Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients
Abstract
:1. Introduction
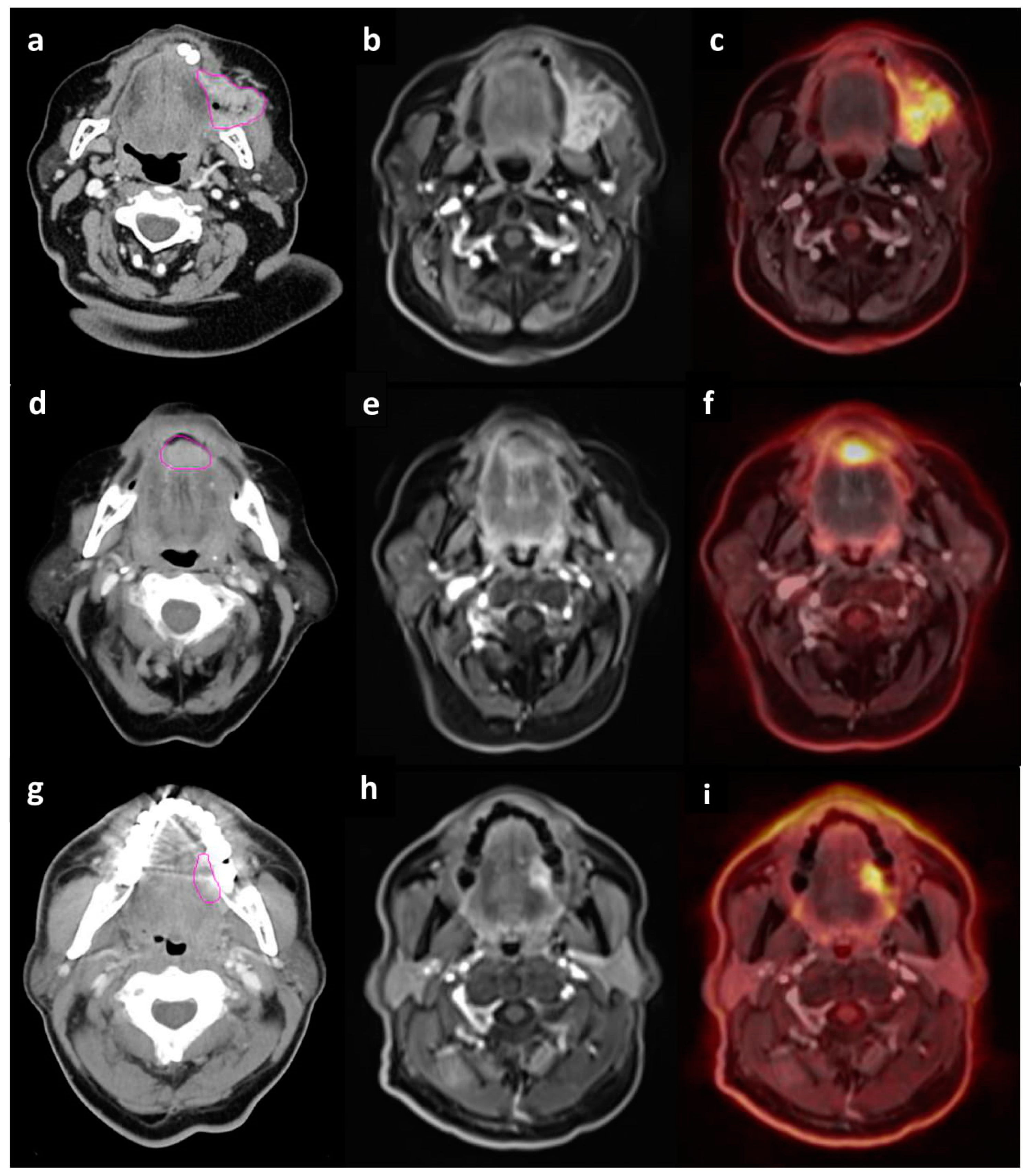
2. Results
2.1. Accuracy of T staging
2.2. Specificity, Sensitivity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of Lymph Node Evaluation in CT and PET/MRI
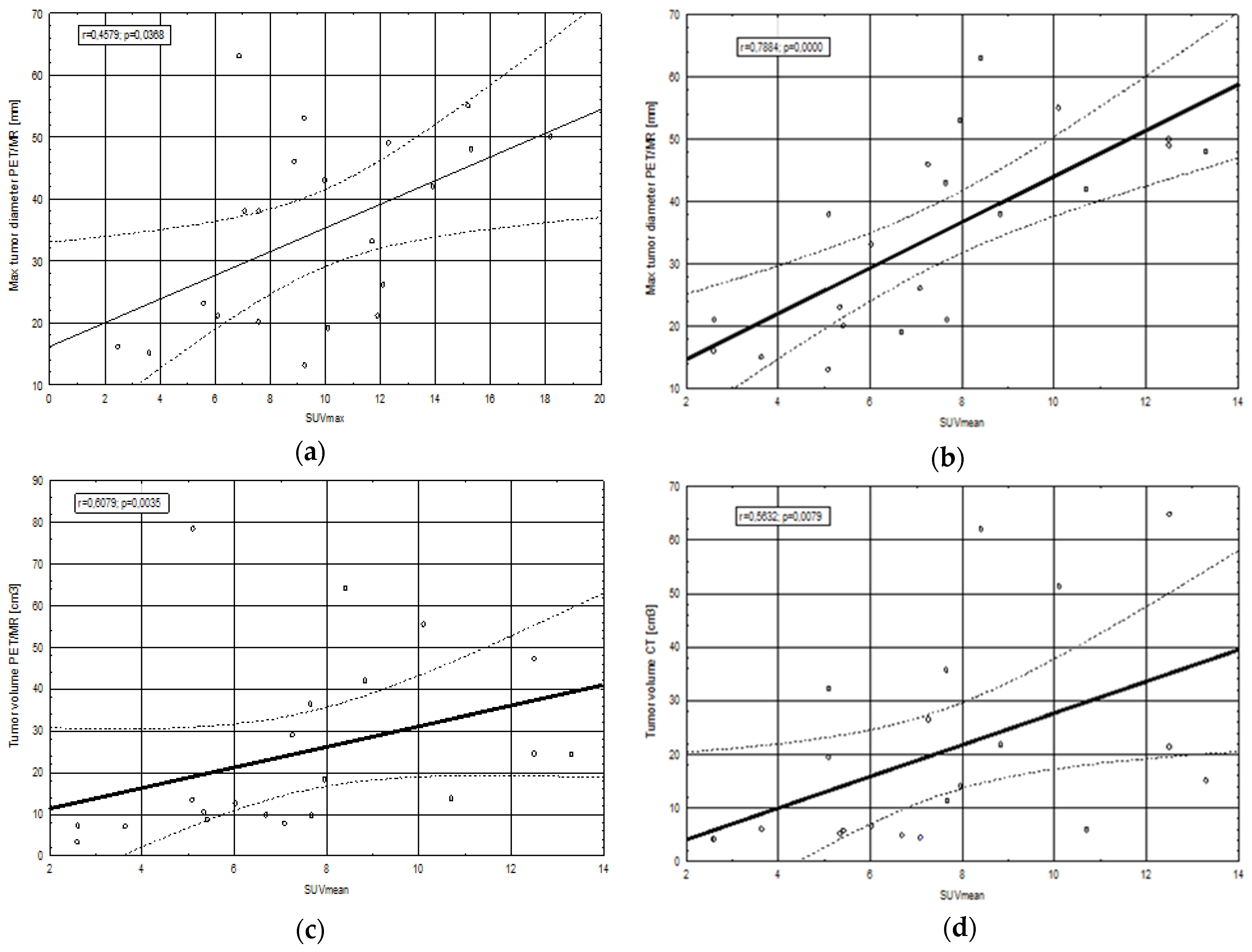
2.3. Correlations between Clinical, Morphological and Metabolic Parameters
2.3.1. SUV Values in Relation to Tumor Geometrical Parameters, Biochemical Parameters and Ki67 Index
2.3.2. Presence of p16, HPV and EBV in Relation to SUV Values, Tumor Geometrical Parameters
2.3.3. Number of Metastatic Lymph Nodes in Relation to SUV Values, Tumor Geometrical Parameters, Ki67 Index and Presence of p16, HPV, EBV
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burela, N.; Soni, T.; Patni, N.; Bhagat, J.; Kumar, T.; Natarajan, T. A quantitative comparison of gross tumor volumes delineated on [18F]-FDG-PET/CT scan and contrast-enhanced computed tomography scan in locally advanced head and neck carcinoma treated with intensity modulated radiotherapy. Adv. Mod. Oncol. Res. 2017, 3, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Friedman, K.; Patel, S.; Hagiwara, M. Potential role of PET/MRI for imaging metastatic lymph nodes in head and neck cancer. Am. J. Roentgenol. 2016, 207, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyszko, T.A.; Cook, G.J.R. PET/CT and PET/MRI in head and neck malignancy. Clin. Radiol. 2018, 73, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Moore, W.; Sumer, B.; Khan, S.; Sher, D.; Subramaniam, R.M. Clinical practice in PET/CT for the management of head and neck squamous cell cancer. Am. J. Roentgenol. 2017, 209, 289–303. [Google Scholar] [CrossRef]
- Zheng, E.; Khariwala, S.S. Do All Patients with Head and Neck Cancer Require a Positron Emission Tomography Scan at Diagnosis? Laryngoscope 2018. [Google Scholar] [CrossRef]
- Leclerc, M.; Lartigau, E.; Lacornerie, T.; Daisne, J.F.; Kramar, A.; Grégoire, V. Primary tumor delineation based on 18FDG PET for locally advanced head and neck cancer treated by chemo-radiotherapy. Radiother. Oncol. 2015, 116, 87–93. [Google Scholar] [CrossRef]
- Ligtenberg, H.; Jager, E.A.; Caldas-Magalhaes, J.; Schakel, T.; Pameijer, F.A.; Kasperts, N.; Philippens, M.E. Modality-specific target definition for laryngeal and hypopharyngeal cancer on FDG-PET, CT and MRI. Radiother. Oncol. 2017, 123, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Tsujikawa, T.; Narita, N.; Kanno, M.; Takabayashi, T.; Fujieda, S.; Okazawa, H. Role of PET/MRI in oral cavity and oropharyngeal cancers based on the 8th edition of the AJCC cancer staging system: A pictorial essay. Ann. Nucl. Med. 2018, 32, 239–249. [Google Scholar] [CrossRef]
