Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer with or without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis
Abstract
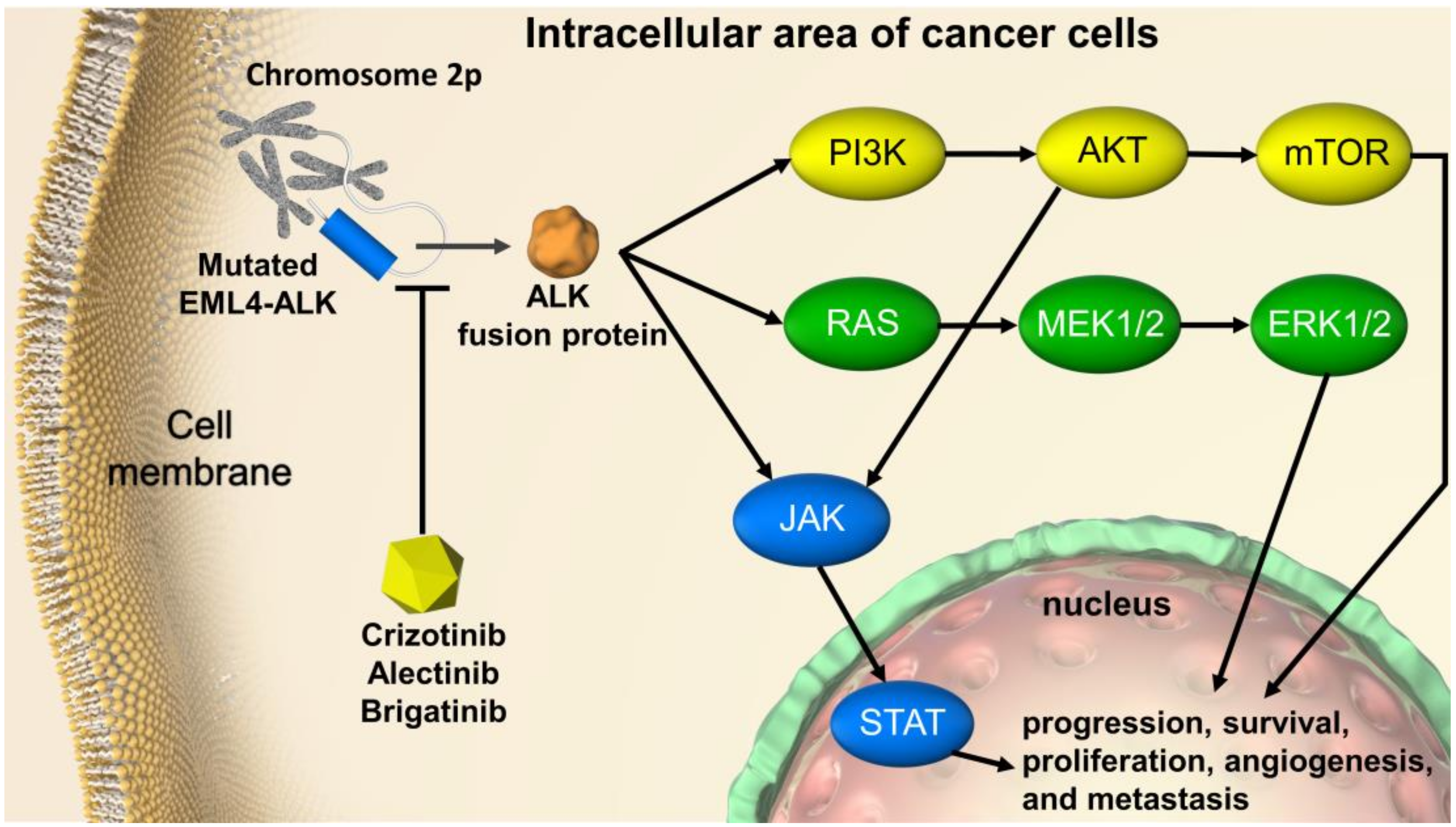
:1. Introduction
2. Methods
2.1. Systematic Review
2.2. Quality Evaluation
2.3. Inclusion and Exclusion Criteria (Predefined PICOS)
2.4. Interventions/Comparisons
2.5. Outcomes
2.6. Study Design
2.7. Statistical Analysis Method of Indirect Comparison
2.8. Ethical Aspects
3. Results
3.1. Systematic Review
3.2. Primary Efficacy Endpoint: Progression-Free Survival
3.3. Incidence of G3–5AAEs
3.4. Bias Assessment
3.5. Comparison with Analysis Using Another Statistical Method
3.6. Sensitivity Analysis
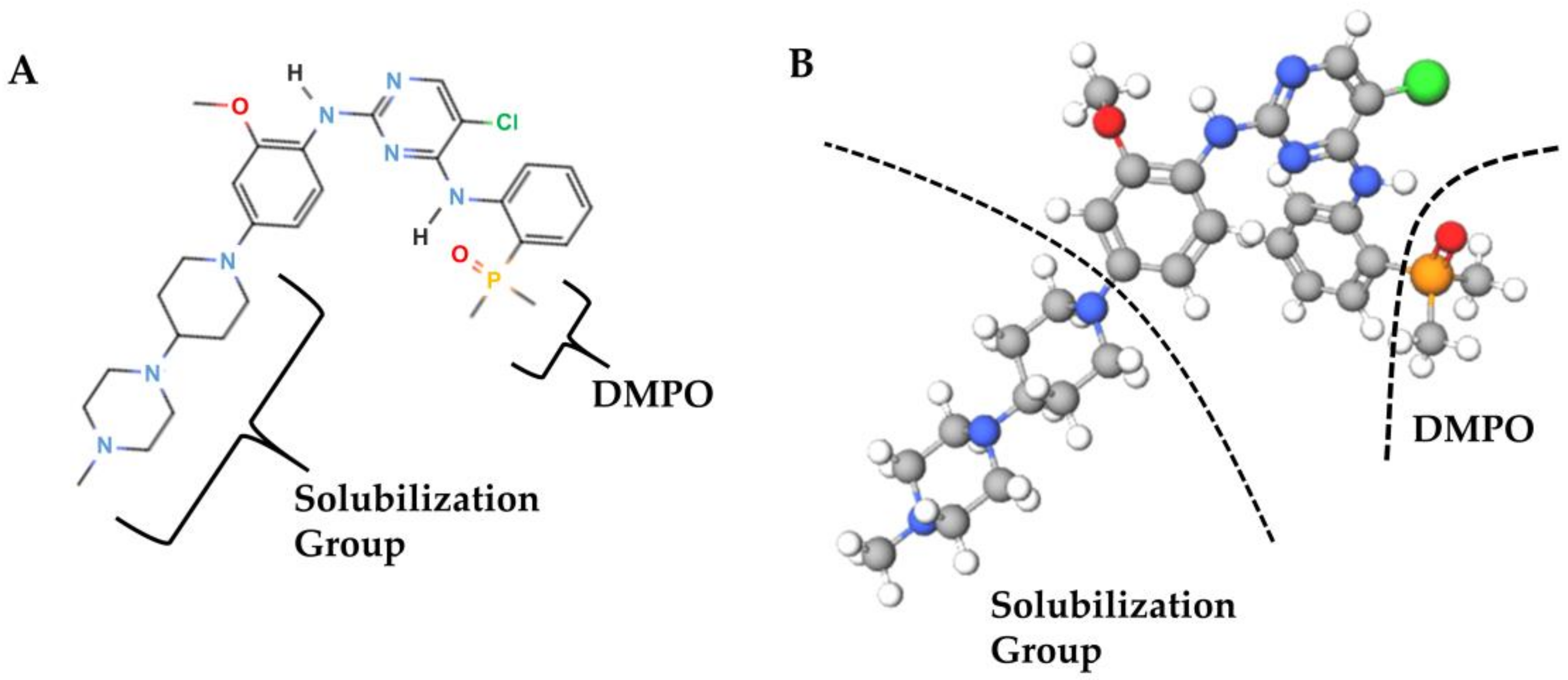
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung cancer. Med. Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Shroff, G.S.; Viswanathan, C.; Carter, B.W. Staging lung cancer: Metastasis. Radiol. Clin. N. Am. 2018, 56, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; DeAngelis, L.M. Brain metastases. Neurol. Clin. 2003, 21, 1–23. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.M.; Goldberg, S.; et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Lim, H.J.; Park, J.S.; Cho, Y.J.; Yoon, H.I.; Chung, J.H.; Lee, J.H.; Lee, C.T. Comparison of clinical characteristics between patients with ALK-positive and EGFR-positive lung adenocarcinoma. Respir. Med. 2014, 108, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Fallet, V.; Cadranel, J.; Doubre, H.; Toper, C.; Monnet, I.; Chinet, T.; Oliviero, G.; Foulon, G.; De Cremoux, H.; Vieira, T.; et al. Prospective screening for ALK: Clinical features and outcome according to ALK status. Eur. J. Cancer 2014, 50, 1239–1246. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar]
- Griesinger, F.; Roeper, J.; Pottgen, C.; Willborn, K.C.; Eberhardt, W.E.E. Brain metastases in ALK-positive NSCLC—Time to adjust current treatment algorithms. Oncotarget 2018, 9, 35181–35194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducray, S.P.; Natarajan, K.; Garland, G.D.; Turner, S.D.; Egger, G. The Transcriptional Roles of ALK Fusion Proteins in Tumorigenesis. Cancers 2019, 11, 1074. [Google Scholar]
- Aubry, A.; Galiacy, S.; Allouche, M. Targeting ALK in Cancer: Therapeutic Potential of Proapoptotic Peptides. Cancers 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, I.; Zaorsky, N.G.; Palmer, J.D.; Mehra, R.; Lu, B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015, 16, e510–e521. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in oNCOLOGY: Non-Small Cell Lung Cancer 2018 (Version 3.2018). Available online: https://www.nccn.org (accessed on 19 March 2020).
