Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update
Abstract
1. Introduction
2. Embolization Techniques
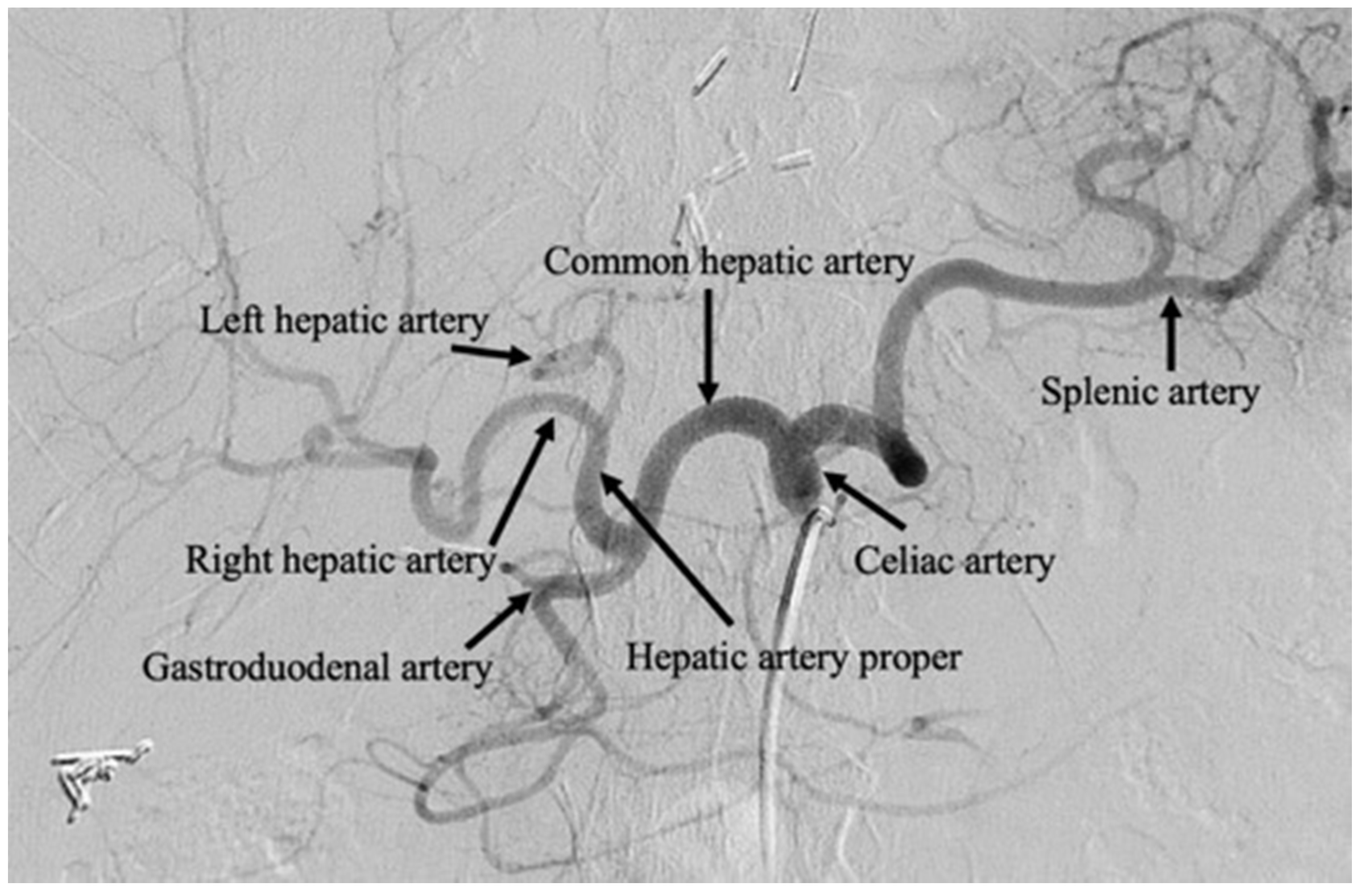
2.1. Technical Overview
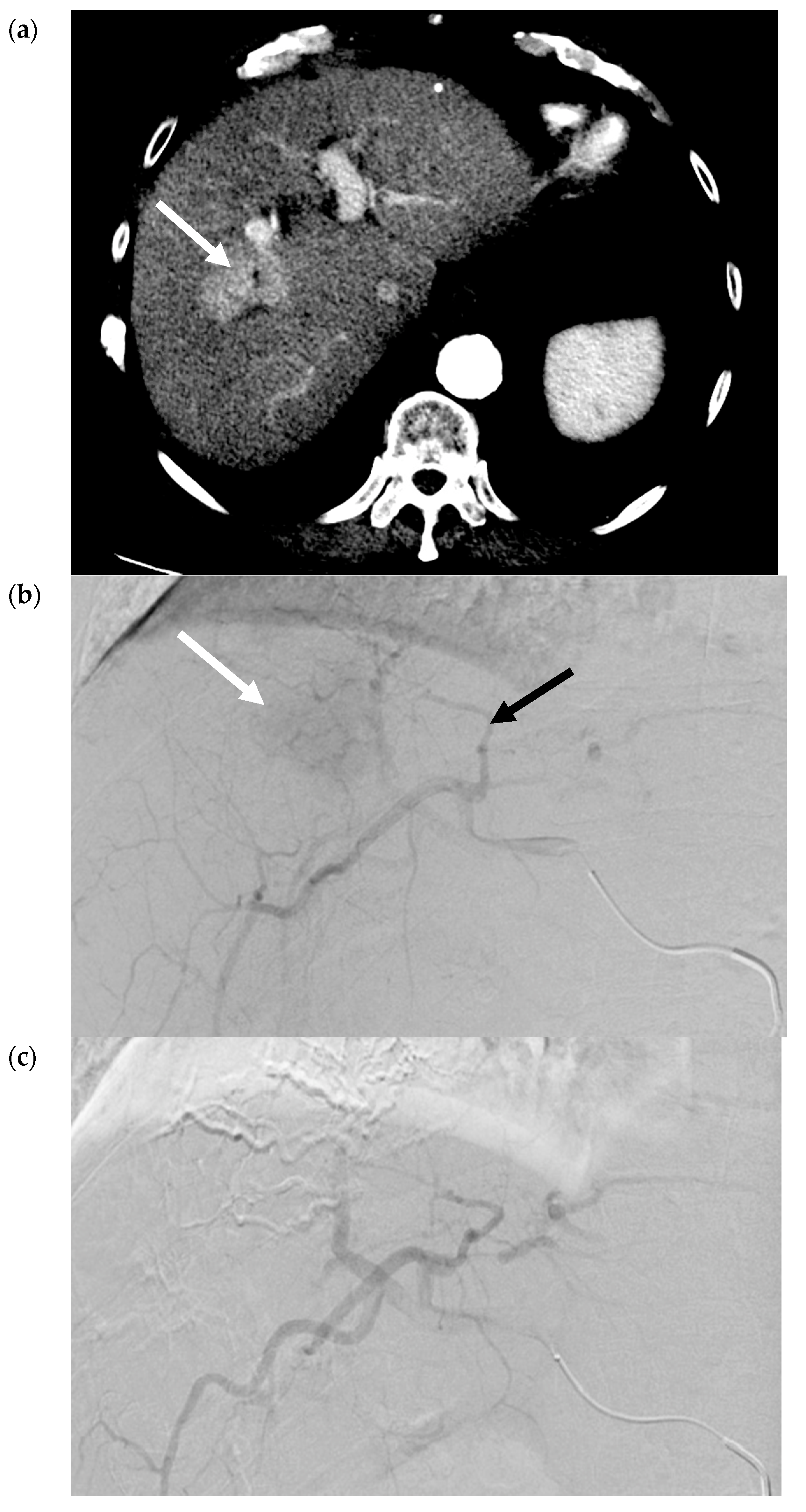
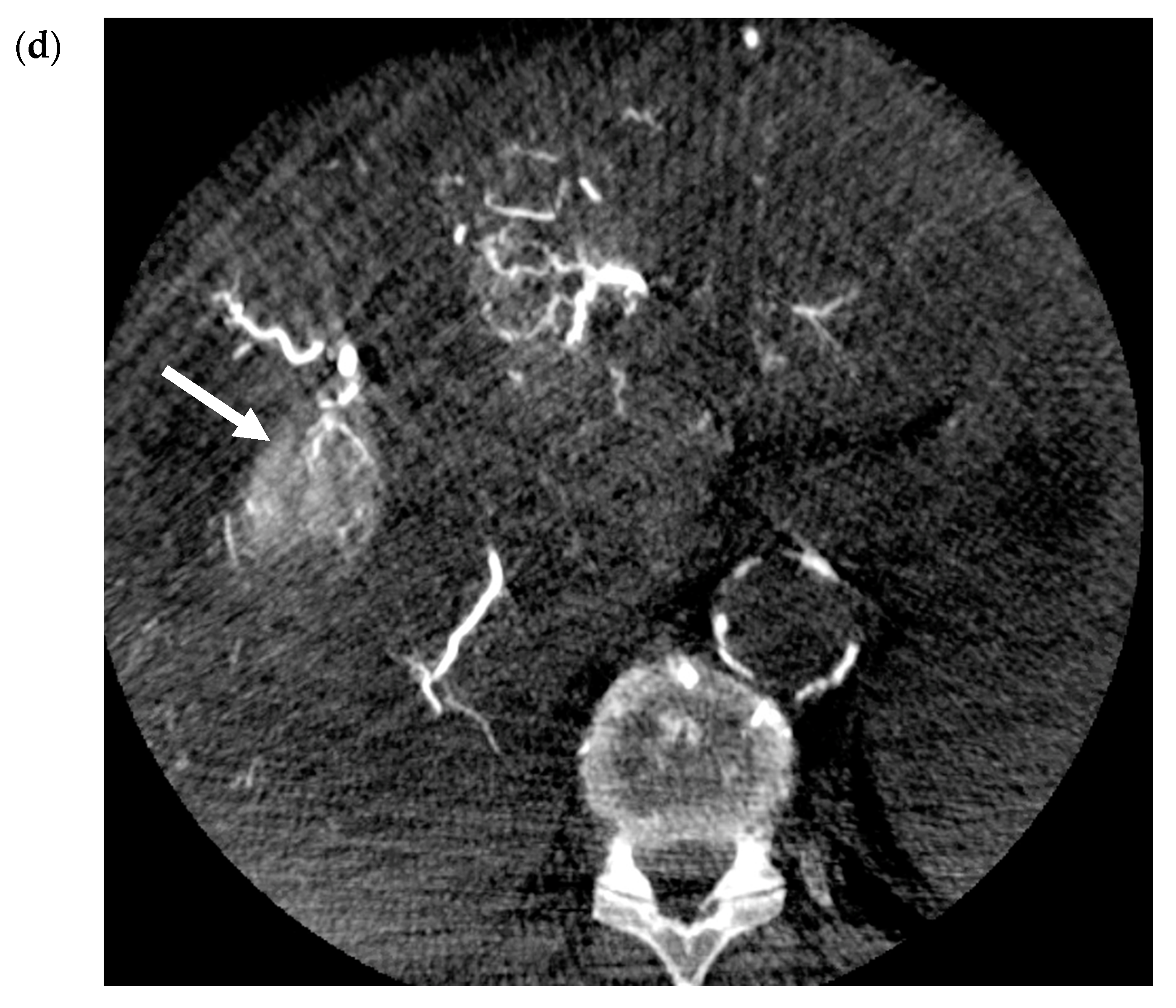
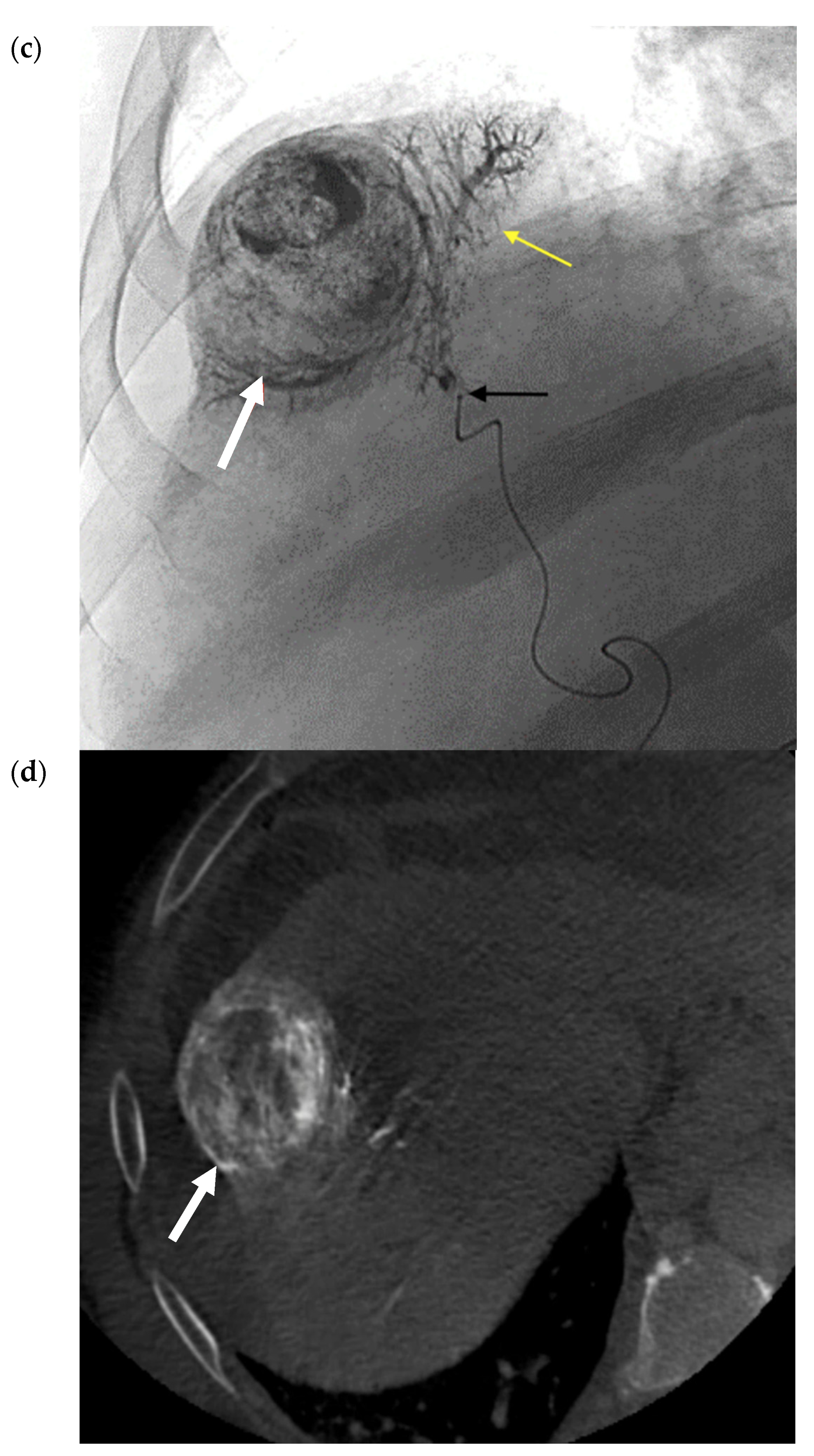
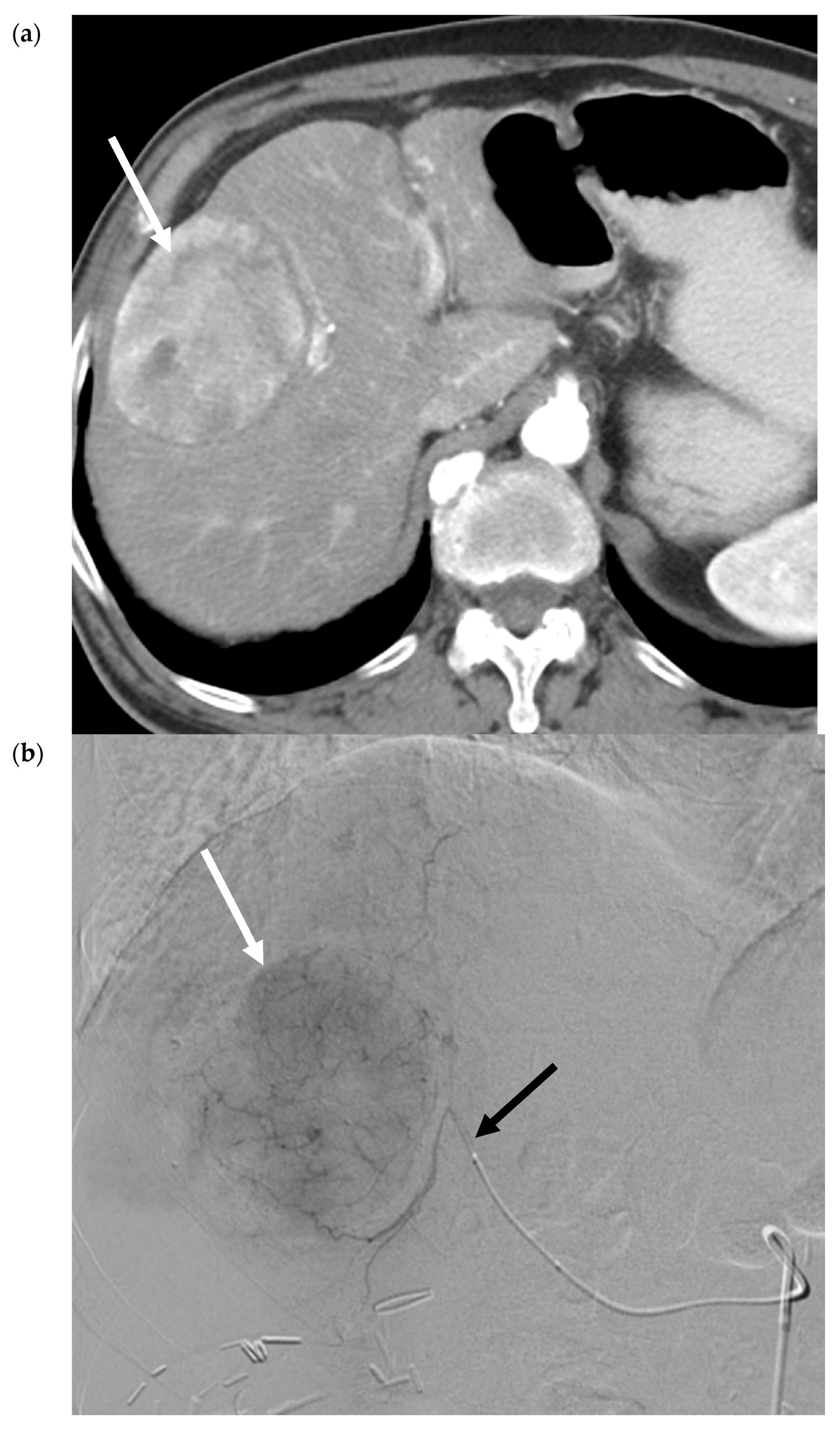
2.2. Transarterial Embolization (TAE)
2.3. Transarterial Chemoembolization (TACE)
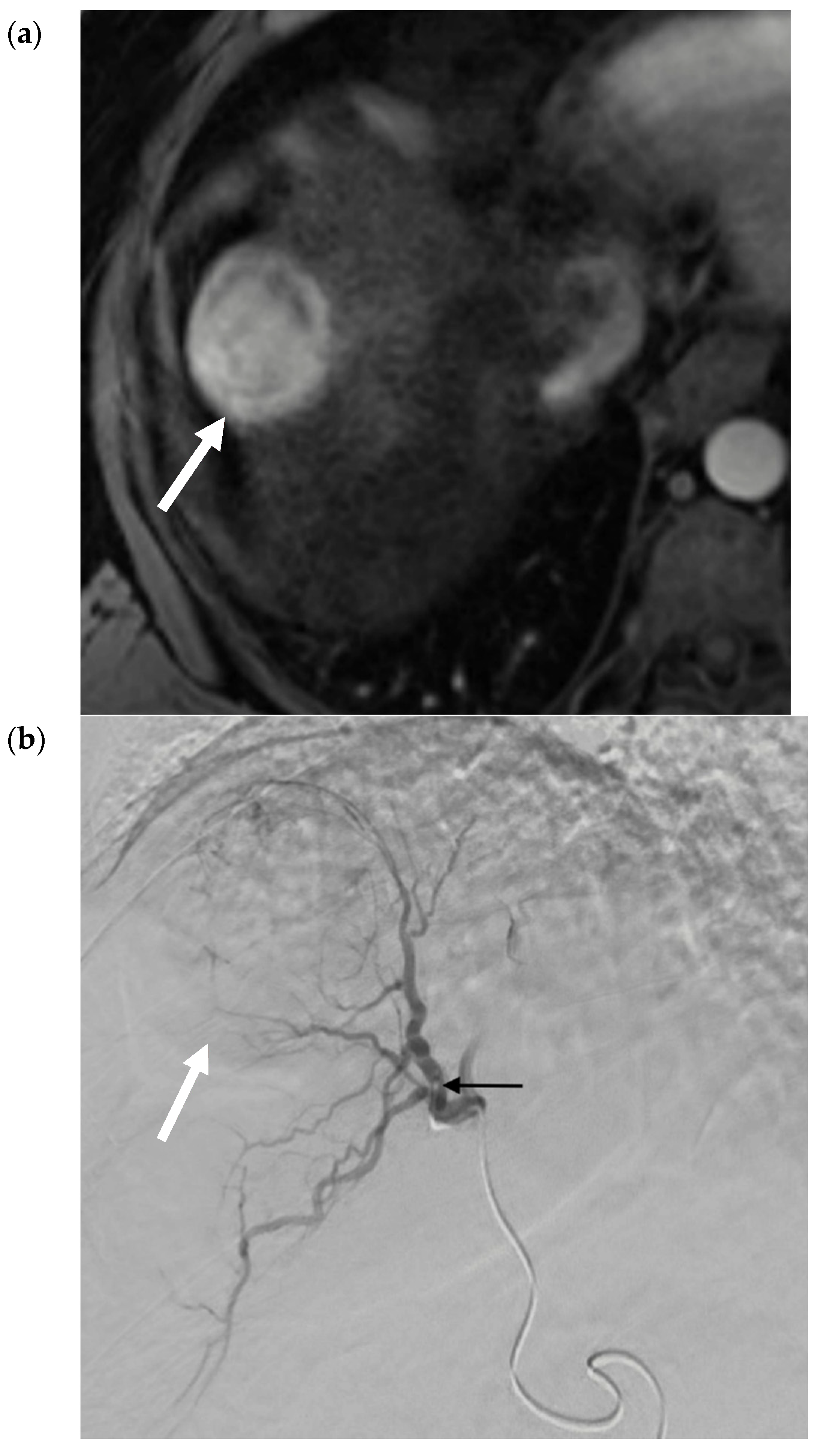
2.4. Selective Internal Radiation Therapy (SIRT)
3. Patient Selection and Applications of Embolotherapy within the Barcelona Clinic Liver Cancer (BCLC) Staging System
- Patient factors
- Performance status (Eastern Cooperative Oncology Group ≥ 2)
- Renal failure (creatinine ≥2.0 mg/dL)
- Unfavorable anatomy (inability to prevent arteriovenous shunting or nontarget embolization)
- Liver factors
- Decompensated liver disease (Child-Pugh B ≥ 8)
- Alterations to portal vein flow (transjugular intrahepatic portosystemic shunt, thrombosis, and hepatofugal flow)