- Differding, S.; Hanin, F.X.; Grégoire, V. PET imaging biomarkers in head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 613–622. [Google Scholar] [CrossRef]
- Huang, S.H.; Chien, C.Y.; Lin, W.C.; Fang, F.M.; Wang, P.W.; Lui, C.C.; Chang, C.C. A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin. Nucl. Med. 2011, 36, 518–525. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Souvatzoglou, M.; Wankerl, V.; Dinges, J.; Ritschl, L.M.; Mücke, T.; Beer, A.J. Diagnostic value of retrospective PET-MRI fusion in head-and-neck cancer. BMC Cancer 2014, 14, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehanna, H.; Jones, T.M.; Gregoire, V.; Ang, K.K. Oropharyngeal carcinoma related to human papillomavirus. BMJ Br. Med. J. 2010, 340. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Shah, J.P. Head and neck cancers—Major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Adelstein, D. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar] [CrossRef]
- Tahari, A.K.; Alluri, K.; Quon, H.; Koch, W.; Wahl, R.L.; Subramaniam, R.M. FDG PET/CT imaging of Oropharyngeal SCC: Characteristics of HPV positive and negative tumors. Clin. Nucl. Med. 2014, 39, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.J.; Wittekindt, C.; Knuth, J.; Steiner, D.; Wuerdemann, N.; Laur, M.; Klussmann, J.P. Intraindividual homogeneity of 18F-FDG PET/CT parameters in HPV-positive OPSCC. Oral Oncol. 2017, 73, 166–171. [Google Scholar] [CrossRef]
- Nesteruk, M.; Lang, S.; Veit-Haibach, P.; Studer, G.; Stieb, S.; Glatz, S.; Guckenberger, M. Tumor stage, tumor site and HPV dependent correlation of perfusion CT parameters and [18F]-FDG uptake in head and neck squamous cell carcinoma. Radiother. Oncol. 2015, 117, 125–131. [Google Scholar] [CrossRef]
- Kim, Y.I.; Cheon, G.J.; Kang, S.Y.; Paeng, J.C.; Kang, K.W.; Lee, D.S.; Chung, J.K. Prognostic value of simultaneous 18 F-FDG PET/MRI using a combination of metabolo-volumetric parameters and apparent diffusion coefficient in treated head and neck cancer. EJNMMI Res. 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Grégoire, V.; Ang, K.; Budach, W.; Grau, C.; Hamoir, M.; Langendijk, J.A.; Lee, A.; Le, Q.T.; Maingon, P.; Nutting, C.; et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 2014, 110, 172–181. [Google Scholar] [CrossRef]
- Schaarschmidt, B.M.; Heusch, P.; Buchbender, C.; Ruhlmann, M.; Bergmann, C.; Ruhlmann, V.; Schlamann, M.; Antoch, G.; Forsting, M.; Wetter, A. Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: A comparison between MRI, PET/CT and integrated PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 92–102. [Google Scholar] [CrossRef]
