Clinical Decision Support Systems in Breast Cancer: A Systematic Review
Abstract
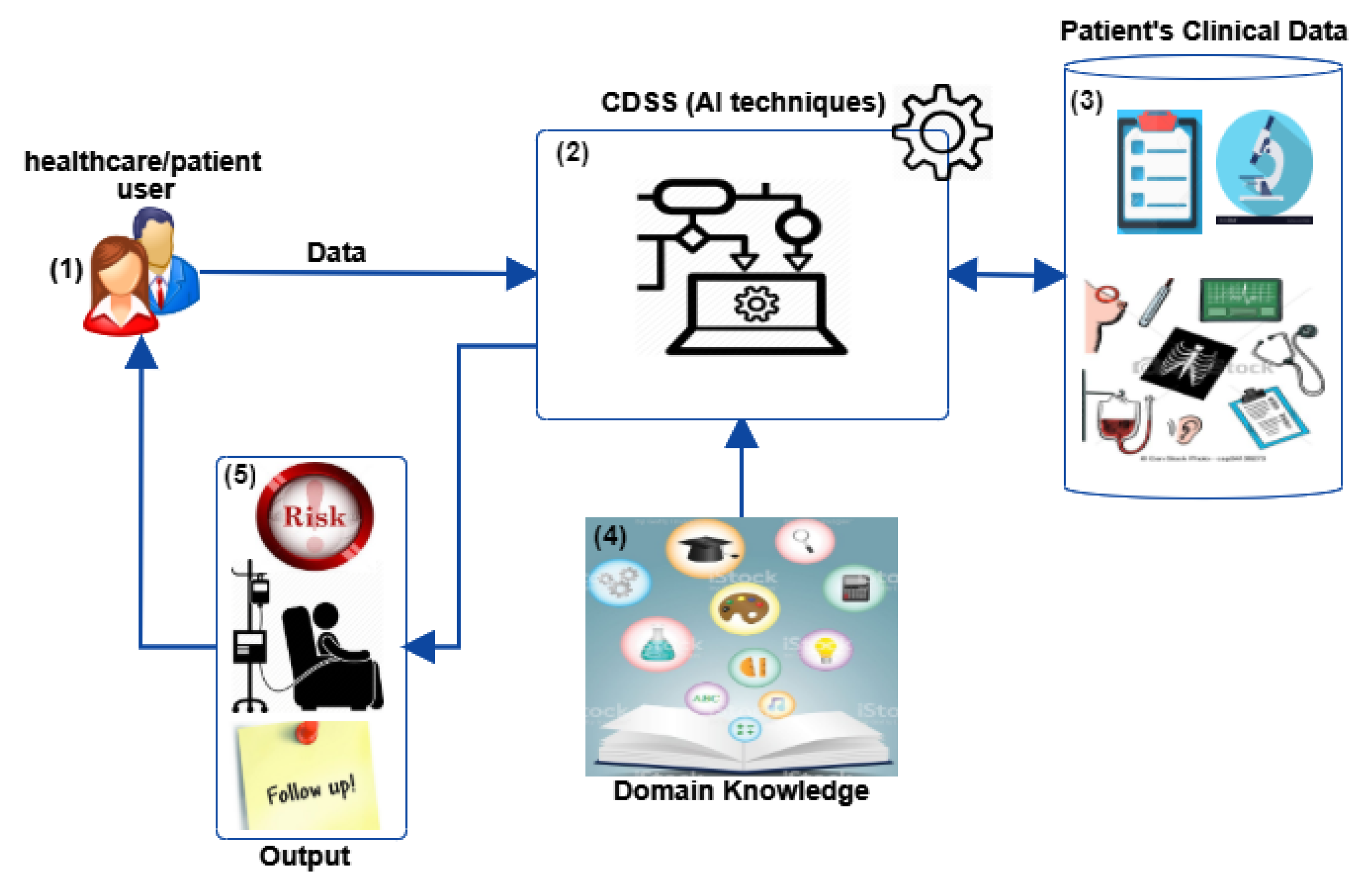
:1. Introduction
2. Method
2.1. Planning the Review
2.1.1. Related Works and Needs for the Review
2.1.2. Research Question
2.2. Conducting the Review
2.2.1. Data Sources
2.2.2. Extracting Data and Synthesis
3. Results
3.1. CDSSs for Treatment Decisions in Breast Cancer
3.2. Advantages and Disadvantages of Using CDSSs
3.3. Effects of the CDSSs in the Population
4. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDSS | Clinical Decision Support System |
| DSS | Decision Support Systems |
| HT | Hormonal Therapy |
| RS | Risk Score |
| DBCP | Digital Breast Cancer Patient |
| WMCIU | West Midlands Cancer Intelligence Unit |
| SEER | Surveillance, Epidemiology, and End Results Program |
| EI | Enterprise Ireland |
| IRC | Irish Research Council |
| SFI | Science Foundation Ireland |
References
- American Cancer Society (ACS). Breast Cancer. 2018. Available online: https://www.cancer.org/cancer/breast-cancer.html (accessed on 29 January 2020).