- Tumor factors
- Extensive tumor with complete replacement of both lobes
3.1. Very Early Stage (0) or Early Stage (A)
3.1.1. Adjunct to Surgery
3.1.2. Bridge to Transplant
3.1.3. Downstage to Transplant
3.1.4. Unsuitable Surgical Candidates
3.2. Intermediate Stage (B)
3.3. Advanced Stage (C)
4. Future Therapies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzmán, N.; A Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- El-Serag, H.B.; Kanwal, F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014, 60, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Chegai, F.; Orlacchio, A.; Merolla, S.; Monti, S.; Mannelli, L. Intermediate hepatocellular carcinoma: The role of transarterial therapy. Hepatic Oncol. 2015, 2, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sung, P.S.; You, Y.K.; Kim, D.G.; Oh, J.S.; Chun, H.J.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. Pathologic complete response to chemoembolization improves survival outcomes after curative surgery for hepatocellular carcinoma: Predictive factors of response. HPB 2019, 21, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Agopian, V.G.; Morshedi, M.M.; McWilliams, J.; Harlander-Locke, M.P.; Markovic, D.; Zarrinpar, A.; Kaldas, F.M.; Farmer, D.G.; Yersiz, H.; Hiatt, J.R.; et al. Complete Pathologic Response to Pretransplant Locoregional Therapy for Hepatocellular Carcinoma Defines Cancer Cure After Liver Transplantation. Ann. Surg. 2015, 262, 536–545. [Google Scholar] [CrossRef]
- Fritsche, M.R.; Watchmaker, J.M.; Lipnik, A.J.; Baker, J.C.; Geevarghese, S.; Banovac, F.; Omary, R.A.; Brown, D. Outpatient Transarterial Chemoembolization of Hepatocellular Carcinoma: Review of a Same-Day Discharge Strategy. J. Vasc. Interv. Radiol. 2018, 29, 550–555. [Google Scholar] [CrossRef]
- Gruber-Rouh, T.; Marko, C.; Thalhammer, A.; Nour-Eldin, N.-E.A.; Langenbach, M.C.; Beeres, M.; Naguib, N.; Zangos, S.; Vogl, T.J. Current strategies in interventional oncology of colorectal liver metastases. Br. J. Radiol. 2016, 89, 20151060. [Google Scholar] [CrossRef]