- Chan, S.C.; Yeh, C.H.; Yen, T.C.; Ng, S.H.; Chang, J.T.; Lin, C.Y.; Yen-Ming, T.; Fan, K.H.; Huang, B.S.; Hsu, C.L.; et al. Clinical utility of simultaneous whole-body 18 F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; de Galiza Barbosa, F.; Kuhn, F.P.; Burger, I.A.; Stolzmann, P.; Huber, G.F.; Kollias, S.S.; von Schulthess, G.K.; Veit-Haibach, P.; Huellner, M.W. PET+ MR versus PET/CT in the initial staging of head and neck cancer, using a trimodality PET/CT+ MR system. Clin. Imaging 2017, 42, 232–239. [Google Scholar] [CrossRef] [PubMed]
- De Bondt, R.B.; Nelemans, P.J.; Hofman, P.A.; Casselman, J.W.; Kremer, B.; van Engelshoven, J.M.; Beets-Tan, R.G. Detection of lymph node metastases in head and neck cancer: A meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur. J. Radiol. 2007, 64, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics Notes: Diagnostic tests 2: Predictive values. BMJ 1994, 309, 102. [Google Scholar] [CrossRef] [Green Version]
- Torabi, M.; Aquino, S.L.; Harisinghani, M.G. Current concepts in lymph node imaging. J. Nucl. Med. 2004, 45, 1509–1518. [Google Scholar]
- Platzek, I.; Beuthien-Baumann, B.; Schneider, M.; Gudziol, V.; Langner, J.; Schramm, G.; Laniado, M.; Kotzerke, J.; van den Hoff, J. PET/MRI in head and neck cancer: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Geraldine, B.E.; Pyatigorskaya, N.; De Laroche, R.; Herve, G.; Zaslavsky, C.; Bertaux, M.; Giron, A.; Soret, M.; Bertolus, C.; Sahli-Amor, M.; et al. Diagnostic performance of 18F-FDG PET/MR in head and neck malignancies. J. Nucl. Med. 2017, 58, 280. [Google Scholar]
- Grégoire, V.; Thorwarth, D.; Lee, J. Molecular imaging-guided radiotherapy for the treatment of head-and-neck squamous cell carcinoma: Does it fulfill the promises? Semin. Radiat. Oncol. 2018, 28, 35–45. [Google Scholar] [CrossRef]
- Samołyk-Kogaczewska, N.; Sierko, E.; Zuzda, K.; Gugnacki, P.; Szumowski, P.; Mojsak, M.; Burzyńska-Śliwowska, J.; Wojtukiewicz, M.Z.; Szczecina, K.; Jurgilewicz, D.H. PET/MRI-guided GTV delineation during radiotherapy planning in patients with squamous cell carcinoma of the tongue. Strahlenther. Onkol. 2019, 195, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Kocher, M.R.; Sharma, A.; Garrett-Mayer, E.; Ravenel, J.G. Pretreatment 18F-Fluorodeoxyglucose Positron Emission Tomography Standardized Uptake Values and Tumor Size in Medically Inoperable Nonsmall Cell Lung Cancer Is Prognostic of Overall 2-Year Survival after Stereotactic Body Radiation Therapy. J. Comput. Assist. Tomo. 2018, 42, 146–150. [Google Scholar] [CrossRef]
- Rutkowski, T. The role of tumor volume in radiotherapy of patients with head and neck cancer. Radiat. Oncol. 2014, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, A.G.; Heilbrun, L.K.; Smith, D.W.; Miller, S.R. Association of 18F-FDG-PET SUV and Tumor Size in Cervical Cancer. Int. J. Radiat. Oncol. 2018, 102, e632. [Google Scholar] [CrossRef]
