrative retinoblastoma: Report of two cases with histologic comparison. AJNR Am. J. Neuroradiol. 2001, 22, 499–504. [Google Scholar] [PubMed]
- de Graaf, P.; Goricke, S.; Rodjan, F.; Galluzzi, P.; Maeder, P.; Castelijns, J.A.; Brisse, H.J.; European Retinoblastoma Imaging, C. Guidelines for imaging retinoblastoma: Imaging principles and MRI standardization. Pediatr. Radiol. 2012, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Mafee, M.F.; Mafee, R.F.; Malik, M.; Pierce, J. Medical imaging in pediatric ophthalmology. Pediatr. Clin. 2003, 50, 259–286. [Google Scholar] [CrossRef]
- Kadom, n.; Sze, R.W. Radiological reasoning: Leukocoria in a child. AJR Am. J. Roentgenol. 2008, 191, S40–S44. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L.; Honavar, S.G.; Demirci, H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am. J. Ophthalmol. 2001, 131, 561–571. [Google Scholar] [CrossRef]
- Munier, F.L.; Beck-Popovic, M.; Chantada, G.L.; Cobrinik, D.; Kivela, T.T.; Lohmann, D.; Maeder, P.; Moll, A.C.; Carcaboso, A.M.; Moulin, A.; et al. Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity”. Prog Retin Eye Res. 2019, 73, 100764. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Yadav, A. Bilateral persistent fetal vasculature: Mimicker of retinoblastoma. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Rodjan, F.; de Graaf, P.; van der Valk, P.; Hadjistilianou, T.; Cerase, A.; Toti, P.; de Jong, M.C.; Moll, A.C.; Castelijns, J.A.; Galluzzi, P.; et al. Detection of calcifications in retinoblastoma using gradient-echo MR imaging sequences: Comparative study between in vivo MR imaging and ex vivo high-resolution CT. AJNR Am. J. Neuroradiol. 2015, 36, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, C.L.; Ghassemi, F.; Tuncer, S.; Thangappan, A.; Shields, J.A. Clinical spectrum of diffuse infiltrating retinoblastoma in 34 consecutive eyes. Ophthalmology 2008, 115, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology 2015, 277, 826–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.W.; de Jong, M.C.; Kooi, I.E.; Sirin, S.; Goricke, S.; Brisse, H.J.; Maeder, P.; Galluzzi, P.; van der Valk, P.; Cloos, J.; et al. MR Imaging Features of Retinoblastoma: Association with Gene Expression Profiles. Radiology 2018, 288, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Kooi, I.E.; Mol, B.M.; Moll, A.C.; van der Valk, P.; de Jong, M.C.; de Graaf, P.; van Mil, S.E.; Schouten-van Meeteren, A.Y.N.; Meijers-Heijboer, H.; Kaspers, G.L.; et al. Loss of photoreceptorness and gain of genomic alterations in retinoblastoma reveal tumor progression. EBioMedicine 2015, 2, 660–670. [Google Scholar] [CrossRef] [Green Version]


| Retinoblastoma | Coats’ Disease | PFV/Retinal Dysplasia | |
|---|---|---|---|
| Patients n (%) | 33 (50%) | 24 (36%) | 9 (14%) (of which n = 2 isolated retinal dysplasia) |
| Assessed eyes n (%) | 34 (50%) | 24 (35%) | 10 (15%) |
| Laterality OD/OS/ODS | 15/17/1 | 10/14/0 | 3/5/1 |
| Stage 1 | B 1/27 (4%) C: 4/27 (15%) D: 7/27 (26%) E: 15/27 (56%) | Advanced stage (3–5): 24/24 (100%) | - |
| Female/male (%) | 15/18 (45/55%) | 3/21 (13/88%) | 2/7 (22/78%) |
| Mean age at MRI scan in months (standard deviation) | 34 (27) | 38 (30) | 16 (24) |
| Mean follow-up duration in months 2 (standard deviation) | 59 (41) | 16 (27) | 13 (16) |
| Mean scan year (range) | 2013 (1997–2019) | 2013 (2009–2019) | 2014 (1999–2019) |
| Imaging Feature | Retinoblastoma 1 | Coats’ Disease 1 | PFV/ Retinal Dysplasia 1 | Pseudoretinoblastoma (Coats’ Disease and PFV) | K 2 | p3 |
|---|---|---|---|---|---|---|
| Eye size 4 | 0.37 | <0.001 | ||||
| Smaller | 9% (3/33) | 67% (16/24) | 80% (8/10) | 71% (24/34) | ||
| Equal | 82% (27/33) | 33% (8/24) | 10% (1/10) | 26% (9/34) | ||
| Larger | 9% (3/33) | 0% (0/24) | 10% (1/10) | 3% (1/34) | ||
| Ciliary deformations | 0% (0/34) | 13% (3/24) | 78% (7/9) | 30% (10/33) | 0.39 | <0.001 |