- International Agency of Research Cancer (IARC). Breast Cancer. 2018. Available online: https://www.iarc.fr/index.php (accessed on 29 January 2020).
- Paraskevi, T. Quality of life outcomes in patients with breast cancer. Oncol. Rev. 2012, 6, 1–4. [Google Scholar] [CrossRef]
- Shachar, S.S.; Muss, H.B. Internet tools to enhance breast cancer care. Npj Breast Cancer 2016, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Entwistle, V.A.; Carter, S.M.; Cribb, A.; McCaffery, K. Supporting Patient Autonomy: The Importance of Clinician-patient Relationships. J. Gen. Intern. Med. 2010, 25, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.E.; Mahant, S. Shared Decision-Making About Assistive Technology for the Child with Severe Neurologic Impairment. Pediatr. Clin. N. Am. 2014, 61, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, S.T.; Bamidis, P.D. Why decision support systems are important for medical education. Healthc. Technol. Lett. 2016, 3, 56–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.R.; Samsa, G.; Hasselblad, V.; Williams, J.W.; Musty, M.D.; et al. Effect of clinical decision-support systems: A systematic review. Ann. Intern. Med. 2012, 157, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Jacob, V.; Thota, A.B.; Chattopadhyay, S.K.; Njie, G.J.; Proia, K.K.; Hopkins, D.P.; Ross, M.N.; Pronk, N.P.; Clymer, J.M. Cost and economic benefit of clinical decision support systems for cardiovascular disease prevention: A community guide systematic review. J. Am. Med. Inform. Assoc. JAMIA 2017, 24, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellodi, E.; Vagnoni, E.; Bonvento, B.; Lamma, E. Economic and organizational impact of a clinical decision support system on laboratory test ordering. BMC Med. Inform. Decis. Mak. 2017, 17, 179. [Google Scholar] [CrossRef] [PubMed]
- Ajami S, A.F. Reduce Medication Errors with Clinical Decision Support Systems. J. Inf. Technol. Soft Eng. 2013, 7, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.; Zhang, L.; Chen, J.; Zhao, P.; Zhang, M. The Effects of Clinical Decision Support Systems on Medication Safety: An Overview. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nurek, M.; Kostopoulou, O.; Delaney, B.C.; Esmail, A. Reducing diagnostic errors in primary care. A systematic meta-review of computerized diagnostic decision support systems by the LINNEAUS collaboration on patient safety in primary care. Eur. J. Gen. Pract. 2015, 21, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.; de Jong, E.E.; van Timmeren, J.E.; Ibrahim, A.; Compter, I.; Peerlings, J.; Sanduleanu, S.; Refaee, T.; Keek, S.; Larue, R.T.; et al. Decision support systems in oncology. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coiera, E. Guide to Health Informatics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Légaré, F.; Robitaille, H.; Gane, C.; Hébert, J.; Labrecque, M.; Rousseau, F. Improving Decision Making about Genetic Testing in the Clinic: An Overview of Effective Knowledge Translation Interventions. PLoS ONE 2016, 11, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Séroussi, B.; Bouaud, J.; Éric Charles Antoine. OncoDoc: A successful experiment of computer-supported guideline development and implementation in the treatment of breast cancer. Artif. Intell. Med. 2001, 22, 43–64. [Google Scholar] [CrossRef]