- Deng, S.M.; Zhang, W.; Zhang, B.; Chen, Y.Y.; Li, J.H.; Wu, Y.W. Correlation between the uptake of 18F-fluorodeoxyglucose (18F-FDG) and the expression of proliferation-associated antigen Ki-67 in cancer patients: A meta-analysis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Xiao, F.; Han, X.; Zhong, L.; Zhong, H.; Xu, L.; Zhu, J.; Ni, B.; Liu, J.; Fang, Y.; et al. Correlation of pretreatment 18F-FDG uptake with clinicopathological factors and prognosis in patients with newly diagnosed diffuse large B-cell lymphoma. Nucl. Med. Commun. 2016, 37, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between PET parameters and expression of Ki-67 in breast cancer. Transl. Oncol. 2019, 12, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Ucar, E.; Yalcin, H.; Kavvasoglu, G.H.; Ilhan, G. Correlations between the maximum standard uptake value of positron emission tomography/computed tomography and laboratory parameters before and after treatment in patients with lymphoma. Chin. Med. J. 2018, 131, 1776–1779. [Google Scholar] [CrossRef]
- Eskian, M.; Alavi, A.; Khorasanizadeh, M.; Viglianti, B.L.; Jacobsson, H. Effect of blood glucose level on standardized uptake value (SUV) in 18 F-FDG PET-scan: A systematic review and meta-analysis of 20, 807 individual SUV measurements. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 224–237. [Google Scholar] [CrossRef] [Green Version]
- Higashi, T.; Saga, T.; Nakamoto, Y.; Ishimori, T.; Fujimoto, K.; Doi, R.; Imamura, M.; Konishi, J. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (fdg pet)—Usefulness and limitations in “clinical reality”. Ann. Nucl. Med. 2003, 17, 261–279. [Google Scholar] [CrossRef]
- Westerterp, M.; Sloof, G.W.; Hoekstra, O.S.; ten Kate, F.J.; Meijer, G.A.; Reitsma, J.B.; Boellaard, R.; van Lanschot, J.J.; Molthoff, C.F. 18 FDG uptake in oesophageal adenocarcinoma: Linking biology and outcome. J. Cancer Res. Clin. 2008, 134, 227–236. [Google Scholar]
- Fleming, J.C.; Woo, J.; Moutasim, K.; Mellone, M.; Frampton, S.J.; Mead, A.; Woelk, C.H. HPV, tumour metabolism and novel target identification in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 356–367. [Google Scholar] [CrossRef]
- Han, M.; Lee, S.J.; Lee, D.; Kim, S.Y.; Choi, J.W. Correlation of human papilloma virus status with quantitative perfusion/diffusion/metabolic imaging parameters in the oral cavity and oropharyngeal squamous cell carcinoma: Comparison of primary tumour sites and metastatic lymph nodes. Clin. Radiol. 2018, 73, 757.e21–757.e27. [Google Scholar] [CrossRef]