- Sakamoto, H.; Tsukaguchi, T.; Hiroshima, S.; Kodama, T.; Kobayashi, T.; Fukami, T.A.; Oikawa, N.; Tsukuda, T.; Ishii, N.; Aoki, Y. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011, 19, 679–690. [Google Scholar]
- Gainor, J.F.; Sherman, C.A.; Willoughby, K.; Logan, J.; Kennedy, E.; Brastianos, P.K.; Chi, A.S.; Shaw, A.T. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J. Thorac. Oncol. 2015, 10, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.H.; Perol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar]
- Markham, A. Brigatinib: First global approval. Drugs 2017, 77, 1131–1135. [Google Scholar] [CrossRef]
- Popat, S.; Liu, G.; Lu, S.; Song, G.; Samnotra, V.; Yang, J.C. Phase III ALTA-3 study of brigatinib (BRG) vs alectinib (ALC) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)−positive non–small cell lung cancer (NSCLC) that progressed on crizotinib (CRZ). Ann. Oncol. 2019, 30, v653–v654. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.; Han, J.Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.; Chang, G.C.; Lee, K.H.; et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Tiseo, M.; Ahn, M.J.; Reckamp, K.L.; Hansen, K.H.; Kim, S.W.; Huber, R.M.; West, H.L.; Groen, H.J.M.; Hochmair, M.J.; et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2017, 35, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Anjum, R.; Squillace, R.; Nadworny, S.; Zhou, T.; Keats, J.; Ning, Y.; Wardwell, S.D.; Miller, D.; Song, Y.; et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin. Cancer Res. 2016, 22, 5527–5538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- Ades, A.E.; Cliffe, S. Markov chain Monte Carlo estimation of a multiparameter decision model: Consistency of evidence and the accurate assessment of uncertainty. Med. Decis. Mak. 2002, 22, 359–371. [Google Scholar] [CrossRef]
- White, I.R. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Ades, A. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2011; ISBN 978-0-470-51845-8. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A.E. Evidence synthesis for decision making 4: Inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. 