Predictive Value of Early Post-Treatment Diffusion-Weighted MRI for Recurrence or Tumor Progression of Head and Neck Squamous Cell Carcinoma Treated with Chemo-Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Outcomes
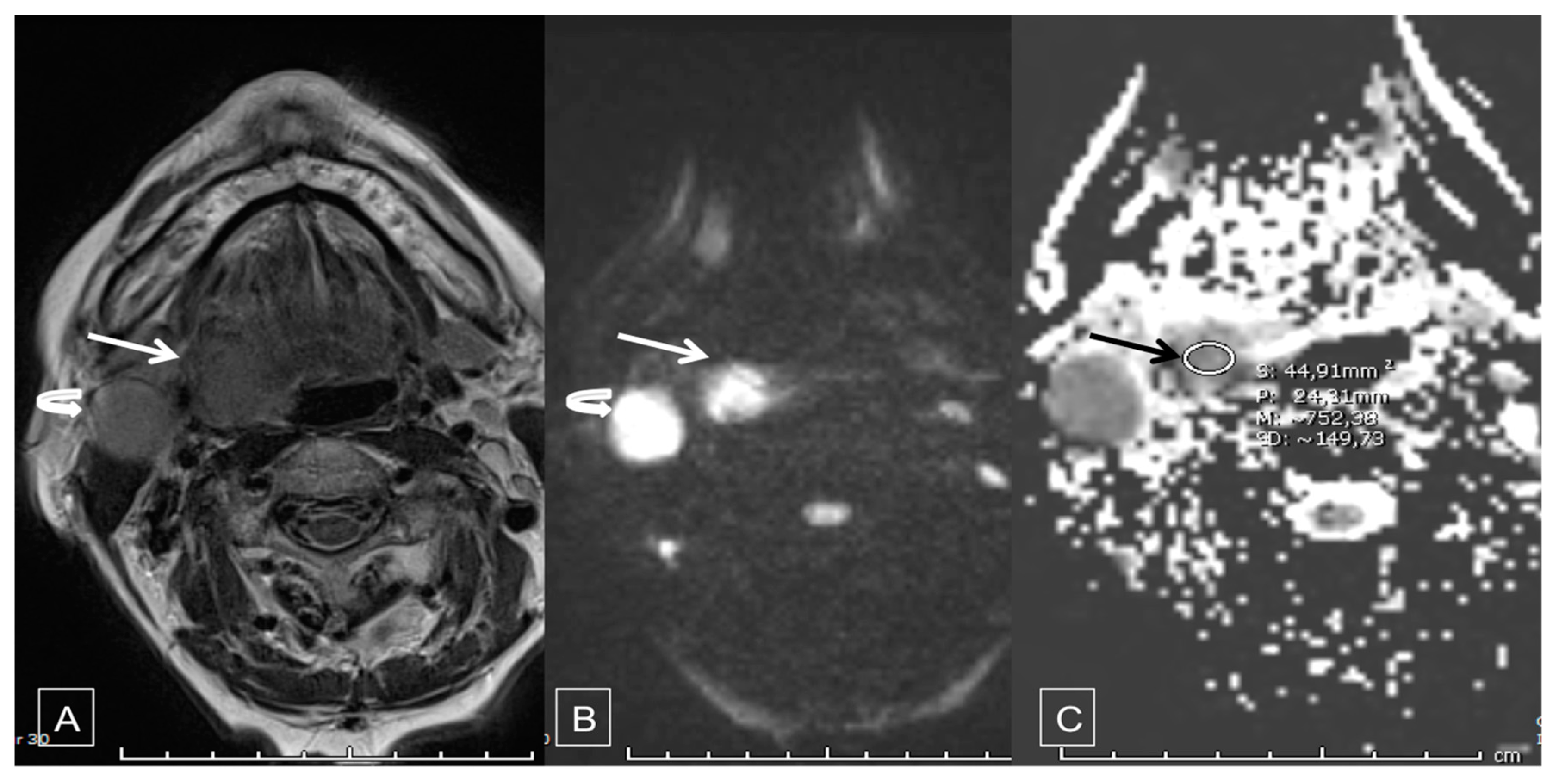
2.3. Diffusion-Weighted MRI
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics, Treatment and Follow-up
3.2. Factors Associated with Tumor Residue
3.3. Factors Associated with Post-Treatment Disease Progression or Early Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aupérin, A. Epidemiology of head and neck cancers. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Périé, S.; Meyers, M.; Mazzaschi, O.; Chanel, O.D.C.; Baujat, B.; Guily, J.L.S. Epidemiology and anatomy of head and neck cancers. Bull Cancer 2014, 101, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Buiret, G.; Combe, C.; Favrel, V.; Pommier, P.; Martin, L.; Ecochard, R.; Fayette, J.; Tartas, S.; Ramade, A.; Ceruse, P. A Retrospective, Multicenter Study of the Tolerance of Induction Chemotherapy With Docetaxel, Cisplatin, and 5-Fluorouracil Followed by Radiotherapy With Concomitant Cetuximab in 46 Cases of Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. 2010, 77, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ceruse, P.; Cosmidis, A.; Belot, A.; Rabilloud, M.; Fuchsmann, C.; Poupart, M.; Ramade, A.; Tartas, S.; Favrel, V.; Pommier, P.; et al. A pyriform sinus cancer organ preservation strategy comprising induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil, followed by potentiated radiotherapy. Anti-Cancer Drugs 2014, 25, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Fayette, J.; Bonnin, N.; Ferlay, C.; Lallemant, B.; Ramade, A.; Favrel, V.; Zrounba, P.; Chabaud, S.; Pommier, P.; Poupart, M.; et al. Neoadjuvant TPF in locally advanced head and neck cancer can be followed by radiotherapy combined with cisplatin or cetuximab. Anti-Cancer Drugs 2013, 24, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Pointreau, Y.; Atean, I.; Fayette, J.; Calais, G.; Lefebvre, J.-L. Induction chemotherapy in head and neck cancer. Anti-Cancer Drugs 2011, 22, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.-L.; Chevalier, D.; Luboinski, B.; Kirkpatrick, A.; Collette, L.; Sahmoud, T. Larynx Preservation in Pyriform Sinus Cancer: Preliminary Results of a European Organization for Research and Treatment of Cancer Phase III Trial. J. Natl. Cancer Inst. 1996, 88, 890–899. [Google Scholar] [CrossRef]