- Kitchenham, B.; Charters, S. Guidelines for performing Systematic Literature Reviews in Software. Engineering 2007, 45, 1051. [Google Scholar]
- Jia, P.L.; Zhang, P.F.; Li, H.D.; Zhang, L.H.; Chen, Y.; Zhang, M.M. Literature review on clinical decision support system reducing medical error. J. Evid.-Based Med. 2014, 7, 219–226. [Google Scholar] [CrossRef]
- Shahsavarani, A.M.; Abadi, E.A.M.; Kalkhoran, M.H.; Jafari, S.; Qaranli, S. Clinical Decision Support Systems (CDSSs): State of the art Review of Literature. Int. J. Med. Rev. 2015, 2, 299–308. [Google Scholar]
- Community Preventive Services Task Force; Njie, G.; Proia, K.; Thota, A.; Finnie, R.; Hopkins, D.; Banks, S.; Callahan, D.; Pronk, N.; Rask, K.; et al. Clinical Decision Support Systems and Prevention: A Community Guide Cardiovascular Disease Systematic Review. Am. J. Prev. Med. 2015, 49, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Rawson, T.; Moore, L.; Hernandez, B.; Charani, E.; Castro-Sanchez, E.; Herrero, P.; Hayhoe, B.; Hope, W.; Georgiou, P.; Holmes, A. A systematic review of clinical decision support systems for antimicrobial management: Are we failing to investigate these interventions appropriately? Clin. Microbiol. Infect. 2017, 23, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Sim, I.; Gorman, P.; Greenes, R.A.; Haynes, R.B.; Kaplan, B.; Lehmann, H.; Tang, P.C. Clinical Decision Support Systems for the Practice of Evidence-based Medicine. J. Am. Med. Inform. Assoc. 2001, 8, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Fillmore, C.L.; Bray, B.E.; Kawamoto, K. Systematic review of clinical decision support interventions with potential for inpatient cost reduction. BMC Med. Inform. Decis. Mak. 2013, 13, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wazir, U.; Mokbel, K.; Carmichael, A.; Mokbel, K. Are online prediction tools a valid alternative to genomic profiling in the context of systemic treatment of ER-positive breast cancer? Cell. Mol. Biol. Lett. 2017, 22, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawloski, P.A.; Brooks, G.A.; Nielsen, M.E.; Olson-Bullis, B.A. A Systematic Review of Clinical Decision Support Systems for Clinical Oncology Practice. J. Natl. Compr. Cancer Netw. J. Natl. Compr. Cancer Netw. 2019, 17, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravdin, P.M.; Siminoff, L.A.; Davis, G.J.; Mercer, M.B.; Hewlett, J.; Gerson, N.; Parker, H.L. Computer Program to Assist in Making Decisions About Adjuvant Therapy for Women With Early Breast Cancer. J. Clin. Oncol. 2001, 19, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Wishart, G.C.; Azzato, E.M.; Greenberg, D.C.; Rashbass, J.; Kearins, O.; Lawrence, G.; Caldas, C.; Pharoah, P.D. PREDICT: A new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010, 12, R1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candido dos Reis, F.J.; Wishart, G.C.; Dicks, E.M.; Greenberg, D.; Rashbass, J.; Schmidt, M.K.; Van den Broek, A.J.; Ellis, I.O.; Green, A.; Rakha, E.; et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 2017, 19, 58. [Google Scholar] [CrossRef]
- Irish mHealth Company Portable Medical Technology Ltd. ONCOassist. 2018. Available online: https://webapp.oncoassist.com/public/index.php/ (accessed on 29 January 2020).