2013, 33, 641–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity—Subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Mak. 2013, 33, 618–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Mak. 2013, 33, 607–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonin, F.S.; Rotta, I.; Mendes, A.M.; Pontarolo, R. Network meta-analysis: A technique to gather evidence from direct and indirect comparisons. Pharm. Pract. (Granada) 2017, 15, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.P.; Crawford, B.; Bergman, G.; Stam, W. Bayesian meta-analysis of multiple treatment comparisons: An introduction to mixed treatment comparisons. Value Health 2008, 11, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 2002, 21, 2313–2324. [Google Scholar] [CrossRef]
- Jansen, J.P.; Fleurence, R.; Devine, B.; Itzler, R.; Barrett, A.; Hawkins, N.; Lee, K.; Boersma, C.; Annemans, L.; Cappelleri, J.C.; et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Value Health 2011, 14, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Hoaglin, D.C.; Hawkins, N.; Jansen, J.P.; Scott, D.A.; Itzler, R.; Cappelleri, J.C.; Boersma, C.; Thompson, D.; Larholt, K.M.; Diaz, M.; et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2. Value Health 2011, 14, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ning, Y.; Cooper, J.; Refoios Camejo, R.; Ni, X.; Yi, B.; Parks, D. Indirect comparison of ropinirole and pramipexole as levodopa adjunctive therapy in advanced Parkinson’s disease: A systematic review and network meta-analysis. Adv. Ther. 2019, 36, 1252–1265. [Google Scholar] [CrossRef]
- Yan, K.; Balijepalli, C.; Sharma, R.; Barakat, S.; Sun, S.X.; Falcao, S.; Druyts, E.; FitzGerald, J.M. Reslizumab and mepolizumab for moderate-to-severe poorly controlled asthma: An indirect comparison meta-analysis. Immunotherapy 2019, 11, 1491–1505. [Google Scholar] [CrossRef]
- Miwa, H.; Igarashi, A.; Teng, L.; Uda, A.; Deguchi, H.; Tango, T. Systematic review with network meta-analysis: Indirect comparison of the efficacy of vonoprazan and proton-pump inhibitors for maintenance treatment of gastroesophageal reflux disease. J. Gastroenterol. 2019, 54, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Ford, J.A.; Elders, A.; Shyangdan, D.; Royle, P.; Waugh, N. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: An indirect comparison in a systematic review. BMJ 2012, 345, e5182. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.P.; Roberts, G.O. Convergence assessment techniques for Markov chain Monte Carlo. Stat. Comput. 1998, 8, 319–335. [Google Scholar] [CrossRef]