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; Laramore, G.E.; Endicott, J.W.; McClatchey, K.; Henderson, W.G.; et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin with or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Bonner, J.A.; Chin, S.; Spencer, S. Cetuximab and Radiotherapy in Laryngeal Preservanion-Reply. JAMA Otolaryngol. Neck Surg. 2017, 143, 526. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Giralt, J.; Harari, P.; Spencer, S.; Schulten, J.; Hossain, A.; Chang, S.-C.; Chin, S.; Baselga, J. Cetuximab and Radiotherapy in Laryngeal Preservation for Cancers of the Larynx and Hypopharynx: A Secondary Analysis of a Randomized Clinical Trial. JAMA Otolaryngol. Neck Surg. 2016, 142, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, N.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- De Raucourt, D.; Rame, J.-P.; Louis, M.-Y. [Principal treatments of head and neck cancer]. La Rev. Prat. 2006, 56, 1662–1666. [Google Scholar]
- Blanchard, D.; Barry, B.; De Raucourt, D.; Choussy, O.; Dessard-Diana, B.; Hans, S.; Lafarge, D. Guidelines update: Post-treatment follow-up of adult head and neck squamous cell carcinoma: Screening for metastasis and metachronous esophageal and bronchial locations. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 217–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halimi, C.; Barry, B.; De Raucourt, D.; Choussy, O.; Dessard-Diana, B.; Hans, S.; Lafarge, D. Guidelines of the French Society of Otorhinolaryngology (SFORL), short version. Diagnosis of local recurrence and metachronous locations in head and neck oncology. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 287–290. [Google Scholar] [CrossRef]
- Boysen, M.; Lövdal, O.; Winther, F.; Tausjö, J. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur. J. Cancer 1992, 28, 426–430. [Google Scholar] [CrossRef]
- Haas, I.; Hauser, U.; Ganzer, U. The dilemma of follow-up in head and neck cancer patients. Eur. Arch. Oto-Rhino-Laryngol. 2001, 258, 177–183. [Google Scholar] [CrossRef]
- Snow, G.B. Follow-up in patients treated for head and neck cancer: How frequent, how thorough and for how long? Eur. J. Cancer 1992, 28, 315–316. [Google Scholar] [CrossRef]
- O’Meara, W.P.; Thiringer, J.K.; Johnstone, P.A. Follow-up of head and neck cancer patients post-radiotherapy. Radiother. Oncol. 2003, 66, 323–326. [Google Scholar] [CrossRef]
- Ritoe, S.C.; Krabbe, P.F.M.; Kaanders, J.H.A.M.; Hoogen, F.J.A.V.D.; Marres, H.A.M. Value of routine follow-up for patients cured of laryngeal carcinoma. Cancer 2004, 101, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Fornasa, F. Diffusion-weighted Magnetic Resonance Imaging: What Makes Water Run Fast or Slow? J. Clin. Imaging Sci. 2011, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Sinkus, R.; Van Beers, B.E.; Vilgrain, V.; DeSouza, N.; Waterton, J. Apparent diffusion coefficient from magnetic resonance imaging as a biomarker in oncology drug development. Eur. J. Cancer 2012, 48, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Rehemtulla, A.; Ross, B.D. Diffusion Magnetic Resonance Imaging: A Biomarker for Treatment Response in Oncology. J. Clin. Oncol. 2007, 25, 4104–4109. [Google Scholar] [CrossRef]