- Noij, D.P.; Martens, R.M.; Zwezerijnen, B.; Koopman, T.; de Bree, R.; Hoekstra, O.S.; Castelijns, J.A. Diagnostic value of diffusion-weighted imaging and 18F-FDG-PET/CT for the detection of unknown primary head and neck cancer in patients presenting with cervical metastasis. Eur. J. Radiol. 2018, 107, 20–25. [Google Scholar] [CrossRef]
- Schouten, C.S.; Hakim, S.; Boellaard, R.; Bloemena, E.; Doornaert, P.A.; Witte, B.I.; de Bree, R. Interaction of quantitative 18F-FDG-PET-CT imaging parameters and human papillomavirus status in oropharyngeal squamous cell carcinoma. Head Neck 2016, 38, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Höhn, A.K.; Winter, K.; Sabri, O.; Purz, S. Associations between [18 F] FDG-PET and complex histopathological parameters including tumor cell count and expression of KI 67, EGFR, VEGF, HIF-1α, and p53 in head and neck squamous cell carcinoma. Mol. Imaging Biol. 2019, 21, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Ozkaya, K.; Tambas, M.; Gezer, U.; Ozgur, E.; Sahin, D.; Altun, M. Is there a correlation between serum Epstein-Barr virus DNA level and tumor metabolic activity, TNM staging and tumor load in nasopharyngeal cancer patients? J. Clin. Oncol. 2017, 35, e17543. [Google Scholar] [CrossRef]
- Alessi, A.; Lorenzoni, A.; Cavallo, A.; Padovano, B.; Iacovelli, N.A.; Bossi, P.; Alfieri, S.; Serafini, G.; Colombo, C.B.; Cicchetti, A.; et al. Role of pretreatment 18F-FDG PET/CT parameters in predicting outcome of non-endemic EBV DNA-related nasopharyngeal cancer (NPC) patients treated with IMRT and chemotherapy. La Radiologia Medica 2019, 124, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Young, R.J.; Fisher, R.; Fox, S.B.; Le, Q.T.; Peters, L.J.; Solomon, B.; Choi, J.; O’Sullivan, B.; Kenny, L.M.; et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J. Clin. Oncol. 2010, 28, 4142–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.Y.; Guo, S.Y.; Tang, L.Q.; Lu, T.Y.; Chen, B.L.; Zhong, Q.Y.; Liu, L.T. Combination of tumor volume and Epstein-Barr virus DNA improved prognostic stratification of stage II nasopharyngeal carcinoma in the intensity modulated radiotherapy era: A large-scale cohort study. Cancer Res. Treat. 2018, 50, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Li, J.; Zhao, C.; Xue, W.; Han, F.; Tao, T.; Lu, T. Prognostic efficacy of combining tumor volume with Epstein-Barr virus DNA in patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma. Oral. Oncol. 2016, 60, 18–24. [Google Scholar] [CrossRef]
- Peng, L.; Yang, Y.; Guo, R.; Mao, Y.P.; Xu, C.; Chen, Y.P.; Tang, L.L. Relationship between pretreatment concentration of plasma Epstein-Barr virus DNA and tumor burden in nasopharyngeal carcinoma: An updated interpretation. Cancer Med. 2018, 7, 5988–5998. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiao, Z.Y.; Wu, R.R. The correlation of EBV in plasma and primary tumor volume of NPC. J. Gannan Med Univ. 2010, 4, 5–11. [Google Scholar]