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Zhang, Z.; Guo, H.; Lu, Y.; Hao, W.; Han, L. Anaplastic lymphoma kinase inhibitors in non-small cell lung cancer patients with brain metastases: A meta-analysis. J. Thorac. Dis. 2019, 11, 1397–1409. [Google Scholar] [CrossRef]
- Elliott, J.; Bai, Z.; Hsieh, S.C.; Kelly, S.E.; Chen, L.; Skidmore, B.; Yousef, S.; Zheng, C.; Stewart, D.J. ALK inhibitors for non-small cell lung cancer: A systematic review and network meta-analysis. PLoS ONE 2020, 15, e0229179. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Fong, T.; Xia, Z.; Zhang, J.; Luo, P. The efficacy and safety of ALK inhibitors in the treatment of ALK-positive non-small cell lung cancer: A network meta-analysis. Cancer Med. 2018, 7, 4993–5005. [Google Scholar] [CrossRef]
- Huang, W.S.; Liu, S.; Zou, D.; Thomas, M.; Wang, Y.; Zhou, T.; Romero, J.; Kohlmann, A.; Li, F.; Qi, J.; et al. Discovery of brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J. Med. Chem. 2016, 59, 4948–4964. [Google Scholar] [CrossRef]
- Sabari, J.K.; Santini, F.C.; Schram, A.M.; Bergagnini, I.; Chen, R.; Mrad, C.; Lai, W.V.; Arbour, K.C.; Drilon, A. The activity, safety, and evolving role of brigatinib in patients with ALK-rearranged non-small cell lung cancers. OncoTargets Ther. 2017, 10, 1983. [Google Scholar] [CrossRef] [Green Version]






| Study | Key Inclusion Criteria |
|---|---|
| ALTA-1L |
|
| |
| |
| ALEX |
|
| |
| |
| |
| J-ALEX |
|
| |
| |
| |
|
| Study | Treatment Arms | n | Age (Years): Median (Range) | Female: No. (%) | ECOG PS: No. (%) | Smoking Status: No. (%) | Histological Type: No. (%) | Stage of Disease at Entry: No. (%) | CNS Metastasis: No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| ALTA-1L | Brigatinib 180 mg | 137 | 58 (27–86) | 69 (50) | PS0–1: 131 (96) | Never: 84 (61) | Adeno: 126 (92) | III B: 8 (6) | 40 (29) |
| once daily | PS2: 6 (4) | Former: 49 (36) | Squamous: 4 (3) | IV: 129 (94) | |||||
| (7-day run-in | Current: 4 (3) | Other: 7 (4) | |||||||
| period of 90 mg | |||||||||
| once daily) | |||||||||
| Crizotinib 250 mg | 138 | 60 (29–89) | 81 (59) | PS0–1: 132 (96) | Never: 75 (54) | Adeno: 137 (99) | III B: 12 (9) | 41 (30) | |
| twice daily | PS2: 6 (4) | Former: 56 (41) | Squamous: 0 (0) | IV: 126 (91) | |||||
| Current: 7 (5) | Other: 1 (1) | ||||||||
| total, 275 | |||||||||
| ALEX | Alectinib 600 mg | 152 | 58 (25–88) | 84 (55) | PS0–1: 142 (93) | Never: 92 (61) | Adeno: 137 (90) | III B: 4 (3) | 64 (42) |