- Gaba, R.C.; Lokken, R.P.; Hickey, R.M.; Lipnik, A.J.; Lewandowski, R.; Salem, R.; Brown, D.B.; Walker, T.G.; Silberzweig, J.E.; Baerlocher, M.O.; et al. Quality Improvement Guidelines for Transarterial Chemoembolization and Embolization of Hepatic Malignancy. J. Vasc. Interv. Radiol. 2017, 28, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef]
- Kritzinger, J.; Klass, D.; Ho, S.; Lim, H.; Buczkowski, A.; Yoshida, E.; Liu, D. Hepatic embolotherapy in interventional oncology: Technology, techniques, and applications. Clin. Radiol. 2013, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Chapiro, J.; Schernthaner, R.E.; Geschwind, J.-F.H. Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: State of the art and future directions. Br. J. Radiol. 2015, 88, 20140564. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañà, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J. Gastroenterol. 2018, 24, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Khan, S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma. Clin. Mol. Hepatol. 2017, 23, 265–272. [Google Scholar] [CrossRef]
- Moore, A.; Cohen-Naftaly, M.; Tobar, A.; Kundel, Y.; Benjaminov, O.; Braun, M.; Issachar, A.; Mor, E.; Sarfaty, M.; Bragilovski, D.; et al. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable Hepatocellular carcinoma. Radiat. Oncol. 2017, 12, 163. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Salem, R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin. Interv. Radiol. 2006, 23, 64–72. [Google Scholar] [CrossRef]
- Yttrium-90 Microspheres (SIR-Spheres®). 2017. Available online: https://www.sirtex.com/media/155126/ssl-us-13.pdf (accessed on 8 December 2019).
- TheraSphere® Yttrium-90 Glass Microspheres. Available online: https://btgplc.com/BTG/media/TheraSphere-Documents/PDF/TheraSphere-Package-Insert_USA_Rev-14.pdf (accessed on 8 December 2019).