- Harry, V.N.; Semple, S.I.; E Parkin, D.; Gilbert, F.J. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010, 11, 92–102. [Google Scholar] [CrossRef]
- Padhani, A.R.; Liu, G.; Koh, D.-M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Surov, A.; Ginat, D.T.; Sanverdi, E.; Lim, C.T.; Hakyemez, B.; Yogi, A.; Cabada, T.; Wienke, A. Use of Diffusion Weighted Imaging in Differentiating Between Maligant and Benign Meningiomas. A Multicenter Analysis. World Neurosurg. 2016, 88, 598–602. [Google Scholar]
- Surov, A.; Meyer, H.-J.; Wienke, A. Can apparent diffusion coefficient (ADC) distinguish breast cancer from benign breast findings? A meta-analysis based on 13 847 lesions. BMC Cancer 2019, 19, 955. [Google Scholar] [CrossRef]
- Wang, J.; Takashima, S.; Takayama, F.; Kawakami, S.; Saito, A.; Matsushita, T.; Momose, M.; Ishiyama, T. Head and Neck Lesions: Characterization with Diffusion-weighted Echo-planar MR Imaging. Radiology 2001, 220, 621–630. [Google Scholar] [CrossRef]
- Driessen, J.P.; Caldas-Magalhaes, J.; Janssen, L.M.; Pameijer, F.A.; Kooij, N.; Terhaard, C.H.J.; Grolman, W.; Philippens, M.E.P. Diffusion-weighted MR Imaging in Laryngeal and Hypopharyngeal Carcinoma: Association between Apparent Diffusion Coefficient and Histologic Findings. Radiology 2014, 272, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.-J.; Leifels, L.; Hamerla, G.; Höhn, A.K.; Surov, A. ADC-histogram analysis in head and neck squamous cell carcinoma. Associations with different histopathological features including expression of EGFR, VEGF, HIF-1α, Her 2 and p53. A preliminary study. Magn. Reson. Imaging 2018, 54, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Wienke, A. Can Imaging Parameters Provide Information Regarding Histopathology in Head and Neck Squamous Cell Carcinoma? A Meta-Analysis. Transl. Oncol. 2018, 11, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Clauser, P.; Chang, Y.-W.; Li, L.; Martincich, L.; Partridge, S.C.; Kim, J.Y.; Meyer, H.-J.; Wienke, A. Can diffusion-weighted imaging predict tumor grade and expression of Ki-67 in breast cancer? A multicenter analysis. Breast Cancer Res. 2018, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Kyriazi, S.; Collins, D.; Messiou, C.; Pennert, K.; Davidson, R.; Giles, S.; Kaye, S.; DeSouza, N. Metastatic Ovarian and Primary Peritoneal Cancer: Assessing Chemotherapy Response with Diffusion-weighted MR Imaging—Value of Histogram Analysis of Apparent Diffusion Coefficients. Radiology 2011, 261, 182–192. [Google Scholar] [CrossRef]
- Meyer, H.-J.; Fiedler, E.; Kornhuber, M.; Spielmann, R.P.; Surov, A. Comparison of diffusion-weighted imaging findings in brain metastases of different origin. Clin. Imaging 2015, 39, 965–969. [Google Scholar] [CrossRef]
