Impact and Diagnostic Gaps of Comprehensive Genomic Profiling in Real-World Clinical Practice
Abstract
:1. Introduction
2. Methods
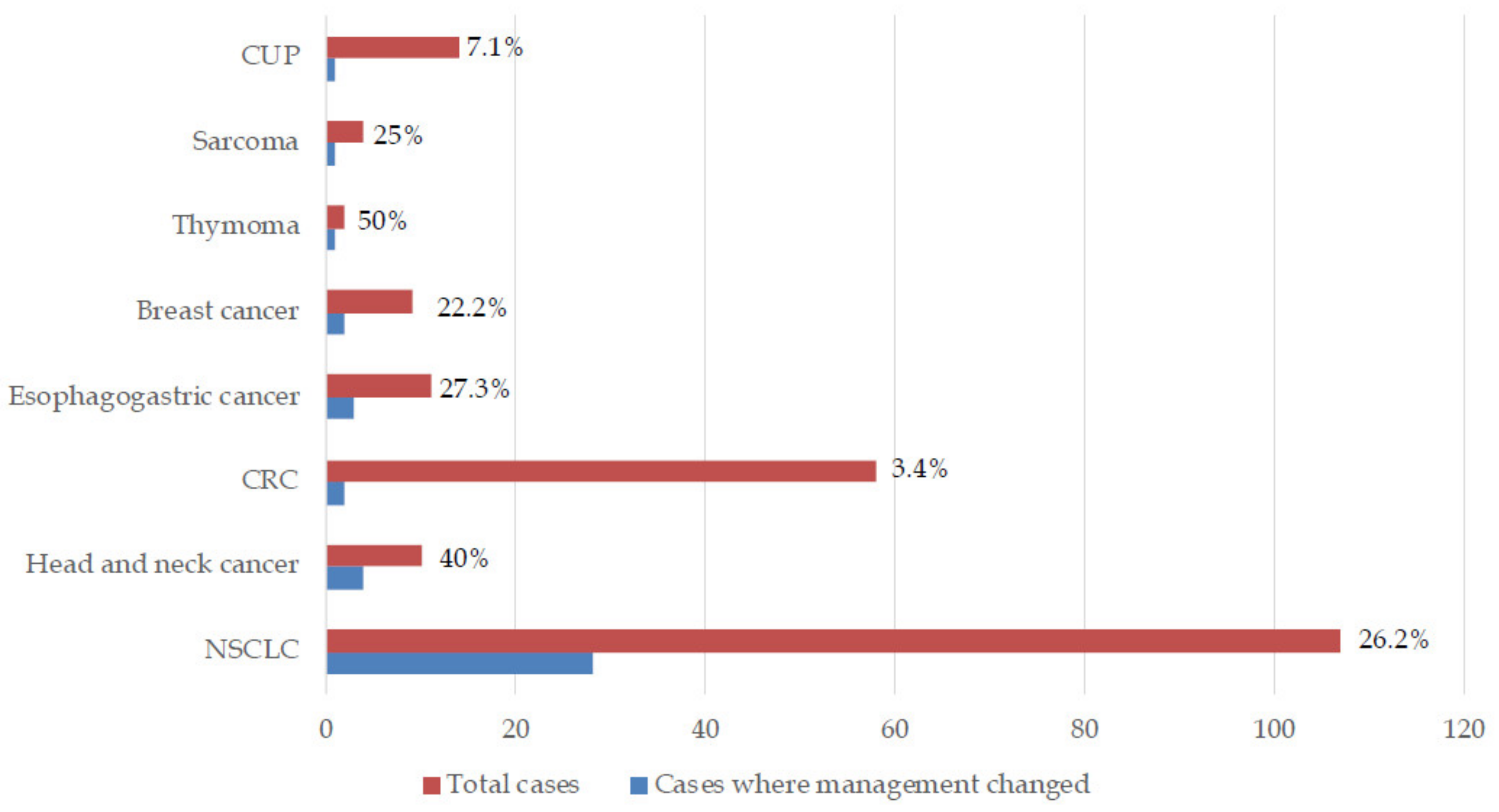
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sholl, L.M.; Do, K.; Shivdasani, P.; Cerami, E.; Dubuc, A.M.; Kuo, F.C.; Garcia, E.P.; Jia, Y.; Davineni, P.; Abo, R.P.; et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016, 1, e87062. [Google Scholar] [CrossRef]
- Signorovitch, J.; Janku, F.; Wheler, J.J.; Miller, V.A.; Ryan, J.; Zhou, Z.; Chawla, A. Estimated cost of anticancer therapy directed by comprehensive genomic profiling (CGP) in a single-center study. J. Clin. Oncol. 2017, 35, 6605. [Google Scholar] [CrossRef]
- Remon, J.; Steuer, C.; Ramalingam, S.; Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018, 29, i20–i27. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.E.; Choi, K.; Lanman, R.B.; Licitra, E.J.; Skrzypczak, S.M.; Benito, R.P.; Wu, T.; Arunajadai, S.; Kaur, S.; Harper, H.; et al. Genomic Profiling of Advanced Non–Small Cell Lung Cancer in Community Settings: Gaps and Opportunities. Clin. Lung Cancer 2017, 18, 651–659. [Google Scholar] [CrossRef]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker Testing for Patients with Advanced Non–Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non Small Cell Lung Cancer Version 3.2020 [11 February 2020]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 16 April 2020).
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Sireci, A.N.; Aggarwal, V.S.; Turk, A.; Gindin, T.; Mansukhani, M.M.; Hsiao, S.J. Clinical Genomic Profiling of a Diverse Array of Oncology Specimens at a Large Academic Cancer Center. J. Mol. Diagn. 2017, 19, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehir, A.; Benayed, R.; Shah, R.; Syed, A.; Middha, S.; Kim, H.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Hirshfield, K.M.; Tolkunov, D.; Zhong, H.; Ali, S.M.; Stein, M.N.; Murphy, S.; Vig, H.; Vázquez, A.; Glod, J.; Moss, R.A.; et al. Clinical Actionability of Comprehensive Genomic Profiling for Management of Rare or Refractory Cancers. Oncologist 2016, 21, 1315–1325. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V. Why the US Centers for Medicare and Medicaid Services (CMS) should have required a randomized trial of Foundation Medicine (F1CDx) before paying for it. Ann. Oncol. 2018, 29, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Institute NC. Genomic Profiling Tests Cleared by FDA Can Help Guide Cancer Treatment, Clinical Trial Enrollment 2017 [29 May 2019]. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2017/genomic-profiling-tests-cancer (accessed on 16 April 2020).
- Inal, C.; Yilmaz, E.; Cheng, H.; Zhu, C.; Pullman, J.; Gucalp, R.; Keller, S.M.; Perez-Soler, R.; Piperdi, B. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J. Clin. Oncol. 2014, 32, 8098. [Google Scholar] [CrossRef]