- Ellis, P.G.; Brufsky, A.M.; Beriwal, S.; Lokay, K.G.; Benson, H.O.; McCutcheon, S.B.; Krebs, M. Pathways Clinical Decision Support for Appropriate Use of Key Biomarkers. J. Oncol. Pract. 2016, 12, e681–e687. [Google Scholar] [CrossRef] [Green Version]
- Larburu, N.; Muro, N.; Macía, I. Augmenting Guideline-based CDSS with Experts’ Knowledge. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies, Volume 5: HEALTHINF, Porto, Portugal, 21–23 February 2017; pp. 370–376. [Google Scholar] [CrossRef]
- Miao, H.; Hartman, M.; Verkooijen, H.M.; Taib, N.A.; Wong, H.S.; Subramaniam, S.; Yip, C.H.; Tan, E.Y.; Chan, P.; Lee, S.C.; et al. Validation of the CancerMath prognostic tool for breast cancer in Southeast Asia. BMC Cancer 2016, 16, 820. [Google Scholar] [CrossRef] [Green Version]
- Dowsett, M.; Sestak, I.; Regan, M.M.; Dodson, A.; Viale, G.; Thürlimann, B.; Colleoni, M.; Cuzick, J. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor–positive breast cancer treated with 5 years of endocrine therapy: CTS5. J. Clin. Oncol. 2018, 36, 1941. [Google Scholar] [CrossRef]
- Yau, C.; van der Noordaa, M.; Wei, J.; Osdoit, M.; Reyal, F.; Hamy, A.S.; Lae, M.; Martin, M.; del Monte, M.; I-SPY TRIAL Consortium; et al. Residual cancer burden after neoadjuvant therapy and long-term survival outcomes in breast cancer: A multi-center pooled analysis. In Proceedings of the 2019 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 12–13 December 2019. [Google Scholar]
- Olivotto, I.A.; Bajdik, C.D.; Ravdin, P.M.; Speers, C.H.; Coldman, A.J.; Norris, B.D.; Davis, G.J.; Chia, S.K.; Gelmon, K.A. Population-Based Validation of the Prognostic Model ADJUVANT! for Early Breast Cancer. J. Clin. Oncol. 2005, 23, 2716–2725. [Google Scholar] [CrossRef]
- Campbell, H.E.; Taylor, M.A.; Harris, A.L.; Gray, A.M. An investigation into the performance of the Adjuvant! Online prognostic programme in early breast cancer for a cohort of patients in the United Kingdom. Br. J. Cancer 2009, 101, 1074–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintyne, K.I.; Woulfe, B.; Coffey, J.C.; Gupta, R.K. Correlation Between Nottingham Prognostic Index and Adjuvant! Online Prognostic Tools in Patients With Early-Stage Breast Cancer in Mid-Western Ireland. Clin. Breast Cancer 2013, 13, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Breast Unit, University of Cambridge Department of Oncology and the Eastern Cancer Information and Registration Centre (ECRIC). PREDICT. 2010. Available online: http://www.predict.nhs.uk/predict_v1.2.html (accessed on 29 January 2020).
- UPMC Cancer Center, University of Pittsburgh Cancer Center, and Via Oncology, Pittsburgh, PA. Oncotype IQ. 2016. Available online: https://online.genomichealth.com/Login.aspx (accessed on 29 January 2020).
- European Consortium. DESIREE. 2015. Available online: http://www.desiree-project.eu/ (accessed on 29 January 2020).
- CancerMath.net Group. Breast Cancer Treatment Outcome Calculator. 2009. Available online: http://www.lifemath.net/cancer/breastcancer/therapy/ (accessed on 29 January 2020).
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef]
- Regan, M.M.; Neven, P.; Giobbie-Hurder, A.; Goldhirsch, A.; Ejlertsen, B.; Mauriac, L.; Forbes, J.F.; Smith, I.; Láng, I.; Wardley, A.; et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8· 1 years median follow-up. Lancet Oncol. 2011, 12, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Anders, C.K.; Fan, C.; Parker, J.S.; Carey, L.A.; Blackwell, K.L.; Klauber-DeMore, N.; Perou, C.M. Breast Carcinomas Arising at a Young Age: Unique Biology or a Surrogate for Aggressive Intrinsic Subtypes? J. Clin. Oncol. 2011, 29, e18–e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, C.K.; Hsu, D.S.; Broadwater, G.; Acharya, C.R.; Foekens, J.A.; Zhang, Y.; Wang, Y.; Marcom, P.K.; Marks, J.R.; Febbo, P.G.; et al. Young Age at Diagnosis Correlates With Worse Prognosis and Defines a Subset of Breast Cancers With Shared Patterns of Gene Expression. J. Clin. Oncol. 2008, 26, 3324–3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [Green Version]
- Azim, H.A.; Partridge, A.H. Biology of breast cancer in young women. Breast Cancer Res. 2014, 16, 427. [Google Scholar] [CrossRef]