- Garbajs, M.; Strojan, P.; Surlan-Popovic, K. Prognostic role of diffusion weighted and dynamic contrast-enhanced MRI in loco-regionally advanced head and neck cancer treated with concomitant chemoradiotherapy. Radiol. Oncol. 2019, 53, 39–48. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, L.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef]
- Galbán, C.J.; Mukherji, S.K.; Chenevert, T.L.; Meyer, C.R.; Hamstra, D.A.; Bland, P.H.; Johnson, T.D.; Moffat, B.A.; Rehemtulla, A.; Eisbruch, A.; et al. A Feasibility Study of Parametric Response Map Analysis of Diffusion-Weighted Magnetic Resonance Imaging Scans of Head and Neck Cancer Patients for Providing Early Detection of Therapeutic Efficacy. Transl. Oncol. 2009, 2, 184–190. [Google Scholar] [CrossRef][Green Version]
- Cui, Y.; Zhang, X.-P.; Sun, Y.-S.; Tang, L.; Shen, L. Apparent Diffusion Coefficient: Potential Imaging Biomarker for Prediction and Early Detection of Response to Chemotherapy in Hepatic Metastases. Radiology 2008, 248, 894–900. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 1: ADCmean. Oncotarget 2017, 8, 75434–75444. [Google Scholar]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 2: ADCmin. Oncotarget 2018, 9, 8675–8680. [Google Scholar] [CrossRef] [PubMed]
- Nunnally, J.C.; Bernstein, I.H.; Berge, J.M.t. Psychom Theory; McGraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Vandecaveye, V.; De Keyzer, F.; Nuyts, S.; Deraedt, K.; Dirix, P.; Hamaekers, P.; Poorten, V.V.; Delaere, P.R.; Hermans, R. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: Correlation between radiologic and histopathologic findings. Int. J. Radiat. Oncol. 2007, 67, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.A.K.A.; Kandeel, A.; Soliman, N.; El-Shenshawy, H.; Kamel, Y.; Nada, N.; Denewar, A. Role of Diffusion-Weighted Echo-Planar MR Imaging in Differentiation of Residual or Recurrent Head and Neck Tumors and Posttreatment Changes. Am. J. Neuroradiol. 2007, 28, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.W.T.; Zbaeren, P.; Geretschlaeger, A.; Vermathen, P.; De Keyzer, F.; Thoeny, H.C. Diffusion-weighted MR imaging including bi-exponential fitting for the detection of recurrent or residual tumour after (chemo)radiotherapy for laryngeal and hypopharyngeal cancers. Eur. Radiol. 2012, 23, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Vandecaveye, V.; Dirix, P.; De Keyzer, F.; De Beeck, K.O.; Poorten, V.V.; Hauben, E.I.; Lambrecht, M.; Nuyts, S.; Hermans, R. Diffusion-Weighted Magnetic Resonance Imaging Early After Chemoradiotherapy to Monitor Treatment Response in Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2012, 82, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Chow, K.-K.; Yu, K.-H.; Mo, F.K.F.; Yeung, D.K.W.; Yuan, J.; Bhatia, K.S.; Vlantis, A.; Ahuja, A.T. Head and Neck Squamous Cell Carcinoma: Diagnostic Performance of Diffusion-weighted MR Imaging for the Prediction of Treatment Response. Radiology 2013, 266, 531–538. [Google Scholar] [CrossRef]