- Nesline, M.K.; DePietro, P.; Dy, G.K.; Early, A.; Papanicolau-Sengos, A.; Conroy, J.M.; Lenzo, F.L.; Glenn, S.T.; Chen, H.; Grand’Maison, A.; et al. Oncologist uptake of comprehensive genomic profile guided targeted therapy. Oncotarget 2019, 10, 4616–4629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilal, T.; Nakazawa, M.; Hodskins, J.; Villano, J.L.; Mathew, A.; Goel, G.; Wagner, L.; Arnold, S.M.; DeSimone, P.; Anthony, L.; et al. Comprehensive genomic profiling in routine clinical practice leads to a low rate of benefit from genotype-directed therapy. BMC Cancer 2017, 17, 602. [Google Scholar] [CrossRef] [PubMed]
- Wheler, J.J.; Janku, F.; Naing, A.; Li, Y.; Stephen, B.; Zinner, R.G.; Subbiah, V.; Fu, S.; Karp, D.D.; Falchook, G.S.; et al. Cancer Therapy Directed by Comprehensive Genomic Profiling: A Single Center Study. Cancer Res. 2016, 76, 3690–3701. [Google Scholar] [CrossRef] [Green Version]
- Schwaederle, M.; Daniels, G.A.; Piccioni, D.E.; Fanta, P.T.; Schwab, R.B.; Shimabukuro, K.A.; Parker, B.A.; Kurzrock, R. On the Road to Precision Cancer Medicine: Analysis of Genomic Biomarker Actionability in 439 Patients. Mol. Cancer Ther. 2015, 14, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Marquart, J.; Chen, E.Y.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Benefit From Genome-Driven Oncology. JAMA Oncol. 2018, 4, 1093. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.-C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Hilal, T.; Nakazawa, M.; Hodskins, J.; Arnold, S.M.; DeSimone, P.A.; Wagner, L.M.; Anthony, L.B.; Chambers, M.D.; Villano, J.L.; Mathew, A.; et al. Utility of comprehensive genomic profiling (CGP) at an NCI-designated cancer center for identifying clinically relevant genomic alterations (CRGA) and implementing genomically directed therapy (GDT). J. Clin. Oncol. 2016, 34, e18018. [Google Scholar] [CrossRef]
- Fakih, M.; O’Neil, B.; Price, T.J.; Falchook, G.S.; Desai, J.; Kuo, J.; Govindan, R.; Rasmussen, E.; Morrow, P.K.H.; Ngang, J.; et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J. Clin. Oncol. 2019, 37, 3003. [Google Scholar] [CrossRef]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Culver, K.W.; Otterson, G.A. Economic impact of next generation sequencing vs sequential single-gene testing modalities to detect genomic alterations in metastatic non-small cell lung cancer using a decision analytic model. J. Clin. Oncol. 2018, 36, 9031. [Google Scholar] [CrossRef]
- Administration USFaD. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm (accessed on 16 April 2020).
- Langer, C.; Gadgeel, S.; Borghaei, H.; Patnaik, A.; Powell, S.; Gentzler, R.; Yang, J.; Gubens, M.; Sequist, L.; Awad, M.; et al. OA04.05 KEYNOTE-021: TMB and Outcomes for Carboplatin and Pemetrexed With or Without Pembrolizumab for Nonsquamous NSCLC. J. Thorac. Oncol. 2019, 14, S216. [Google Scholar] [CrossRef]