- Liu, D.; Westcott, M.; Garcia-Monaco, R.; Abraham, R.; Gandhi, R. Down and dirty with Dosimetry A practical understanding and approach to radioembolization. Endovasc. Today 2016, 15, 70–76. [Google Scholar]
- Vouche, M.; Lewandowski, R.J.; Atassi, R.; Memon, K.; Gates, V.; Ryu, R.K.; Gaba, R.C.; Mulcahy, M.F.; Baker, T.; Sato, K.; et al. Radiation lobectomy: Time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J. Hepatol. 2013, 59, 1029–1036. [Google Scholar] [CrossRef]
- Riaz, A.; Gates, V.; Atassi, B.; Lewandowski, R.; Mulcahy, M.F.; Ryu, R.K.; Sato, K.T.; Baker, T.; Kulik, L.; Gupta, R.; et al. Radiation Segmentectomy: A Novel Approach to Increase Safety and Efficacy of Radioembolization. Int. J. Radiat. Oncol. 2011, 79, 163–171. [Google Scholar] [CrossRef]
- Padia, S.A.; Kwan, S.W.; Roudsari, B.; Monsky, W.L.; Coveler, A.; Harris, W.P. Superselective Yttrium-90 Radioembolization for Hepatocellular Carcinoma Yields High Response Rates with Minimal Toxicity. J. Vasc. Interv. Radiol. 2014, 25, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Gabr, A.; Abouchaleh, N.; Ali, R.; Baker, T.; Caicedo, J.; Katariya, N.; Abecassis, M.; Riaz, A.; Lewandowski, R.; Salem, R. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 1502–1510. [Google Scholar] [CrossRef]
- Lewandowski, R.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.; Kulik, L.; Ganger, D.; Desai, K.; Thornburg, B.; et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018, 68, 1429–1440. [Google Scholar] [CrossRef]
- Sofocleous, C.T.; Boas, F.E. Radiation Segmentectomy for Hepatocellular Carcinoma: Ready for Prime Time? Radiology 2018, 287, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Gaba, R.C.; Lewandowski, R.; Hickey, R.M.; Baerlocher, M.O.; Cohen, E.I.; Dariushnia, S.R.; D’Othée, B.J.; Padia, S.A.; Salem, R.; Wang, D.S.; et al. Transcatheter Therapy for Hepatic Malignancy: Standardization of Terminology and Reporting Criteria. J. Vasc. Interv. Radiol. 2016, 27, 457–473. [Google Scholar] [CrossRef]
- Song, S.-Y.; Chung, J.W.; Han, J.K.; Lim, H.G.; Koh, Y.H.; Park, J.H.; Lee, H.-S.; Kim, C.Y. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: Incidence, predisposing factors, and clinical outcome. J. Vasc. Interv. Radiol. 2001, 12, 313–320. [Google Scholar] [CrossRef]
- Brown, D.B.; Nikolic, B.; Covey, A.M.; Nutting, C.W.; Saad, W.E.; Salem, R.; Sofocleous, C.T.; Sze, D.Y. Quality Improvement Guidelines for Transhepatic Arterial Chemoembolization, Embolization, and Chemotherapeutic Infusion for Hepatic Malignancy. J. Vasc. Interv. Radiol. 2012, 23, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Ogasawara, S. Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer 2018, 7, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.-L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef]
- Sieghart, W.; Hucke, F.; Peck-Radosavljevic, M. Transarterial chemoembolization: Modalities, indication, and patient selection. J. Hepatol. 2015, 62, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer EASL–EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Chapman, W.C.; Klintmalm, G.; Hemming, A.; Vachharajani, N.; Doyle, M.B.; DeMatteo, R.; Zaydfudim, V.M.; Chung, H.; Cavaness, K.; Goldstein, R.; et al. Surgical Treatment of Hepatocellular Carcinoma in North America: Can Hepatic Resection Still Be Justified? J. Am. Coll. Surg. 2015, 220, 628–637. [Google Scholar] [CrossRef]
- Fayek, S.A.; Quintini, C.; Chavin, K.D.; Marsh, C.L. The Current State of Liver Transplantation in the United States. Am. J. Transplant. 2016, 16, 3093–3104. [Google Scholar] [CrossRef]
- Xu, X.-F.; Xing, H.; Han, J.; Li, Z.-L.; Lau, W.-Y.; Zhou, Y.-H.; Gu, W.-M.; Wang, H.; Chen, T.-H.; Zeng, Y.-Y.; et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2018, 154, 209. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Avritscher, R.; De Baere, T.; Murthy, R.; Deschamps, F.; Madoff, D.C. Percutaneous transhepatic portal vein embolization: Rationale, technique, and outcomes. Semin. Interv. Radiol. 2008, 25, 132–145. [Google Scholar] [CrossRef]
- Capussotti, L.; Muratore, A.; Baracchi, F.; Lelong, B.; Ferrero, A.; Regge, D.; Delpero, J.-R. Portal Vein Ligation as an Efficient Method of Increasing the Future Liver Remnant Volume in the Surgical Treatment of Colorectal Metastases. Arch. Surg. 2008, 143, 978. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Benhaïm, L. Portal Vein Occlusion Prior to Extensive Resection in Colorectal Liver Metastasis: A Necessity Rather than an Option! Ann. Surg. Oncol. 2009, 16, 1098–1099. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Kim, J.S.; Watanabe, G.; Mohuczy, D.; Behrns, K.E. Liver regeneration and the atrophy-hypertrophy complex. Semin. Interv. Radiol. 2008, 25, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; Ernani, L.; Coelho, F.; Bernardo, W.M.; Junior, S.S.; Krüger, J.A.P.; Fonseca, G.; Jeismann, V.B.; Cecconello, I.; Herman, P. Preoperative strategies to improve resectability for hepatocellular carcinoma: A systematic review and meta-analysis. HPB 2018, 20, 1109–1118. [Google Scholar] [CrossRef]
- Ogata, S.; Belghiti, J.; Farges, O.; Varma, D.; Sibert, A.; Vilgrain, V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br. J. Surg. 2006, 93, 1091–1098. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tokidis, E.; Basourakos, S.-P.; Ntzani, E.E.; Lianos, G.D.; Pentheroudakis, G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur. J. Surg. Oncol. 2017, 43, 32–41. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, J.-H.; Ko, G.-Y.; Kim, K.W.; Gwon, N.I.; Lee, S.-G.; Hwang, S. Sequential Transcatheter Arterial Chemoembolization and Portal Vein Embolization versus Portal Vein Embolization Only before Major Hepatectomy for Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2010, 18, 1251–1257. [Google Scholar] [CrossRef]
- Teo, J.-Y.; Allen, J.C.; Ng, D.C.; Choo, S.-P.; Tai, D.W.; Chang, J.P.; Cheah, F.-K.; Chow, P.K.H.; Goh, B.K.P. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB 2015, 18, 7–12. [Google Scholar] [CrossRef]
- Labgaa, I.; Tabrizian, P.; Titano, J.; Kim, E.; Thung, S.N.; Florman, S.; Schwartz, M.; Melloul, E. Feasibility and safety of liver transplantation or resection after transarterial radioembolization with Yttrium-90 for unresectable hepatocellular carcinoma. HPB 2019, 21, 1497–1504. [Google Scholar] [CrossRef]
- Mehta, N.; Dodge, J.L.; Goel, A.; Roberts, J.P.; Hirose, R.; Yao, F.Y. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: Implications for the current organ allocation policy. Liver Transplant. 2013, 19, 1343–1353. [Google Scholar] [CrossRef]
- Lee, H.A.; Cho, E.Y.; Kim, T.H.; Lee, Y.-S.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; An, H.; Seo, Y.S.; Kim, N.-S.; et al. Risk Factors for Dropout From the Liver Transplant Waiting List of Hepatocellular Carcinoma Patients Under Locoregional Treatment. Transplant. Proc. 2018, 50, 3521–3526. [Google Scholar] [CrossRef]
- Parikh, N.D.; Singal, A.G. Model for end-stage liver disease exception points for treatment-responsive hepatocellular carcinoma. Clin. Liver Dis. 2016, 7, 97–100. [Google Scholar] [CrossRef] [PubMed]
- OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. Changes to HCC Criteria for Auto Approval. 2016. Available online: https://optn.transplant.hrsa.gov/media/1922/liver_hcc_criteria_for_auto_approval_20160815.pdf (accessed on 1 December 2019).
