Efficacy of Crizotinib, Ceritinib, and Alectinib in ALK-Positive Non-Small Cell Lung Cancer Treatment: A Meta-Analysis of Clinical Trials
Abstract
:1. Introduction
2. Results
2.1. Selection of Relevant Studies
2.2. General Characteristics of Studies
2.3. Risk of Bias for Randomized, Double-Blind, Placebo-Controlled Trials
2.4. Efficacy of ALK Inhibitors in Patients with ALK-Positive NSCLC by Type of Outcomes and Type of ALK Inhibitors
2.5. Efficacy of ALK Inhibitors Compared with Chemotherapy in Patients with ALK-Positive NSCLC by Type of Outcomes and Type of ALK Inhibitors
3. Discussion
3.1. Summary of Findings
3.2. Comparison with Previous Studies
3.3. Possible Mechanisms
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Literature Search
4.2. Study Selection and Eligibility Criteria
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, L.; Lin, Z.; Cao, Y.; Chen, Y.; Xu, Z.; Qin, Z. Targeting EIF4F complex in non-small cell lung cancer cells. Oncotarget 2017, 8, 55731–55735. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, G.M.; Karuri, S.W.; Zhang, H.; Zhang, L.; Khozin, S.; Kazandjian, D.; Tang, S.; Sridhara, R.; Keegan, P.; Pazdur, R. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J. Clin. Oncol. 2015, 33, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Kanteti, R.; El-Hashani, E.; Dhanasingh, I.; Tretiakova, M.; Husain, A.N.; Sharma, S.; Sharma, J.; Vokes, E.E.; Salgia, R. Role of PAX8 in the regulation of MET and RON receptor tyrosine kinases in non-small cell lung cancer. BMC Cancer 2014, 14, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, C.C.; Kelly, J.J. Development of crizotinib, a rationally designed tyrosine kinase inhibitor for non-small cell lung cancer. Int. J. Cancer 2017, 140, 1945–1954. [Google Scholar] [CrossRef] [Green Version]
- Bendaly, E.; Dalal, A.A.; Culver, K.; Galebach, P.; Bocharova, I.; Foster, R.; Sasane, M.; Macalalad, A.R.; Guerin, A. Treatment Patterns and Early Outcomes of ALK-Positive Non-Small Cell Lung Cancer Patients Receiving Ceritinib: A Chart Review Study. Adv. Ther. 2017, 34, 1145–1156. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann-La, R. Alecensa (Alectinib): Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf (accessed on 4 July 2019).
- Norvatis. Zykadia (Ceritinib): Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205755s003s004lbl.pdf (accessed on 4 July 2019).
- Camidge, D.R.; Bang, Y.J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.; Ou, S.H.I.; Kim, D.W.; et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Crino, L.; Ahn, M.J.; De Marinis, F.; Groen, H.J.; Wakelee, H.; Hida, T.; Mok, T.; Spigel, D.; Felip, E.; Nishio, M.; et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND-2. J. Clin. Oncol. 2016, 34, 2866–2873. [Google Scholar] [CrossRef]
- Cui, S.; Zhao, Y.; Gu, A.; Ge, X.; Song, Y.; Zhang, W.; Lou, Y.; Dong, L.; Han, B.; Jiang, L. Efficacy and tolerability of crizotinib in the treatment of ALK-positive, advanced non-small cell lung cancer in Chinese patients. Med. Oncol. 2015, 32, 626. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Hoffmann-La, R. A Study of RO5424802 in Patients with Non-Small Cell Lung Cancer Who Have ALK Mutation and Failed Crizotinib Treatment. Available online: https://ClinicalTrials.gov/show/NCT01801111 (accessed on 19 September 2018).
- Iwama, E.; Goto, Y.; Murakami, H.; Harada, T.; Tsumura, S.; Sakashita, H.; Mori, Y.; Nakagaki, N.; Fujita, Y.; Seike, M.; et al. Alectinib for patients with ALK rearrangement–positive non–small cell lung cancer and a poor performance status (Lung Oncology Group in Kyushu 1401). J. Thorac. Oncol. 2017, 12, 1161–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; Riely, G.J.; et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016, 17, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N. Eng. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Pfizer. An Investigational Drug, PF-02341066 Is Being Studied versus Standard of Care in Patients with Advanced Non-Small Cell Lung Cancer with a Specific Gene Profile Involving the Anaplastic Lymphoma Kinase (ALK) Gene. Available online: https://ClinicalTrials.gov/show/NCT00932893 (accessed on 19 September 2018).