- Gletsou, E.; Papadas, T.A.; Baliou, E.; Tsiambas, E.; Ragos, V.; Armata, I.E.; Fotiades, P.P. HPV infection in oropharyngeal squamous cell carcinomas: Correlation with tumor size. J. BUON 2018, 23, 433–438. [Google Scholar] [PubMed]
- Liang, S.B.; Deng, Y.M.; Zhang, N.; Lu, R.L.; Zhao, H.; Chen, H.Y.; Chen, Y. Prognostic significance of maximum primary tumor diameter in nasopharyngeal carcinoma. BMC Cancer 2013, 13, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kågedal, Å.; Rydberg Millrud, C.; Häyry, V.; Kumlien Georén, S.; Lidegran, M.; Munck-Wikland, E.; Cardell, L.O. Oropharyngeal squamous cell carcinoma induces an innate systemic inflammation, affected by the size of the tumour and the lymph node spread. Clin. Otolaryngol. 2018, 43, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- De Bree, R.; Takes, R.P.; Castelijns, J.A.; Medina, J.E.; Stoeckli, S.J.; Mancuso, A.A.; Hunt, J.L.; Rodrigo, J.P.; Triantafyllou, A.; Teymoortash, A.; et al. Advances in diagnostic modalities to detect occult lymph node metastases in head and neck squamous cell carcinoma. Head Neck 2015, 37, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Z.; Cao, H.; Yan, J.; Wang, Z.; Le, H.; Weng, J.; Zhang, Y. A retrospective clinicopathological study of lung adenocarcinoma: Total tumor size can predict subtypes and lymph node involvement. Clin. Imaging 2018, 47, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Grigsby, P.W. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int. J. Radiat. Oncol. 2002, 53, 353–359. [Google Scholar] [CrossRef]
- Ouyang, M.L.; Xia, H.W.; Xu, M.M.; Lin, J.; Wang, L.L.; Zheng, X.W.; Tang, K. Prediction of occult lymph node metastasis using SUV, volumetric parameters and intratumoral heterogeneity of the primary tumor in T1-2N0M0 lung cancer patients staged by PET/CT. Ann. Nucl. Med. 2019, 33, 671–680. [Google Scholar] [CrossRef]
- Kim, S.J.; Pak, K.; Chang, S. Determination of regional lymph node status using 18F-FDG PET/CT parameters in oesophageal cancer patients: Comparison of SUV, volumetric parameters and intratumoral heterogeneity. Br. J. Radiol. 2016, 89. [Google Scholar] [CrossRef] [Green Version]
- Raman, A.; Sen, N.; Ritz, E.; Fidler, M.J.; Revenaugh, P.; Stenson, K.; Al-khudari, S. Heterogeneity in the clinical presentation, diagnosis, and treatment initiation of p16-positive oropharyngeal cancer. Am. J. Otol. 2019, 40, 626–630. [Google Scholar] [CrossRef]