- Garassino, M.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; Reck, M.; Hui, R.; Boyer, M.; Cristescu, R.; et al. OA04.06 Evaluation of TMB in KEYNOTE-189: Pembrolizumab Plus Chemotherapy vs Placebo Plus Chemotherapy for Nonsquamous NSCLC. J. Thorac. Oncol. 2019, 14, S216–S217. [Google Scholar] [CrossRef]
- Patel, J.M.; Knopf, J.; Reiner, E.; Bossuyt, V.; Epstein, L.; DiGiovanna, M.; Chung, G.; Silber, A.; Sanft, T.; Hofstatter, E.; et al. Mutation based treatment recommendations from next generation sequencing data: A comparison of web tools. Oncotarget 2016, 7, 22064–22076. [Google Scholar] [CrossRef] [Green Version]
- Tafe, L.J.; Gorlov, I.P.; De Abreu, F.B.; Lefferts, J.A.; Liu, X.; Pettus, J.R.; Marotti, J.D.; Bloch, K.J.; Memoli, V.A.; Suriawinata, A.A.; et al. Implementation of a Molecular Tumor Board: The Impact on Treatment Decisions for 35 Patients Evaluated at Dartmouth-Hitchcock Medical Center. Oncologist 2015, 20, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Dalton, W.B.; Forde, P.M.; Kang, H.; Connolly, R.M.; Stearns, V.; Gocke, C.D.; Eshleman, J.R.; Axilbund, J.; Petry, D.; Geoghegan, C.; et al. Personalized Medicine in the Oncology Clinic: Implementation and Outcomes of the Johns Hopkins Molecular Tumor Board. JCO Precis. Oncol. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Gupta, R.; Othman, T.; Chen, C.; Sandhu, J.; Ouyang, C.; Fakih, M. Guardant360 Circulating Tumor DNA Assay Is Concordant with FoundationOne Next-Generation Sequencing in Detecting Actionable Driver Mutations in Anti-EGFR Naive Metastatic Colorectal Cancer. Oncologist 2019, 25, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Perez, D.D.M.; Gunasekaran, M.; Scilla, K.; Lapidus, R.; Cooper, B.; Mehra, R.; Adamo, V.; Malapelle, U.; Rolfo, C.D. Liquid biopsy tracking of lung tumor evolutions over time. Expert Rev. Mol. Diagn. 2019, 19, 1099–1108. [Google Scholar] [CrossRef]
- Imperial, R.; Nazer, M.; Ahmed, Z.; Kam, A.E.; Pluard, T.J.; Bahaj, W.; Levy, M.; Kuzel, T.M.; Hayden, D.M.; Pappas, S.G.; et al. Matched Whole-Genome Sequencing (WGS) and Whole-Exome Sequencing (WES) of Tumor Tissue with Circulating Tumor DNA (ctDNA) Analysis: Complementary Modalities in Clinical Practice. Cancers 2019, 11, 1399. [Google Scholar] [CrossRef] [Green Version]