- Haybittle, J.L.; Blamey, R.W.; Elston, C.W.; Johnson, J.; Doyle, P.J.; Campbell, F.C.; Nicholson, R.I.; Griffiths, K. A prognostic index in primary breast cancer. Br. J. Cancer 1982, 45, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Aaltomaa, S.; Lipponen, P.; Eskelinen, M.; Kosma, V.M.; Marin, S.; Alhava, E.; Syrjänen, K. Mitotic indexes as prognostic predictors in female breast cancer. J. Cancer Res. Clin. Oncol. 1992, 118, 75–81. [Google Scholar] [CrossRef]
- Mollon, B.; Chong, J.J.; Holbrook, A.M.; Sung, M.; Thabane, L.; Foster, G. Features predicting the success of computerized decision support for prescribing: A systematic review of randomized controlled trials. BMC Med. Inform. Decis. Mak. 2009, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, A.; Xu, S.; Banting, J. What factors determine the success of clinical decision support systems? AMIA Annu. Symp. Proc. 2003, 2003, 862. [Google Scholar]
- Kawamoto, K.; Houlihan, C.A.; Balas, E.A.; Lobach, D.F. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005, 330, 765. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Padman, R.; Johnson, M.P.; Diamond, H.S. Understanding technology adoption in clinical care: Clinician adoption behavior of a point-of-care reminder system. Int. J. Med. Inform. 2005, 74, 535–543. [Google Scholar] [CrossRef]
- Brender, J.; Ammenwerth, E.; Nykänen, P.; Talmon, J. Factors influencing success and failure of health informatics systems: A pilot Delphi study. Methods Inf. Med. 2006, 45, 125–136. [Google Scholar]
- Varonen, H.; Kortteisto, T.; Kaila, M.; EBMeDS Study Group. What may help or hinder the implementation of computerized decision support systems (CDSSs): A focus group study with physicians. Fam. Pract. 2008, 25, 162–167. [Google Scholar] [CrossRef]
- Lin, G.A.; Aaronson, D.S.; Knight, S.J.; Carroll, P.R.; Dudley, R.A. Patient Decision Aids for Prostate Cancer Treatment: A Systematic Review of the Literature. CA: A Cancer J. Clin. 2009, 59, 379–390. [Google Scholar] [CrossRef]
- Ufer, T. Which tools can I use in daily clinical practice to improve tailoring of treatment for breast cancer?The 2007 St Gallen guidelines and/or Adjuvant! Online. Ann. Oncol. 2008, 19, 41–45. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Sotiriou, C. Adjuvant chemotherapy—Yes or no? Prognostic markers in early breast cancer. Ann. Oncol. 2007, 18, 2–7. [Google Scholar] [CrossRef]
- Marcial, L.H.; Richardson, J.E.; Lasater, B.; Middleton, B.; Osheroff, J.A.; Kawamoto, K.; Blumenfeld, B.H. The Imperative for Patient-Centered Clinical Decision Support. EGEMs (Generating Evid. Methods Improv. Patient Outcomes) 2018, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fathima, M.; Peiris, D.; Naik-Panvelkar, P.; Saini, B.; Armour, C.L. Effectiveness of computerized clinical decision support systems for asthma and chronic obstructive pulmonary disease in primary care: A systematic review. BMC Pulm. Med. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaarup, C.; Pape-Haugaard, L.B.; Hejlesen, O.K. Models Used in Clinical Decision Support Systems Supporting Healthcare Professionals Treating Chronic Wounds: Systematic Literature Review. JMIR Diabetes 2018, 3, e11. [Google Scholar] [CrossRef] [PubMed]
- Hemens, B.J.; Holbrook, A.; Tonkin, M.; Mackay, J.A.; Weise-Kelly, L.; Navarro, T.; Wilczynski, N.L.; Haynes, R.B.; CCDSS Systematic Review Team. Computerized clinical decision support systems for drug prescribing and management: A decision-maker-researcher partnership systematic review. Implement. Sci. 2011, 6, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wijk, Y.; Halilaj, I.; van Limbergen, E.; Walsh, S.; Lutgens, L.; Lambin, P.; Vanneste, B.G.L. Decision Support Systems in Prostate Cancer Treatment: An Overview. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, K.; Lobach, D.F. Clinical decision support provided within physician order entry systems: A systematic review of features effective for changing clinician behavior. AMIA Annu. Symp. Proc. 2003, 2003, 361. [Google Scholar]