- Mirzamani, N.; Salehian, P.; Farhadi, M.; Amin Tehran, E. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp. Mol. Pathol. 2007, 81, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Niedobitek, G. Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Mol. Pathol. 2000, 53, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Fujimoto, Y.; Ozawa, H.; Bun, A.; Fukui, R.; Miyagawa, Y.; Imamura, M.; Kitajima, K.; Yamakado, K.; Miyoshi, Y. Significance of Metabolic Tumor Volume at Baseline and Reduction of Mean Standardized Uptake Value in 18 F-FDG-PET/CT Imaging for Predicting Pathological Complete Response in Breast Cancers Treated with Preoperative Chemotherapy. Ann. Surg. Oncol. 2019, 26, 2175–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śnietura, M.; Jaworska, M.; Pigłowski, W.; Goraj-Zając, A.; Woźniak, G.; Lange, D. High-risk HPV DNA status and p16 (INK4a) expression as prognostic markers in patients with squamous cell cancer of oral cavity and oropharynx. Pol. J. Pathol. 2010, 61, 133–139. [Google Scholar] [PubMed]
- Begum, S.; Gillison, M.L.; Ansari-Lari, M.A.; Shah, K.; Westra, W.H. Detection of human papillomavirus in cervical lymph nodes: A highly effective strategy for localizing site of tumor origin. Clin. Cancer Res. 2003, 9, 6469–6475. [Google Scholar] [PubMed]
- Guzińska-Ustymowicz, K.; Pryczynicz, A.; Kemona, A.; Czyżewska, J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009, 29, 3049–3052. [Google Scholar]
- Altman, D.G.; Bland, J.M. Diagnostic tests. 1: Sensitivity and specificity. BMJ Br. Med. J. 1994, 308, 1552. [Google Scholar] [CrossRef] [Green Version]
| No. of pts. | Localization of Primary Tumor | TNM Stage | Ki67 Index [%] | p16 Status | HPV Status | EBV Status | Smoking | Biopsy before PET/MR (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | OT | T2 N1 M0 | 30 | 0 | 0 | 0 | 1 | 28 |
| 2 | BM | T2 N1 M0 | 30 | 0 | 0 | 0 | 0 | 25 |
| 3 | LG | T3 N2b M0 | 20 | 0 | 0 | 0 | 1 | 21 |
| 4 | BoT | T2 N0 M0 | 20 | 0 | 0 | 0 | 1 | 20 |
| 5 | BoT | T3 N2c M0 | 40 | 1 | 0 | 0 | 0 | 14 |
| 6 | BoT | T2 N2c M0 | 30 | 0 | 0 | 0 | 1 | 18 |
| 7 | OT | T1 N1 M0 | 50 | 1 | 1 | 1 | 0 | 14 |
| 8 | FoM | T2 N2c M0 | 30 | 1 | 1 | 0 | 1 | 21 |
| 9 | FoM | T1 N2b M0 | 20 | 0 | 0 | 0 | 0 | 18 |
| 10 | OT | T2 N1 M0 | 40 | 1 | 1 | 0 | 0 | 16 |
| 11 | MAR | T3 N0 M0 | 40 | 1 | 1 | 0 | 0 | 23 |
| 12 | FoM | T3 N0 M0 | 20 | 0 | 0 | 0 | 1 | 20 |
| 13 | FoM | T2 N2b M0 | 50 | 1 | 0 | 1 | 1 | 30 |
| 14 | LG | T2 N0 M0 | 60 | 1 | 0 | 0 | 0 | 14 |
| 15 | SSG | T3 N0 M0 | 30 | 0 | 0 | 0 | 0 | 15 |
| 16 | OT | T1 N0 M0 | 30 | 0 | 0 | 0 | 0 | 15 |
| 17 | OT | T3 N2b M0 | 30 | 0 | 0 | 0 | 1 | 21 |
| 18 | FoM | T1 N0 M0 | 20 | 0 | 0 | 0 | 0 | 17 |
| 19 | BM | T3 N2b M0 | 30 | 0 | 0 | 1 | 1 | 18 |
| 20 | BoT | T1 N0 M0 | 20 | 0 | 0 | 0 | 0 | 28 |
| 21 | FoM | T4a N2c M0 | 50 | 1 | 0 | 0 | 1 | 24 |