- Kelley, S.; Pantel, K. A New Era in Liquid Biopsy: From Genotype to Phenotype. Clin. Chem. 2019, 66, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bin Lim, S.; Di Lee, W.; Vasudevan, J.; Lim, W.-T.; Lim, C.T. Liquid biopsy: One cell at a time. NPJ Precis. Oncol. 2019, 3, 23–29. [Google Scholar] [CrossRef]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batth, I.S.; Mitra, A.; Rood, S.; Kopetz, S.; Menter, D.; Li, S. CTC analysis: An update on technological progress. Transl. Res. 2019, 212, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 185–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Stockley, T.L.; Oza, A.M.; Berman, H.K.; Leighl, N.B.; Knox, J.J.; Shepherd, F.A.; Chen, E.X.; Krzyzanowska, M.K.; Dhani, N.; Joshua, A.M.; et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Singal, G.; Miller, P.G.; Agarwala, V.; Li, G.; Kaushik, G.; Backenroth, D.; Gossai, A.; Frampton, G.M.; Torres, A.Z.; Lehnert, E.M.; et al. Association of Patient Characteristics and Tumor Genomics with Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 2019, 321, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Spizzo, G.; Siebert, U.; Gastl, G.; Voss, A.; Schuster, K.; Leonard, R.; Seeber, A. Cost-comparison analysis of a multiplatform tumour profiling service to guide advanced cancer treatment. Cost Eff. Resour. Alloc. 2019, 17, 23–25. [Google Scholar] [CrossRef] [Green Version]
- Signorovitch, J.; Zhou, Z.; Ryan, J.; Anhorn, R.; Chawla, A. Budget impact analysis of comprehensive genomic profiling in patients with advanced non-small cell lung cancer. J. Med. Econ. 2018, 22, 140–150. [Google Scholar] [CrossRef]
- Pezo, R.C.; Chen, T.W.-W.; Berman, H.K.; Mulligan, A.M.; Razak, A.A.; Siu, L.L.; Cescon, D.W.; Amir, E.; Elser, C.; Warr, D.G.; et al. Impact of multi-gene mutational profiling on clinical trial outcomes in metastatic breast cancer. Breast Cancer Res. Treat. 2017, 168, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, M.; Tsai, L.; Lo, C.; Yen, T.; Huang, T.Y.; Chen, C.; Fan, S.; Kuo, S.; Huang, C. Using next-generation sequencing to redefine BRCAness in triple-negative breast cancer. Cancer Sci. 2020, 111, 1375–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
| Type of Tumor | Number | Type of Tumor | Number |
|---|---|---|---|
| NSCLC | 107 | Gastric cancer | 5 |
| Colon cancer | 47 | Sarcoma | 4 |
| Ovarian cancer | 15 | Cervical cancer | 4 |
| Carcinoma of unknown primary | 14 | Myelodysplastic syndrome | 4 |
| Pancreatic cancer | 11 | Hepatocellular carcinoma | 3 |
| Uterine cancer | 11 | Thyroid cancer | 3 |
| Head and Neck cancer | 10 | Lymphoma | 3 |
| Renal cancer | 9 | Pancreatobiliary cancer | 3 |
| Breast cancer | 9 | Parotid cancer | 3 |
| Brain tumors | 8 | Multiple myeloma | 2 |
| Prostate cancer | 7 | Small cell lung cancer | 2 |
| Gallbladder cancer | 7 | Thymoma | 2 |
| Rectal cancer | 11 | B-ALL | 2 |
| Bladder cancer | 6 | Melanoma | 2 |
| Esophageal cancer | 6 | Germ cell tumors | 2 |
| Cholangiocarcinoma | 5 | Others | 25 |
| OncoKB Level of Evidence | Number | Percentage |
|---|---|---|
| Level 1 | 32 | 7.8 |
| Level 2A | 9 | 2.2 |
| Level 2B | 31 | 7.6 |
| Level 3A | 4 | 0.9 |
| Level 3B | 31 | 7.6 |
| Level 4 | 72 | 17.6 |