- Pfizer. A Clinical Trial Testing the Efficacy of Crizotinib versus Standard Chemotherapy Pemetrexed Plus Cisplatin or Carboplatin in Patients with ALK Positive Non Squamous Cancer of the Lung. Available online: https://ClinicalTrials.gov/show/NCT01154140 (accessed on 19 September 2018).
- Pfizer. A Study of Crizotinib versus Chemotherapy in Previously Untreated ALK Positive East Asian Non-Small Cell Lung Cancer Patients. Available online: https://ClinicalTrials.gov/show/NCT01639001 (accessed on 19 September 2018).
- Pfizer. An Investigational Drug, PF-02341066, Is Being Studied in Patients with Advanced Non-Small Cell Lung Cancer with a Specific Gene Profile Involving the Anaplastic Lymphoma Kinase (ALK) Gene. Available online: https://ClinicalTrials.gov/show/NCT00932451 (accessed on 19 September 2018).
- Shaw, A.; Kim, T.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus Chemotherapy in Patients with ALK-Rearranged Non-Small-Cell Lung Cancer Previously Given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Shaw, A.T.; Gandhi, L.; Gadgeel, S.; Riely, G.J.; Cetnar, J.; West, H.; Camidge, D.R.; Socinski, M.A.; Chiappori, A.; Mekhail, T.; et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016, 17, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Soria, J.-C.; Tan, D.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Wu, X.; Li, J. Therapeutic effects of crizotinib in EML4-ALK-positive patients with non-small-cell lung cancer. J. South. Med Univ. 2015, 35, 753–757. [Google Scholar]
- Yang, J.; Lei, Y.; Zhang, X.; Zhou, Q.; Yan, H.H.; Chen, H.J.; Tu, H.; Wang, Z.; Xu, C.; Su, J.; et al. First-line versus second or further-line crizotinib for trial patients with advanced non-small-cell lung cancer harboring ALK rearrangements. J. Clin. Oncol. 2015, 33, e19139. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, K.; Zhang, L.Y.; Wang, H. Clinical efficacy of crizotinib in advanced ALK positive non-small cell lung cancer. Chin. J. Lung Cancer 2015, 18, 616–620. [Google Scholar] [CrossRef]
- Hida, T.; Seto, T.; Horinouchi, H.; Maemondo, M.; Takeda, M.; Hotta, K.; Hirai, F.; Kim, Y.H.; Matsumoto, S.; Ito, M.; et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci. 2018, 109, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-La, R. Alectinib versus Pemetrexed or Docetaxel in Anaplastic Lymphoma Kinase (ALK)-Positive Advanced Non-Small Cell Lung Cancer (NSCLC) Participants Previously Treated with Platinum-Based Chemotherapy and Crizotinib. Available online: https://ClinicalTrials.gov/show/NCT02604342 (accessed on 19 September 2018).