| Lens deformations | 3% (1/34) | 17% (4/24) | 90% (9/10) | 38% (13/34) | 0.53 | <0.001 |
| Optic nerve atrophy | 0% (0/34) | 0% (0/24) | 70% (7/10) | 21% (7/34) | 0.49 | <0.001 |
| Central stalk 5 | 0% (0/34) | 4% (1/23) | 70% (7/10) | 24% (8/33) | 0.43 | <0.001 |
| Shape of retinal detachment 6 | 0.40 | 0.007 | ||||
| T | 22% (7/32) | 25% (5/20) | 44% (4/9) | 31% (9/29) | ||
| Y | 0% (0/32) | 20% (4/20) | 44% (4/9) | 28% (8/29) | ||
| Sharp-V | 25% (8/32 | 5% (1/20) | 11% (1/9) | 7% (2/29) | ||
| Wide-V | 53% (17/32) | 50% (10/20) | 0% (0/9) | 34% (10/29) | ||
| Intraretinal macrocyst | 0% (0/34) | 38% (9/24) | 20% (2/10) | 32% (11/34) | 0.55 | 0.005 |
| Absence of calcifications | 0% (0/34) | 67% (14/21) | 50% (4/8) | 62% (18/29) | 0.35 | <0.001 |
| Lesion islands/seeding | 0.44 | <0.001 | ||||
| Vitreous | 30% (10/33) | 4% (1/23) | 0% (0/10) | 3% (1/33) | ||
| Subretinal | 64% (21/33) | 30% (7/23) | 10% (1/10) | 24% (8/33) | ||
| No/minimal enhancement in solid lesion | 3% (1/34) | 31% (7/22) | 57% (4/7) | 38% (11/29) | 0.35 | 0.008 |
| Enhancement outside solid lesion (mismatch) | 0% (0/34) | 30% (7/23) | 57% (4/7) | 37% (11/30) | 0.15 | <0.001 |
| Features Favoring | Imaging Feature 1 | Sensitivity (95%CI) | Specificity (95%CI) | Accuracy (95%CI) | |
|---|---|---|---|---|---|
| Retinoblastoma | Larger eye size 2 | 9% (2–25%) | 97% (85–100%) | 54% (41–66%) | |
| Narrow V-shape | 25% (11–43%) | 93% (77–99%) | 57% (44–70%) | ||
| Lesion islands/vitreous | 30% (16–49%) | 96% (78–100%) | 58% (44–71%) | ||
| Pseudoretinoblastoma | Smaller eye size 1 | 71% (53–85%) | 91% (76–98%) | 81% (69–89%) | |
| Y-shaped retinal detachment | 28% (13–47%) | 100% (89–100%) | 65% (52–77%) | ||
| Absence of calcifications | 62% (42–79%) | 100% (90–100%) | 83% (81–91%) | ||
| Intraretinal macrocysts | 32% (17–51%) | 100% (90–100%) | 66% (54–77%) | ||
| No/minimal enhancement in solid lesion | 38% (21–57%) | 97% (85–100%) | 70% (57–81%) | ||
| Enhancement outside solid lesion (mismatch) | 37% (20–56%) | 100% (90–100%) | 70% (58–81%) | ||
| Ciliary body deformations | 30% (65–93%) | 100% (89–100%) | 66% (53–77%) | ||
| Lens deformations | 38% (22–56%) | 97% (84–100%) | 68% (55–78%) | ||
| Optic nerve atrophy | 22% (9–40%) | 100% (89–100%) | 62% (49–74%) | ||
| Central stalk 3 | 24% (11–42%) | 100% (89–100%) | 63% (50–74%) | ||
| Individual pseudoretinoblastoma | PFV/retinal dysplasia | Ciliary body deformations | 78% (40–97%) | 95% (86–99%) | 93% (83–98%) |
| Lens deformations | 90% (56–100%) | 91% (81–97%) | 91% (82–97%) | ||
| Optic nerve atrophy | 70% (35–93%) | 100% (94–100%) | 96% (88–99%) | ||
| Central stalk 2 | 70% (35–93%) | 98% (91–100%) | 94% (85–98%) | ||
| Coats’ disease | Intraretinal macrocysts | 38% (19–59%) | 95% (85–99%) | 38% (19–59%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, R.W.; de Bloeme, C.M.; Brisse, H.J.; Galluzzi, P.; Cardoen, L.; Göricke, S.; Maeder, P.; Cassoux, N.; Gauthier, A.; Schlueter, S.; et al. MR Imaging Features to Differentiate Retinoblastoma from Coats’ Disease and Persistent Fetal Vasculature. Cancers 2020, 12, 3592. https://doi.org/10.3390/cancers12123592
Jansen RW, de Bloeme CM, Brisse HJ, Galluzzi P, Cardoen L, Göricke S, Maeder P, Cassoux N, Gauthier A, Schlueter S, et al. MR Imaging Features to Differentiate Retinoblastoma from Coats’ Disease and Persistent Fetal Vasculature. Cancers. 2020; 12(12):3592. https://doi.org/10.3390/cancers12123592
Chicago/Turabian StyleJansen, Robin W., Christiaan M. de Bloeme, Hervé J. Brisse, Paolo Galluzzi, Liesbeth Cardoen, Sophia Göricke, Philippe Maeder, Nathalie Cassoux, Arnaud Gauthier, Sabrina Schlueter, and et al. 2020. "MR Imaging Features to Differentiate Retinoblastoma from Coats’ Disease and Persistent Fetal Vasculature" Cancers 12, no. 12: 3592. https://doi.org/10.3390/cancers12123592