| Level R1 | 35 | 8.6 |
| No level assigned, oncogenic | 71 | 17.4 |
| No level assigned, likely oncogenic | 57 | 14.0 |
| No level assigned, oncogenic function unknown | 39 | 9.6 |
| No level assigned, no information available | 8 | 2.0 |
| No level assigned, Tumor mutational burden | 8 | 4.4 |
| No level assigned, Microsatellite Instability | 18 | 0.2 |
| Diagnosis | Molecular Alterations | Management Change | Observed Benefit | |
|---|---|---|---|---|
| 1. | Lung adenocarcinoma | EML4-ALK fusion (Variant 1) | Crizotinib | Lost to follow-up |
| 2. | Lung adenocarcinoma | EGFR amplification, G719A | Erlotinib continued | Yes |
| 3. | Lung adenocarcinoma | EGFR G719A, Q701L, amplification | Erlotinib continued | Yes |
| 4. | Lung adenocarcinoma | EGFR E746_A750del MET amplification | Crizotinib-Erlotinib | Lost to follow-up |
| 5. | Poorly differentiated NSCLC, sarcomatoid morphology | NTRK1 TPM3-NTRK1 fusion | Died prior to giving Crizotinib | N/A |
| 6. | Lung adenocarcinoma | EGFR amplification, exon 19 deletion | Afatinib | Yes |
| 7. | Lung adenocarcinoma | EGFR exon 19 deletion, T790M | Osimertinib | Yes |
| 8. | Lung adenocarcinoma | ALK EML4-ALK fusion (Variant 1) | Alectinib | Yes |
| 9. | Lung adenocarcinoma | BRCA2 S1099* MET amplification | Declined participation in MATCH study | N/A |
| 10. | Lung adenocarcinoma | EGFR L858R | Died prior to starting EGFR-TKI | N/A |
| 11. | Lung adenocarcinoma | EGFR exon 19 deletion, T790M, L792F, C797S | Osimertinib continued beyond progression | N/A |
| 12. | Medullary thyroid cancer | RET V804M | Cabozantinib Lenvatinib Phase 1 study of MGCD516 | Yes |
| 13. | Poorly differentiated NSCLC | MET amplification | Died prior to planned phase 1 trial of MGCD516 | N/A |
| 14. | Poorly differentiated NSCLC | MET amplification | Died prior to planned phase 1 trial of MGCD516 | N/A |
| 15. | Lung adenocarcinoma | EGFR exon 19 deletion (L747_S752del) T790M | Osimertinib | Yes |
| 16. | Lung adenocarcinoma Urothelial bladder cancer | Numerous | Clarified primary tumor to be urothelial in origin | Yes |
| 17. | Gastric adenocarcinoma | MSI-High | Pembrolizumab | Yes |
| 18. | Lung adenocarcinoma | EGFR exon 19 deletion (E746_A750del), T790M | Osimertinib | Yes |
| 19. | Lung adenocarcinoma | EGFR amplification, L858R, R776C, T790M MET amplification | Osimertinib + crizotinib | Yes |
| 20. | Lacrimal duct carcinoma | ERBB2 amplification | Trastuzumab | No |
| 21. | Nasopharyngeal adenoid cystic carcinoma | PIK3CA H1047R | Taselisib on MATCH study | Yes |
| 22. | Lung adenocarcinoma | EGFR exon 19 deletion | Erlotinib after clearance of T790M | No |
| 23. | Thymoma | CDKN2A/B loss KDM6A W1194* | Phase I/IIa trial of ALRN-6924 in patients with wild typeTP53 | Yes |
| 24. | Invasive ductal breast cancer | CCND1 amplification | Abemaciclib | No |