- Fan, J.; Fong, T.; Xia, Z.; Zhang, J.; Luo, P. The efficacy and safety of ALK inhibitors in the treatment of ALK-positive non-small cell lung cancer: A network meta-analysis. Cancer Med. 2018, 7, 4993–5005. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xia, Z.; Zhang, X.; Chen, Y.; Qian, R.; Liu, S.; You, D.; Zhang, J.; Luo, P. The efficacy and safety of alectinib in the treatment of ALK+ NSCLC: A systematic review and meta-analysis. Onco. Targets Ther. 2018, 11, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.; Hsieh, C.C.; Lee, Y.L.; Li, C.Y. Which should be used first for ALK-positive non-small-cell lung cancer: Chemotherapy or targeted therapy? A meta-analysis of five randomized trials. Medicina (Kaunas) 2019, 55, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Dai, W.R.; Shao, F.C. Effect of ALK-inhibitors in the treatment of non-small cell lung cancer: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3496–3503. [Google Scholar]
- El Din, A.M.M.; Rasmy, A.A. Efficacy and safety of crizotinib in patients with anaplastic lymphoma kinase-positive stage IV non-small cell lung cancer. J. Clin. Oncol. 2017, 35, e20516. [Google Scholar] [CrossRef]
- DiBonaventura, M.; Higginbottom, K.; Meyers, A.; Morimoto, Y.; Ilacqua, J. Comparative effectiveness of crizotinib among ALK+ NSCLC patients across the United States, Western Europe, and Japan. Value Health 2016, 19, A711. [Google Scholar] [CrossRef]
- Galetta, D.; Rossi, A.; Pisconti, S.; Colucci, G. The emerging role of ALK inhibitors in the treatment of advanced non-small cell lung cancer. Expert Opin. Ther. Targets 2012, 16 (Suppl. S2), S45–S54. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Crizotinib resistance: Implications for therapeutic strategies. Ann. Oncol. 2016, 27 (Suppl. S3), iii42–iii50. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.G.; Russo, A.; Scilla, K.A.; Mehra, R.; Rolfo, C. How I treat ALK-positive non-small cell lung cancer. ESMO Open 2019, 4, e000524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: http://www.handbook.cochrane.org (accessed on 4 July 2019).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothenstein, J.M.; Chooback, N. ALK inhibitors, resistance development, clinical trials. Curr. Oncol. 2018, 25, S59–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, D.; Kay, A.; Berg, W.; Baladi, J.F.; Zheng, J. Progression-free survival: Gaining on overall survival as a gold standard and accelerating drug development. Cancer J. 2009, 15, 386–394. [Google Scholar] [CrossRef]
- Macaskill, P.; Walter, S.D.; Irwig, L. A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 2001, 20, 641–654. [Google Scholar] [CrossRef]