- Coletta, M.; Nicolini, D.; Benedetti Cacciaguerra, A.; Mazzocato, S.; Rossi, R.; Vivarelli, M. Bridging patients with hepatocellular cancer waiting for liver transplant: All the patients are the same? Transl. Gastroenterol. Hepatol. 2017, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Sukato, D.; Chen, H.-W.; Amesur, N.; Zajko, A.B.; Humar, A.; Geller, D.A.; Marsh, J.W.; Tsung, A. Yttrium-90 Radioembolization as a Bridge to Liver Transplantation: A Single-Institution Experience. J. Vasc. Interv. Radiol. 2013, 24, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, X.; Azahaf, M.; Lassailly, G.; Sergent, G.; Buob, D.; Truant, S.; Boleslawski, E.; Louvet, A.; Gnemmi, V.; Canva, V.; et al. Drug-Eluting Beads Loaded With Doxorubicin (DEBDOX) Chemoembolisation Before Liver Transplantation for Hepatocellular Carcinoma: An Imaging/Histologic Correlation Study. Cardiovasc. Interv. Radiol. 2014, 38, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Hodavance, M.S.; Vikingstad, E.M.; Griffin, A.S.; Pabon-Ramos, W.M.; Berg, C.L.; Suhocki, P.V.; Kim, C.Y. Effectiveness of Transarterial Embolization of Hepatocellular Carcinoma as a Bridge to Transplantation. J. Vasc. Interv. Radiol. 2016, 27, 39–45. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163. [Google Scholar] [CrossRef]
- Clavien, P.-A.; Lesurtel, M.; Bossuyt, P.M.M.; Gores, G.J.; Langer, B.; Perrier, A. OLT for HCC Consensus Group Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2011, 13, e11–e22. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Senthilnathan, S.; Mulcahy, M.F.; Ryu, R.K.; Ibrahim, S.M.; Sato, K.T.; Baker, T.; Miller, F.H.; et al. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization Versus Radioembolization. Arab. Archaeol. Epigr. 2009, 9, 1920–1928. [Google Scholar] [CrossRef]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transplant. 2015, 21, 1142–1152. [Google Scholar] [CrossRef]
- Toso, C.; Meeberg, G.; Andres, A.; Shore, C.; Saunders, C.; Bigam, D.L.; Shapiro, A.M.J.; Compagnon, P.; Berney, T.; Majno, P.; et al. Downstaging prior to liver transplantation for hepatocellular carcinoma: Advisable but at the price of an increased risk of cancer recurrence—A retrospective study. Transpl. Int. 2018, 32, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Goldberg, S.N.; Lazzaroni, S.; Meloni, M.F.; Ierace, T.; Solbiati, L.; Gazelle, G.S. Hepatocellular Carcinoma: Radio-frequency Ablation of Medium and Large Lesions. Radiology 2000, 214, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Foltz, G. Image-Guided Percutaneous Ablation of Hepatic Malignancies. Semin. Interv. Radiol. 2014, 31, 180–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ni, J.-Y.; Liu, S.-S.; Xu, L.-F.; Sun, H.-L.; Chen, Y.-T. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 3872–3882. [Google Scholar] [CrossRef] [PubMed]
- Elnekave, E.; Erinjeri, J.P.; Brown, K.; Thornton, R.H.; Petre, E.N.; Maybody, M.; Maluccio, M.A.; Hsu, M.; Sofocleous, C.T.; Getrajdman, G.I.; et al. Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC <7 cm. Ann. Surg. Oncol. 2013, 20, 2881–2886. [Google Scholar] [PubMed]
- Yang, H.-J.; Lee, J.; Lee, D.; Yu, S.J.; Kim, Y.J.; Yoon, J.-H.; Kim, H.-C.; Lee, J.M.; Chung, J.W.; Yi, N.-J.; et al. Small Single-Nodule Hepatocellular Carcinoma: Comparison of Transarterial Chemoembolization, Radiofrequency Ablation, and Hepatic Resection by Using Inverse Probability Weighting. Radiology 2014, 271, 909–918. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.-H.; Sung, K.-B.; Ko, H.-K.; Shin, J.H.; Kim, P.N.; Choi, H.-K.; Ko, G.-Y.; Yoon, H.-K.; Chun, S.-Y.; et al. Transarterial Chemoembolization vs. Radiofrequency Ablation for the Treatment of Single Hepatocellular Carcinoma 2 cm or Smaller. Am. J. Gastroenterol. 2014, 109, 1234–1240. [Google Scholar] [CrossRef]
- Vouche, M.; Habib, A.; Ward, T.J.; Kim, E.; Kulik, L.; Ganger, D.; Mulcahy, M.; Baker, T.; Abecassis, M.; Sato, K.T.; et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: Multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014, 60, 192–201. [Google Scholar] [CrossRef]
- Biederman, D.M.; Titano, J.J.; Korff, R.; Fischman, A.; Patel, R.S.; Nowakowski, F.S.; Lookstein, R.; Kim, E. Radiation Segmentectomy versus Selective Chemoembolization in the Treatment of Early-Stage Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 30–37. [Google Scholar] [CrossRef]
- Rostambeigi, N.; Dekarske, A.S.; Austin, E.E.; Golzarian, J.; Cressman, E.N. Cost Effectiveness of Radioembolization Compared with Conventional Transarterial Chemoembolization for Treatment of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2014, 25, 1075–1084. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, Y.; Chau, G.-Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Lewandowski, R.; Hickey, R.; Kallini, J.; Gabr, A.; Sato, K.; Desai, K.; Thornburg, B.; Gates, V.; Ganger, D.; et al. Prospective randomized phase 2 study of chemoembolization versus radioembolization in hepatocellular carcinoma: Results from the PREMIERE trial. J. Vasc. Interv. Radiol. 2016, 27, S61–S62. [Google Scholar] [CrossRef]
- Casadei Gardini, A.; Tamburini, E.; Iñarrairaegui, M.; Frassineti, G.L.; Sangro, B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: A meta-analysis of randomized trials. Oncol. Targets Ther. 2018, 11, 7315–7321. [Google Scholar] [CrossRef] [PubMed]
- Klompenhouwer, E.G.; Dresen, R.C.; Verslype, C.; Laenen, A.; De Hertogh, G.; Deroose, C.M.; Bonne, L.; Vandevaveye, V.; Maleux, G. Safety and Efficacy of Transarterial Radioembolisation in Patients with Intermediate or Advanced Stage Hepatocellular Carcinoma Refractory to Chemoembolisation. Cardiovasc. Interv. Radiol. 2017, 40, 1882–1890. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Abdalla, E. Overview of Treatment Approaches for Hepatocellular Carcinoma—Up to Date. Available online: https://www-uptodate-com.proxy.library.rcsi.ie/contents/overview-of-treatment-approaches-for-hepatocellular-carcinoma?search=hcc&topicRef=3599&source=see_link#H4 (accessed on 10 November 2019).