| 25. | Lung adenocarcinoma | RET KIF5B-RET fusion, RET-KIF5B fusion | Phase 1/1b MGCD516 | Not documented, patient withdrew from study |
| 26. | Lung adenocarcinoma | BRAF V600E | Vemurafenib Dabrafenib +Trametinib | Yes |
| 27. | Lung adenocarcinoma | EGFR exon 19 deletion | Erlotinib after clearance of T790M | No |
| 28. | Lung adenocarcinoma | EGFR amplification, exon 19 deletion (E746_A750del), T790M | Osimertinib | Yes |
| 29. | Rectal adenocarcinoma | ERBB2 amplification, V777L | Ado-trastuzumab emtansine on MATCH study | Yes |
| 30. | Esophageal adenocarcinoma | ERBB2 amplification | Trastuzumab | Patient lost to follow-up/did not complete treatment |
| 31. | Carcinoma of unknown primary, likely upper GI/pancreaticobiliary origin | MET amplification | Planned for crizotinib but not approved by insurance | N/A |
| 32. | Lung adenocarcinoma | EGFR amplification, L858R, T790M | Osimertinib | Yes |
| 33. | Invasive ductal breast cancer | CCND1 amplification | Palbociclib | No |
| 34. | Lung adenocarcinoma | EGFR exon 19 deletion | Afatinib | Yes |
| 35. | Salivary ductal carcinoma | VEGFA amplification | Sorafenib | No |
| 36. | Colon adenocarcinoma | Numerous | Pembrolizumab based on numerous mutations detected and concern for MSI status | No |
| 37. | Esophageal squamous cell carcinoma | EGFR amplification | Panitumumab | No |
| 38. | Lung adenocarcinoma | ALK EML4-ALK fusion (Variant 3a/b) | Alectinib | Yes |
| 39. | Lung adenocarcinoma | EGFR L964L | Erlotinib | Yes |
| 40. | Follicular dendritic cell sarcoma | AKT2 amplification | Everolimus | Yes |
| 41. | Lung adenocarcinoma | MET H1094R | Died prior to starting crizotinib | N/A |
| 42. | Lung adenocarcinoma | MET exon 14 splice site (D1010N) | Crizotinib | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.P.; Shum, E.; Rajdev, L.; Cheng, H.; Goel, S.; Perez-Soler, R.; Halmos, B. Impact and Diagnostic Gaps of Comprehensive Genomic Profiling in Real-World Clinical Practice. Cancers 2020, 12, 1156. https://doi.org/10.3390/cancers12051156
Singh AP, Shum E, Rajdev L, Cheng H, Goel S, Perez-Soler R, Halmos B. Impact and Diagnostic Gaps of Comprehensive Genomic Profiling in Real-World Clinical Practice. Cancers. 2020; 12(5):1156. https://doi.org/10.3390/cancers12051156
Chicago/Turabian StyleSingh, Aditi P., Elaine Shum, Lakshmi Rajdev, Haiying Cheng, Sanjay Goel, Roman Perez-Soler, and Balazs Halmos. 2020. "Impact and Diagnostic Gaps of Comprehensive Genomic Profiling in Real-World Clinical Practice" Cancers 12, no. 5: 1156. https://doi.org/10.3390/cancers12051156
APA StyleSingh, A. P., Shum, E., Rajdev, L., Cheng, H., Goel, S., Perez-Soler, R., & Halmos, B. (2020). Impact and Diagnostic Gaps of Comprehensive Genomic Profiling in Real-World Clinical Practice. Cancers, 12(5), 1156. https://doi.org/10.3390/cancers12051156