| twice daily | PS2: 10 (7) | Former: 48 (32) | Squamous: 5 (3) | IV: 148 (97) | |||||
| Current: 12 (8) | Other: 10 (7) | ||||||||
| Crizotinib 250 mg | 151 | 54 (18–91) | 87 (58) | PS0–1: 141 (93) | Never: 98 (65) | Adeno: 142 (94) | III B: 6 (4) | 58 (38) | |
| twice daily | PS2: 10 (7) | Former: 48 (32) | Squamous: 2 (1) | IV: 145 (96) | |||||
| Current: 5 (3) | Other: 7 (5) | ||||||||
| total, 303 | |||||||||
| J-ALEX | Alectinib 300 mg | 103 | 61.0 (27–85) | 62 (60) | PS0–1: 101 (98) | Never: 56 (54) | Adeno: 100 (97) | III B: 3 (3) | 16 (16) |
| twice daily | PS2: 2 (2) | Former: 45 (44) | Squamous: 2 (2) | IV: 76 (74) | |||||
| Current: 2 (2) | Other: 1 (1) | postoperative | |||||||
| recurrence: 24 (23) | |||||||||
| Crizotinib 250 mg | 104 | 59.5 (25–84) | 63 (61) | PS0–1: 102 (98) | Never: 61 (59) | Adeno: 103 (99) | III B: 3 (3) | 31 (30) | |
| twice daily | PS2: 2 (2) | Former: 40 (38) | Squamous: 0 (0) | IV: 75 (72) | |||||
| Current: 3 (3) | Other: 1 (1) | postoperative | |||||||
| recurrence: 26 (25) | |||||||||
| total, 207 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, K.; Akimoto, K.; Sato, H.; Manabe, R.; Kishino, Y.; Homma, T.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; et al. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer with or without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers 2020, 12, 942. https://doi.org/10.3390/cancers12040942
Ando K, Akimoto K, Sato H, Manabe R, Kishino Y, Homma T, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, et al. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer with or without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers. 2020; 12(4):942. https://doi.org/10.3390/cancers12040942
Chicago/Turabian StyleAndo, Koichi, Kaho Akimoto, Hiroki Sato, Ryo Manabe, Yasunari Kishino, Tetsuya Homma, Sojiro Kusumoto, Toshimitsu Yamaoka, Akihiko Tanaka, Tohru Ohmori, and et al. 2020. "Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer with or without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis" Cancers 12, no. 4: 942. https://doi.org/10.3390/cancers12040942
APA StyleAndo, K., Akimoto, K., Sato, H., Manabe, R., Kishino, Y., Homma, T., Kusumoto, S., Yamaoka, T., Tanaka, A., Ohmori, T., & Sagara, H. (2020). Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer with or without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers, 12(4), 942. https://doi.org/10.3390/cancers12040942