- De Perrot, T.; Lenoir, V.; Ayllón, M.D.; Dulguerov, N.; Pusztaszeri, M.; Becker, M. Apparent Diffusion Coefficient Histograms of Human Papillomavirus–Positive and Human Papillomavirus–Negative Head and Neck Squamous Cell Carcinoma: Assessment of Tumor Heterogeneity and Comparison with Histopathology. Am. J. Neuroradiol. 2017, 38, 2153–2160. [Google Scholar] [CrossRef]
- Ryan, W.R.; Fee, W.E.; Le, Q.-T.; Pinto, H.A. Positron-Emission Tomography for Surveillance of Head and Neck Cancer. Laryngoscope 2005, 115, 645–650. [Google Scholar] [CrossRef]
- Yao, M.; Luo, P.; Hoffman, H.T.; Chang, K.; Graham, M.M.; Menda, Y.; Tan, H.; Buatti, J.M. Pathology and FDG PET Correlation of Residual Lymph Nodes in Head and Neck Cancer After Radiation Treatment. Am. J. Clin. Oncol. 2007, 30, 264–270. [Google Scholar] [CrossRef]
- Subramaniam, R.M.; Truong, M.; Peller, P.; Sakai, O.; Mercier, G. Fluorodeoxyglucose–Positron-Emission Tomography Imaging of Head and Neck Squamous Cell Cancer. Am. J. Neuroradiol. 2009, 31, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Yoon, D.Y.; Moon, J.Y.; Baek, S.; Han, Y.M.; Seo, Y.L.; Yun, E.J. Detection of loco-regional recurrence in malignant head and neck tumors: A comparison of CT, MRI, and FDG PET-CT. Acta Radiol. 2018, 60, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Preda, L.; Conte, G.; Bonello, L.; Giannitto, C.; Travaini, L.L.; Raimondi, S.; Summers, P.; Mohssen, A.; Alterio, D.; Rocca, M.C.; et al. Combining standardized uptake value of FDG-PET and apparent diffusion coefficient of DW-MRI improves risk stratification in head and neck squamous cell carcinoma. Eur. Radiol. 2016, 26, 4432–4441. [Google Scholar] [CrossRef] [PubMed]
- Yom, S.S.; Machtay, M.; Biel, M.A.; Sinard, R.J.; El-Naggar, A.K.; Weber, R.S.; Rosenthal, D.I. Survival Impact of Planned Restaging and Early Surgical Salvage Following Definitive Chemoradiation for Locally Advanced Squamous Cell Carcinomas of the Oropharynx and Hypopharynx. Am. J. Clin. Oncol. 2005, 28, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P.; Waltonen, J.D.; Ozer, E.; Hall, N.C.; Schuller, D.E.; Agrawal, A. Results of Salvage Treatment of the Neck in Patients with Oral Cancer. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Villaret, U.B.; Amdur, R.; Hinerman, R.W.; Mancuso, A.A. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck 2002, 24, 1012–1018. [Google Scholar] [CrossRef]