- Jao, C.S.; Hier, D.B. Clinical Decision Support Systems: An Effective Pathway to Reduce Medical Errors and Improve Patient Safety. In Decision Support Systems; Jao, C.S., Ed.; IntechOpen: Rijeka, Croatia, 2010; Chapter 8. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, R.; Kern, L.M.; Barrón, Y.; Quaresimo, J.; Abramson, E.L. Electronic prescribing improves medication safety in community-based office practices. J. Gen. Intern. Med. 2010, 25, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Séroussi, B.; Bouaud, J. Using OncoDoc as a computer-based eligibility screening system to improve accrual onto breast cancer clinical trials. Artif. Intell. Med. 2003, 29, 153–167. [Google Scholar] [CrossRef]
- Briss, P.A.; Zaza, S.; Pappaioanou, M.; Fielding, J.; Aguero, L.W.D.; Truman, B.I.; Hopkins, D.P.; Mullen, P.D.; Thompson, R.S.; Woolf, S.H.; et al. Developing an evidence-based guide to community preventive services methods. Am. J. Prev. Med. 2000, 18, 35–43. [Google Scholar] [CrossRef]

| CDSS | Users | Sample | Clinical Question | Clinical & Pathological Input | Method | Access | URL |
|---|---|---|---|---|---|---|---|
| Adjuvant! Online [27] | HC | 631 | Risk of recurrence and death at 10 years; Estimated benefits of adjuvant endocrine and chemotherapy | Surveillance, epidemiology, SEER, and efficacy of adjuvant therapy | Kaplan Meier analysis | Not available | www.adjuvantonline.com |
| PREDICT [28,29] | HC/P | 5694 | Prediction of survival at 5, 10 and 15 years with different treatment options | Age, tumour size and grade, PN, ER, HER2, Ki67 and mode of detection | Cox regression models | Online | https://breast.predict.nhs.uk/ |
| ONCOassist [30] | HC | 5694 | What is the most appropriate treatment following breast cancer surgery? Prediction of survival at 5, 10 and 15 years | Patient age, tumour size, tumour grade, number of positive nodes, ER status, HER2 status, Ki67 status and mode of detection | PREDICT method (Multivariable Cox regression models) | Online /smartphone app | https://oncoassist.com/ |
| Oncotype IQ [31] | HC | 643 | Do I have aggressive disease? Do I need radiation? Do I need surgery? Do I need chemo? What stage is my cancer? | HER2-, ER+, PN | OncoType DX | Online | https://online.genomichealth.com |
| DESIREE [32] | HC/P | 400 | What are the available therapy options? | Medical imaging, biological and genetic data, diagnostic and biomarkers, risk factors, environmental or social aspects, clinical trials | Image processing, ontology and datamining | Online | http://www.desiree-project.eu/ |
| CancerMath [33] | HC/P | 779,999 | Prediction of survival at 15 years with different treatment options | SEER, HER-2, tumour size, nodal, tumour phenotype, and grade | Statistical algorithms | Online | http://www.lifemath.net/cancer/ |
| CTS5 Calculator [34] | HC/P | 4000+ | Prediction of survival at 10 years with different treatment options | Tumour size, tumour grade, patient age, and number of nodes | Statistical algorithms | Online | www.cts5-calculator.com/ |
| Residual Cancer Burden Calculator [35] | HC/P | 5000+ | Prognostic for event-free and distant relapse-free survival | Primary tumour bed area, percentage of cancer cellularity, percentage of cancer that is in situ disease, lymph nodes, number of positive lymph nodes, and diameter of largest metastasis | Statistical algorithms | Online | http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazo, C.; Kearns, C.; Mooney, C.; Gallagher, W.M. Clinical Decision Support Systems in Breast Cancer: A Systematic Review. Cancers 2020, 12, 369. https://doi.org/10.3390/cancers12020369
Mazo C, Kearns C, Mooney C, Gallagher WM. Clinical Decision Support Systems in Breast Cancer: A Systematic Review. Cancers. 2020; 12(2):369. https://doi.org/10.3390/cancers12020369
Chicago/Turabian StyleMazo, Claudia, Cathriona Kearns, Catherine Mooney, and William M. Gallagher. 2020. "Clinical Decision Support Systems in Breast Cancer: A Systematic Review" Cancers 12, no. 2: 369. https://doi.org/10.3390/cancers12020369
APA StyleMazo, C., Kearns, C., Mooney, C., & Gallagher, W. M. (2020). Clinical Decision Support Systems in Breast Cancer: A Systematic Review. Cancers, 12(2), 369. https://doi.org/10.3390/cancers12020369