| Study | Enrollment Period | Regimen | No. pts | OS (95% CI) (Months) | PFS (95% CI) (Months) | ORR (%) | DCR (%) | 1-Year Survival Rate | 2-Year Survival Rate |
|---|---|---|---|---|---|---|---|---|---|
| Single-arm study | |||||||||
| Camidge 2012 [10] (PROFILE 1001) | 08/2008–06/2011 (US, Australia, South Korea) | Crizotinib | 143 | - | 9.7 (7.7–12.8) | 60.8 | 82.5 | 74.8 | - |
| Cui 2015 [12] | 06/2013–10/2014 (China) | Crizotinib | 67 | - | 10.3 (8.6–12.0) | 52.2 | 64.2 | 77.6 | - |
| Yang 2015 [26] | 12/2010–08/2014 (China) | Crizotinib | 22 46 | - | 13.8 (7.6–19.9) 7.0 (3.8–10.2) | 81.8 69.6 | - | - | 65.0 50.0 |
| Crino 2016 [11] (ASCEND-2) | 12/2012–09/2013 (51 global sites) | Ceritinib | 140 | 15.6 (13.6–24.2) | 5.8 (5.4–7.6) | 38.6 | 77.1 | 63.8 | - |
| Kim 2016 [16] (ASCEND-1) | 01/2011–07/2013 (11 countries) | Ceritinib | 83 163 | - | - 6.9 (5.6–8.7) | 72 56 | - | 83 67 | - |
| Shaw 2016 [23] (NCT01871805) | 09/2013–08/2014 (US, Canada) | Alectinib | 87 | - | 8.1 (6.2–12.6) | 52.9 | 66.7 | 71 | - |
| Iwana 2017 [15] | 09/2014–12/2015 (Japan) | Alectinib | 18 | - | 10.1 (7.1–17.8) | 72.2 | 77.8 | - | - |
| Hida 2018 [28] (ASCEND-9) | 08/2015–03/2017 (Japan) | Ceritinib | 20 | - | 3.7 (1.9–5.3) | 25 | - | - | - |
| NCT00932451 [21] (PROFILE 1005) | 01/2010–03/2015 (21 countries) | Crizotinib | 908 158 | 21.8 (19.4–24.0) 16.9 (13.4–21.5) | 8.4 (7.1–9.7) 6.9 (5.6–9.4) | 54.1 40.5 | 70.8 61.4 | 66.5 62.4 | - |
| NCT01801111 [14] | 06/2013–10/2014 (16 countries) | Alectinib | 138 | - | 9.1 (7.4–11.2) | 47.8 | 68.8 | - | - |
| Double-arm study | |||||||||
| Wu 2015 [25] | 06/2010–11/2014 (China) | Crizotinib vs. Pemetrexed/docetaxel/gemcitabine/paclitaxel + platinum | 21 21 | - | - | 61.9 28.6 | - | - | - |
| Zhao 2015 [27] | 01/2012–12/2013 (China) | Crizotinib vs. Dexamethasone + docetaxel | 14 14 | - | - | 64.3 21.4 | - | - | - |
| Hida 2017 [13] (J-ALEX) | 11/2014–08/2015 (Japan) | Alectinib vs. Crizotinib | 103 104 | - | - 10.2 (8.2–12.0) | 85 70 | 98.1 88.5 | - | - |
| Peters 2017 [17] (ALEX) | 08/2014–01/2016 (98 global sites) | Alectinib vs. Crizotinib | 152 151 | - | - 10.4 (7.7–14.6) | 82.9 75.5 | - | 84.3 82.5 | - |
| Shaw 2017 [22] (ASCEND-5) | 06/2013–11/2015 (20 countries) | Ceritinib vs. Pemetrexed/docetaxel | 115 116 | 18.1 (13.4–23.9) 20.1 (11.9–25.1) | 5.4 (4.1–6.9) 1.6 (1.4–2.8) | 39.1 6.9 | 76.5 36.3 | - | - |
| Soria 2017 [24] (ASCEND-4) | 08/2013–05/2015 (28 countries) | Ceritinib vs. Cisplatin/carboplatin | 189 187 | - | 16.6 (12.6–27.2) 8.1 (5.8–11.1) | 72.5 26.7 | - | - | 70.6 58.2 |