- Sivan, V.; Vozenin, M.-C.; Tricaud, Y.; Lefaix, J.-L.; Cosset, J.-M.; DuBray, B.; Martin, M.T. Altered proliferation and differentiation of human epidermis in cases of skin fibrosis after radiotherapy. Int. J. Radiat. Oncol. 2002, 53, 385–393. [Google Scholar] [CrossRef]
- Temam, S.; Koka, V.; Mamelle, G.; Julieron, M.; Carmantrant, R.; Marandas, P.; Janot, F.; Bourhis, J.; Luboinski, B. Treatment of the N0 neck during salvage surgery after radiotherapy of head and neck squamous cell carcinoma. Head Neck 2005, 27, 653–658. [Google Scholar] [CrossRef]



| Characteristics 1 | Value |
|---|---|
| Patients | |
| Age at inclusion (year) − Mean ± SD | 60.8 ± 12.1 |
| Sexe Ratio (M/F) | 50/9 |
| Tobacco use | |
| No | 8 (13.6) |
| Yes | 51 (86.4) |
| Active | 42 (82.4) |
| weaned | 9 (17.6) |
| Alcohol | 25 (42.4) |
| Body Mass Index (kg/m2) − Mean ± SD | 23.9 ± 4.9 |
| Head and Neck Tumor | |
| Unique location | 59 (100) |
| Location | |
| Oropharynx | 33 (55.9) |
| Oral cavity | 8 (13.6) |
| Larynx | 7 (11.9) |
| Hypopharynx | 8 (13.6) |
| Cavum | 1 (1.7) |
| Sinus | 1 (1.7) |
| Parotid gland | 1 (1.7) |
| HPV status (among oropharynx location) | |
| Positive | 13/33 (39.4) |
| Negative | 20/33 (60.6) |
| TNM classification | |
| T1 | 4 (6.8) |
| T2 | 5 (8.5) |
| T3 | 9 (15.2) |
| T4 | 41 (69.5) |
| N0 | 9 (15.2) |
| N1 | 8 (13.6) |
| N2a | 1 (1.7) |
| N2b | 10 (17.0) |
| N2c | 22 (37.3) |
| N3 | 9 (15.2) |
| M0 | 57 (96.6) |
| M1 | 2 (3.4) |
| Histology | |
| Squamous cell carcinoma | 59 (100) |
| Différentiation | |
| Well | 28 (47.5) |
| Middle | 24 (40.7) |
| Poor | 7 (11.9) |
| Therapeutic sequence decided | |
| Induction chemotherapy | 10 (17.0) |
| Radiotherapy | 59 (100) |
| Exclusive | 19 |
| With Cetuximab | 25 |
| With Cisplatine | 15 |
| Variables 1 | Variables 1 | Absence of Residue N = 27 | Presence of Residue N = 19 | Univariate Analysis | Multivariate Analysis 2 | |
|---|---|---|---|---|---|---|
| p Value | OR [CI 95%] | p Value | ||||
| Tumor diameter on MRI (mm) − Mean ± SD | 44.3 ± 18.8 | 52.6 ± 21.0 | 0.20 | |||
| Median ADC tumor (s/mm2) − Mean ± SD | 0.79 ± 0.13 | 0.56 ± 0.11 | <0.0001 | |||
| Patients with ADC tumor ≥0.7 | 24 (88.9) | 5 (26.3) | <0.0001 | 22.6 [4.9–103.6] | <0.0001 | |
| <0.7 | 3 (11.1) | 14 (73.7) | ||||
| Tumor location | Oral cavity | 2 (7.4) | 4 (21.0) | 0.23 | ||
| Oropharynx | 16 (59.3) | 9 (47.4) | ||||
| Larynx | 5 (18.5) | 1 (5.3) | ||||
| Hypopharynx | 4 (14.8) | 2 (10.5) | ||||
| Sinus | 0 (0.0) | 1 (5.3) | ||||
| Cavum | 0 (0.0) | 1 (5.3) | ||||
| Parotid | 0 (0.0) | 1 (5.3) | ||||
| Initial T status | T1 | 2 (7.4) | 1 (5.3) | 0.64 | ||
| T2 | 3 (11.1) | 1 (5.3) | ||||
| T3 | 6 (22.2) | 2 (10.5) | ||||
| T4 | 16 (59.3) | 15 (79.0) | ||||
| Initial N status | N0 | 3 (11.1) | 6 (31.6) | 0.13 | ||
| N+ | 24 (88.9) | 13 (68.4) | ||||
| Initial M status | M0 | 27 (100.0) | 18 (94.7) | 0.41 | ||
| M+ | 0 (0.0) | 1 (5.3) | ||||