| No of pts | SUVmax [g/mL] | SUVmean [g/mL] | Maximal Tumor Diameter CT [mm] | Maximal Tumor Diameter PET/MR [mm] | Tumor Volume PET/MR [cm3] | Tumor Volume CT [cm3] | No of mts Lymph Nodes in CT | No of mts Lymph Nodes in PET/MR | CRP [mg/L] | Glucose [mg/dL] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11.7 | 6.03 | 34 | 33 | 12.63 | 6.65 | 0 | 1 | 1.9 | 92 |
| 2 | 11.9 | 7.68 | 15 | 21 | 9.62 | 11.24 | 2≤ | 0 | 0.5 | 110 |
| 3 | 9.25 | 7.96 | 45 | 53 | 18.23 | 13.97 | 2≤ | 2≤ | 3.1 | 99 |
| 4 | 12.1 | 7.09 | 27 | 26 | 7.76 | 4.35 | 0 | 1 | 9.1 | 139 |
| 5 | 15.2 | 10.1 | 50 | 55 | 55.48 | 51.26 | 2≤ | 2≤ | 1.1 | 88 |
| 6 | 9.26 | 5.09 | 28 | 13 | 13.38 | 19.51 | 2≤ | 2≤ | 0.9 | 99 |
| 7 | 10.1 | 6.7 | 24 | 19 | 9.76 | 4.78 | 0 | 1 | 0.4 | 93 |
| 8 | 7.08 | 5.1 | 51 | 38 | 78.35 | 32.12 | 2≤ | 2≤ | 1.1 | 117 |
| 9 | 15.3 | 13.3 | 10 | 48 | 24.27 | 15.06 | 0 | 2≤ | 4.2 | 122 |
| 10 | 5.59 | 5.35 | 25 | 23 | 10.31 | 5.09 | 0 | 1 | 1.2 | 104 |
| 11 | 7.59 | 8.84 | 35 | 38 | 41.97 | 21.82 | 2≤ | 2≤ | 44.4 | 91 |
| 12 | 10 | 7.65 | 41 | 43 | 36.2 | 35.65 | 0 | 0 | 0.9 | 83 |
| 13 | 3.62 | 3.64 | 18 | 15 | 6.91 | 6.07 | 2≤ | 2≤ | 0.7 | 92 |
| 14 | 13.9 | 10.7 | 18 | 42 | 13.79 | 5.89 | 2≤ | 1 | 3.6 | 118 |
| 15 | 12.3 | 12.5 | 39 | 49 | 47.08 | 64.8 | 1 | 0 | 0.3 | 93 |
| 16 | 6.11 | 2.61 | 25 | 21 | 7.16 | 4.08 | 0 | 0 | 16 | 85 |
| 17 | 8.9 | 7.26 | 0 | 46 | 28.85 | 26.4 | 2≤ | 2≤ | 120.5 | 98 |
| 18 | 7.59 | 5.43 | 19 | 20 | 8.71 | 5.66 | 0 | 0 | 17.4 | 122 |
| 19 | 18.2 | 12.5 | 39 | 50 | 24.37 | 21.34 | 2≤ | 2≤ | 2.1 | 104 |
| 20 | 2.5 | 2.6 | 20 | 16 | 3.28 | 4.12 | 0 | 0 | 0.6 | 87 |
| 21 | 6.9 | 8.41 | 60 | 63 | 64.14 | 62.03 | 1 | 0 | 5.1 | 101 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samolyk-Kogaczewska, N.; Sierko, E.; Dziemianczyk-Pakiela, D.; Nowaszewska, K.B.; Lukasik, M.; Reszec, J. Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients. Cancers 2020, 12, 511. https://doi.org/10.3390/cancers12020511
Samolyk-Kogaczewska N, Sierko E, Dziemianczyk-Pakiela D, Nowaszewska KB, Lukasik M, Reszec J. Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients. Cancers. 2020; 12(2):511. https://doi.org/10.3390/cancers12020511
Chicago/Turabian StyleSamolyk-Kogaczewska, Natalia, Ewa Sierko, Dorota Dziemianczyk-Pakiela, Klaudia Beata Nowaszewska, Malgorzata Lukasik, and Joanna Reszec. 2020. "Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients" Cancers 12, no. 2: 511. https://doi.org/10.3390/cancers12020511
APA StyleSamolyk-Kogaczewska, N., Sierko, E., Dziemianczyk-Pakiela, D., Nowaszewska, K. B., Lukasik, M., & Reszec, J. (2020). Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients. Cancers, 12(2), 511. https://doi.org/10.3390/cancers12020511