| NCT00932893 [18] (PROFILE 1007) | 09/2009–03/2012 (22 countries) | Crizotinib vs. Pemetrexed/docetaxel | 173 174 | 21.7 (18.9–30.5) 21.9 (16.8–26.0) | 7.7 (6.0–8.8) 3.0 (2.6–4.3) | 65.3 19.5 | 64.2 38.5 | 70.4 66.7 | - |
| NCT01154140 [19] (PROFILE 1014) | 01/2011–11/2013 (31 countries) | Crizotinib vs. Pemetrexed + cisplatin/carboplatin | 172 171 | - | 10.9 (8.3–13.9) 7.0 (6.8–8.2) | 74.4 45.0 | 78.5 68.4 | 83.5 78.4 | - |
| NCT01639001 [20] | 09/2012–06/2015 (5 Asia countries) | Crizotinib vs. Pemetrexed + cisplatin/carboplatin | 104 103 | - | 11.1 (8.3–12.6) 6.8 (5.7–7.0) | 87.5 45.6 | 82.7 73.8 | 79.3 79.5 | - |
| NCT02604342 [29] | 11/2015–01/2017 (15 countries) | Alectinib vs. Pemetrexed/docetaxel | 72 35 | - - | 9.6 (6.9–12.2) 1.4 (1.3–1.6) | 37.5 2.9 | 80.6 28.6 | - - | - - |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | No. of Low Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Wu 2015 [25] | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | 2 |
| Zhao 2015 [27] | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | 2 |
| Hida 2017 [13] (J-ALEX) | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| Peters 2017 [17] (ALEX) | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| Shaw 2017 [22] (ASCEND-5) | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| Soria 2017 [24] (ASCEND-4) | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| NCT00932893 [18] (PROFILE 1007) | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | 6 |
| NCT01154140 [19] (PROFILE 1014) | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| NCT01639001 [20] | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| NCT02604342 [29] | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | 4 |
| Outcome | No. of Groups | Period/Rate (95% CI) | I2 (%) |
|---|---|---|---|
| Time period (months) | |||
| OS [11,18,21,22] | 5 | 19.14 (16.42–21.85) | 50.5 |
| Crizotinib [18,21] | 3 | 20.22 (16.94–23.50) | 54.3 |
| Ceritinib [11,22] | 2 | 16.86 (13.13–20.59) | 0.0 |
| PFS (months) [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,26,28,29] | 20 | 8.47 (7.43–9.52) | 80.1 |
| Crizotinib [10,12,13,17,18,19,20,21,26] | 11 | 9.27 (8.28–10.26) | 56.1 |
| Ceritinib [11,16,22,24,28] | 5 | 5.92 (4.36–7.48) | 75.6 |
| Alectinib [14,15,23,29] | 4 | 9.12 (7.77–10.46) | 0.0 |
| Rate (%) | |||
| ORR [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] | 25 | 62 (56–68) | 93.4 |
| Crizotinib [10,12,13,17,18,19,20,21,25,26,27] | 13 | 66 (58–74) | 92.2 |
| Ceritinib [11,16,22,24,28] | 6 | 52 (38–66) | 93.3 |
| Alectinib [13,14,15,17,23,29] | 6 | 63 (46–80) | 95.4 |