- Geschwind, J.-F.; Kudo, M.; Marrero, J.A.; Venook, A.P.; Chen, X.-P.; Bronowicki, J.-P.; Dagher, L.; Furuse, J.; De Guevara, L.L.; Papandreou, C.; et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology 2016, 279, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, W.; Wang, M.; Hu, J.; Wang, E.; Zhao, Y.; Liu, L. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Elsayed, Z. Yttrium-90 microsphere radioembolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst. Rev. 2016, 2. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Palmer, D.H.; Hawkins, N.S.; Vilgrain, V.; Pereira, H.; Chatellier, G.; Ross, P.J. Tumor burden and liver function in HCC patient selection for selective internal radiation therapy: SARAH post-hoc study. Future Oncol. 2020, 16, 4315–4325. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Finn, R.S. Negative phase 3 study of 90Y microspheres versus sorafenib in HCC. Lancet Oncol. 2018, 19, e69. [Google Scholar] [CrossRef]
- Sposito, C.; Mazzaferro, V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: Reasons for a failure, and perspectives for the future. Hepatobiliary Surg. Nutr. 2018, 7, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Klumpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; De Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Makuuchi, M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J. Gastroenterol. 2006, 12, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Crouzet, L.; Campillo-Gimenez, B.; Rolland, Y.; Pracht, M.; Guillygomarc’H, A.; Boudjema, K.; Lenoir, L.; Adhoute, X.; Rohou, T.; et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Kokabi, N.; Camacho, J.C.; Kim, H.S. Prospective longitudinal quality of life and survival outcomes in patients with advanced infiltrative hepatocellular carcinoma and portal vein thrombosis treated with Yttrium-90 radioembolization. BMC Cancer 2018, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Couri, T.; Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Gerbes, A.L.; Zoulim, F.; Tilg, H.; Dufour, J.-F.; Bruix, J.; Paradis, V.; Salem, R.; Peck-Radosavljevic, M.; Galle, P.R.; Greten, T.F.; et al. Gut roundtable meeting paper: Selected recent advances in hepatocellular carcinoma. Gut 2017, 67, 380–388. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; Elgindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2016, 66, 545–551. [Google Scholar] [CrossRef]
- Dendy, M.S.; Ludwig, J.M.; Stein, S.M.; Kim, H.S. Locoregional Therapy, Immunotherapy and the Combination in Hepatocellular Carcinoma: Future Directions. Liver Cancer 2019, 8, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Ruohoniemi, D.; Shanbhogue, K.P.; Wei, J.; Welling, T.H.; Gu, P.; Park, J.; Dagher, N.; Taslakian, B.; Hickey, R.M. Safety of Combined Yttrium-90 Radioembolization and Immune Checkpoint Inhibitor Immunotherapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020, 31, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.-Y.; Breder, V.V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2019, 37, 4004. [Google Scholar] [CrossRef]
- De Toni, E.N. Immune checkpoint inhibitors: Use them early, combined and instead of TACE? Gut 2019. [Google Scholar] [CrossRef]
- Murata, S.; Mine, T.; Sugihara, F.; Yasui, D.; Yamaguchi, H.; Ueda, T.; Onozawa, S.; Kumita, S.-I. Interventional treatment for unresectable hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 13453–13465. [Google Scholar] [CrossRef]
- Morshid, A.; Elsayes, K.; Khalaf, A.M.; Elmohr, M.M.; Yu, J.; Kaseb, A.O.; Hassan, M.; Mahvash, A.; Wang, Z.; Hazle, J.D.; et al. A machine learning model to predict hepatocellular carcinoma response to transcatheter arterial chemoembolization. Radiol. Artif. Intell. 2019, 1, e180021. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishore, S.A.; Bajwa, R.; Madoff, D.C. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers 2020, 12, 791. https://doi.org/10.3390/cancers12040791
Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers. 2020; 12(4):791. https://doi.org/10.3390/cancers12040791
Chicago/Turabian StyleKishore, Sirish A., Raazi Bajwa, and David C. Madoff. 2020. "Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update" Cancers 12, no. 4: 791. https://doi.org/10.3390/cancers12040791
APA StyleKishore, S. A., Bajwa, R., & Madoff, D. C. (2020). Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers, 12(4), 791. https://doi.org/10.3390/cancers12040791