| Induction chemotherapy | Yes | 5 (18.5) | 3 (15.8) | 0.99 | ||
| No | 22 (81.5) | 16 (84.2) | ||||
| Therapeutic sequence | Radiotherapy exclusive | 6 (22.2) | 5 (26.3) | 0.18 | ||
| Radiotherapy with Cetuximab | 10 (37.1) | 11 (57.9) | ||||
| Radiotherapy with Cisplatine | 11 (40.7) | 3 (15.8) | ||||
| Variables | Variables 1 | Time of Disease-Free Survival (Days) 1 | Univariate Analysis p Value |
|---|---|---|---|
| Tumor diameter at first MRI (mm) Mean ± SD | 0.31 | ||
| Delta ADC (ADC2–ADC1) Mean ± SD | 0.0009 | ||
| Patients with Delta ADC (ADC2–ADC1) | ≥0.7 | 377.5 [286–402] | <0.0001 |
| <0.7 | 253 [198–370] | ||
| Induction chemotherapy | Yes | 343 [253–396] | 0.82 |
| No | 353 [198–402] | ||
| Therapeutic sequence | Radiotherapy exclusive | 336 [243–386] | 0.35 |
| Radiotherapy with Cetuximab | 338 [198–396] | ||
| Radiotherapy with Cisplatin | 370 [225–402] | ||
| Tumor location | Oral cavity | 342.5 [315–370] | 0.71 |
| Oropharynx | 346 [198–402] | ||
| Larynx | 348 [243–391] | ||
| Hypopharynx | 383 [343–394] | ||
| Initial T status | T1 | 319.5 [253–386] | 0.69 |
| T2 | 381 [280–389] | ||
| T3 | 340.5 [243–391] | ||
| T4 | 357 [198–402] | ||
| Initial N status | N0 | 350 [198–402] | 0.28 |
| N+ | 300 [243–357] | ||
| Initial M status | M0 | 350.5 [198–402] | - |
| M+ | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenet, E.; Barbe, C.; Hoeffel, C.; Dubernard, X.; Merol, J.-C.; Fath, L.; Servagi-Vernat, S.; Labrousse, M. Predictive Value of Early Post-Treatment Diffusion-Weighted MRI for Recurrence or Tumor Progression of Head and Neck Squamous Cell Carcinoma Treated with Chemo-Radiotherapy. Cancers 2020, 12, 1234. https://doi.org/10.3390/cancers12051234
Brenet E, Barbe C, Hoeffel C, Dubernard X, Merol J-C, Fath L, Servagi-Vernat S, Labrousse M. Predictive Value of Early Post-Treatment Diffusion-Weighted MRI for Recurrence or Tumor Progression of Head and Neck Squamous Cell Carcinoma Treated with Chemo-Radiotherapy. Cancers. 2020; 12(5):1234. https://doi.org/10.3390/cancers12051234
Chicago/Turabian StyleBrenet, Esteban, Coralie Barbe, Christine Hoeffel, Xavier Dubernard, Jean-Claude Merol, Léa Fath, Stéphanie Servagi-Vernat, and Marc Labrousse. 2020. "Predictive Value of Early Post-Treatment Diffusion-Weighted MRI for Recurrence or Tumor Progression of Head and Neck Squamous Cell Carcinoma Treated with Chemo-Radiotherapy" Cancers 12, no. 5: 1234. https://doi.org/10.3390/cancers12051234
APA StyleBrenet, E., Barbe, C., Hoeffel, C., Dubernard, X., Merol, J.-C., Fath, L., Servagi-Vernat, S., & Labrousse, M. (2020). Predictive Value of Early Post-Treatment Diffusion-Weighted MRI for Recurrence or Tumor Progression of Head and Neck Squamous Cell Carcinoma Treated with Chemo-Radiotherapy. Cancers, 12(5), 1234. https://doi.org/10.3390/cancers12051234