| DCR [10,11,12,13,14,15,18,19,20,21,22,23,25,28,29] [10,11,12,13,14,15,18,19,20,21,22,23,25,28,29] | 16 | 78 (71–84) | 94.8 |
| Crizotinib [10,12,13,18,19,20,21,25] | 8 | 78 (71–85) | 90.9 |
| Ceritinib [11,22,28] | 3 | 76 (71–81) | 0.0 |
| Alectinib [13,14,15,23,29] | 5 | 79 (63–95) | 95.4 |
| 1-year survival rate [10,11,12,16,17,18,19,20,21,23] | 13 | 74 (70–79) | 85.3 |
| Crizotinib [10,12,17,18,19,20,21] | 8 | 75 (69–81) | 86.7 |
| Ceritinib [11,16] | 3 | 71 (60–83) | 85.0 |
| Alectinib [17,23] | 2 | 81 (76–86) | 0.0 |
| 2-year survival rate [24,26] | 3 | 62 (49–76) | 69.0 |
| Crizotinib [26] | 2 | 55 (43–66) | 0.0 |
| Ceritinib [24] | 1 | 70 (64–76) | NA |
| Outcome | No of Groups | Effect size (95% CI) | I2 (%) |
|---|---|---|---|
| Effect size: Hazard ratio | |||
| OS [18,19,20,22,24] | 5 | 0.83 (0.72–0.97) | 0.0 |
| Crizotinib [18,19,20] | 3 | 0.83 (0.69–1.00) | 0.0 |
| Ceritinib [22,24] | 2 | 0.85 (0.62–1.16) | 19.1 |
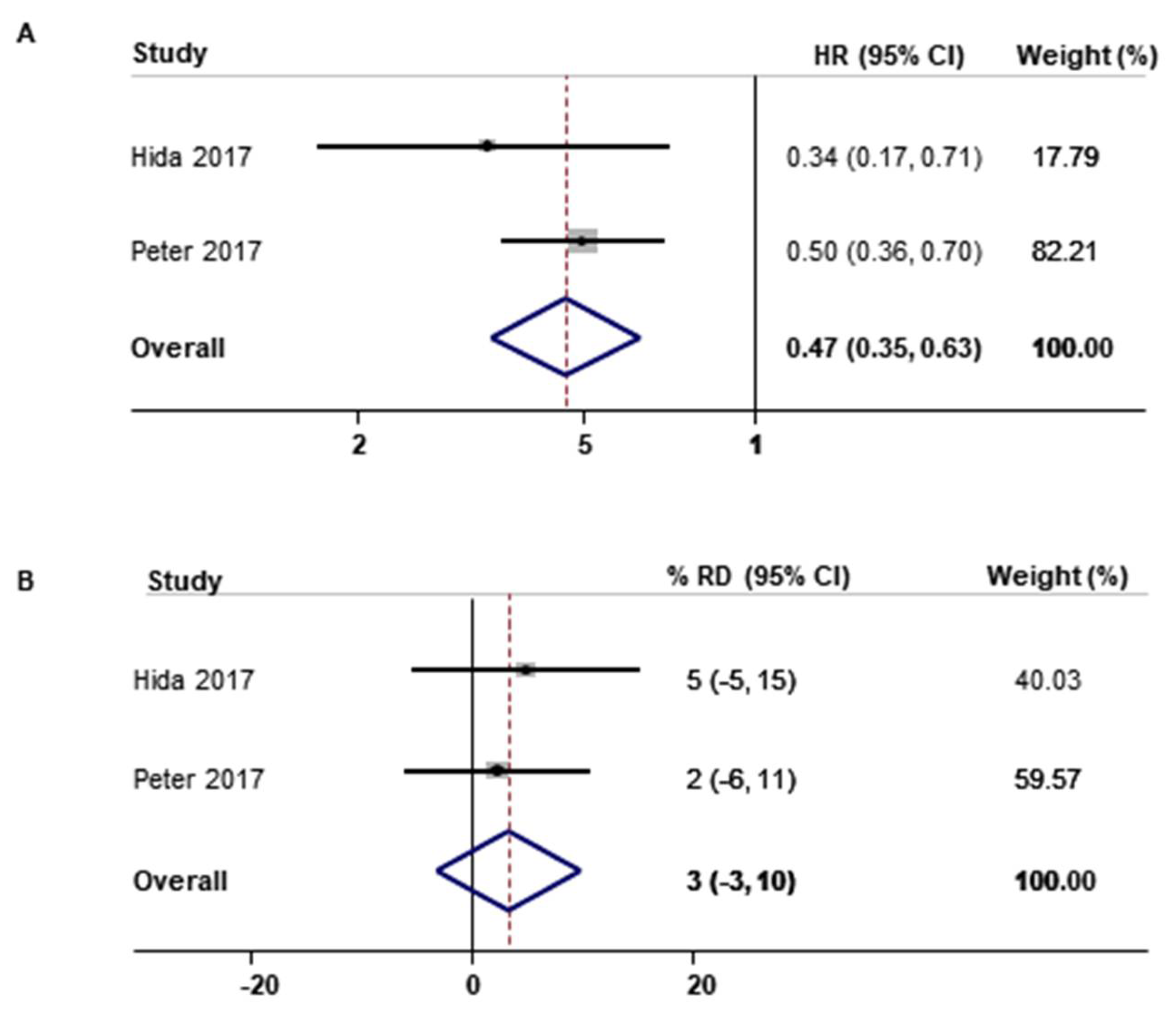
| PFS [18,19,20,22,24,29] | 6 | 0.43 (0.35–0.54) | 64.7 |
| Crizotinib [18,19,20] | 3 | 0.45 (0.38–0.54) | 0.0 |
| Ceritinib [22,24] | 2 | 0.52 (0.43–0.64) | 0.0 |
| Alectinib [29] | 1 | 0.15 (0.08–0.29) | NA |
| Effect size: Rate difference (%) | |||
| ORR [18,19,20,22,24,25,27,29] | 8 | 23 (17–29) | 52.7 |
| Crizotinib [18,19,20,25,27] | 5 | 19 (12–26) | 36.5 |
| Ceritinib [22,24] | 2 | 28 (16–40) | 65.4 |
| Alectinib [29] | 1 | 29 (18–40) | NA |
| DCR [18,19,20,22,25,29] | 6 | 10 (4–16) | 44.8 |
| Crizotinib [18,19,20,25] | 4 | 6 (1–11) | 0.0 |
| Ceritinib [22] | 1 | 18 (08–28) | NA |
| Alectinib [29] | 1 | 18 (06–30) | NA |
| 1-year survival rate [18,19,20] | |||
| Crizotinib [18,19,20] | 3 | 1 (−4, 6) | 0.0 |
| 2-year survival rate [22] | |||
| Ceritinib [22] | 1 | 5 (−3, 13) | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.; Myung, S.-K.; Pham, T.T.; Park, B. Efficacy of Crizotinib, Ceritinib, and Alectinib in ALK-Positive Non-Small Cell Lung Cancer Treatment: A Meta-Analysis of Clinical Trials. Cancers 2020, 12, 526. https://doi.org/10.3390/cancers12030526
Hoang T, Myung S-K, Pham TT, Park B. Efficacy of Crizotinib, Ceritinib, and Alectinib in ALK-Positive Non-Small Cell Lung Cancer Treatment: A Meta-Analysis of Clinical Trials. Cancers. 2020; 12(3):526. https://doi.org/10.3390/cancers12030526
Chicago/Turabian StyleHoang, Tung, Seung-Kwon Myung, Thu Thi Pham, and Boyoung Park. 2020. "Efficacy of Crizotinib, Ceritinib, and Alectinib in ALK-Positive Non-Small Cell Lung Cancer Treatment: A Meta-Analysis of Clinical Trials" Cancers 12, no. 3: 526. https://doi.org/10.3390/cancers12030526






