Relapse-Free Survival and PD-L1 Expression in First High- and Low-Grade Relapsed Luminal, Basal and Double-Negative P53-Mutant Non-Muscular Invasive Bladder Cancer Depending on Previous Chemo- and Immunotherapy
Abstract
:1. Introduction
2. Results
2.1. PD-L1 Expression in Primary and Relapsed Non-Invasive Urothelial Tumors
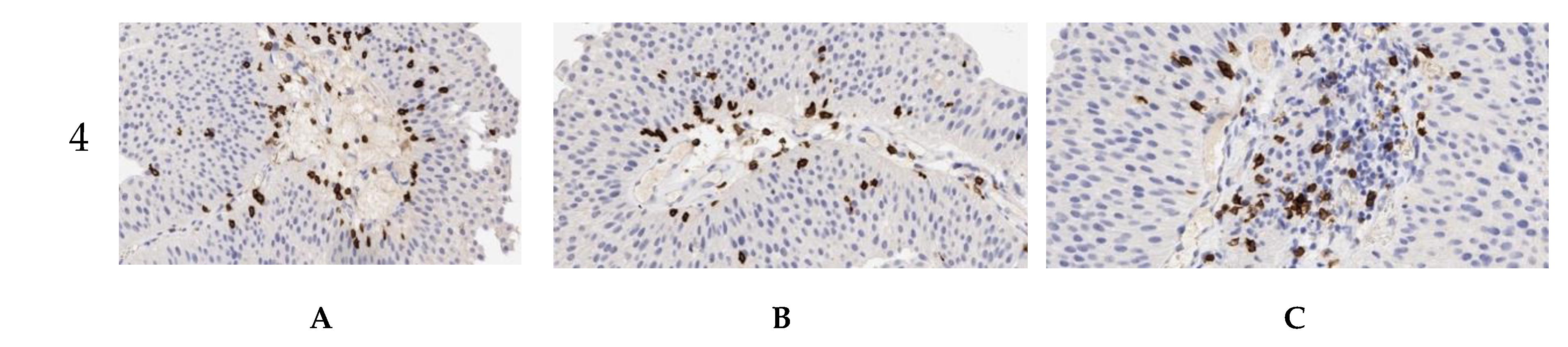
2.2. Intensity of Tumor-Associated Immune Cells’ Infiltration in Primary and Relapsed NMIBC
2.3. Mapping of PD-L1 Expressing Status of Relapsed NMIBCs
2.4. Association Between PD-L1 Expression and Relapse-Free Survival in Chemotherapy/Immunotherapy-Naive and Frontline Treated NMIBC of Main Molecular Subtypes with High and Low Malignant Potential
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Ethic Statement
4.3. Data Sources and Study Population
4.3.1. Sample Size and Study Population
- Age > 18 years at the time of first diagnosis;
- Diagnosis of NMIBC;
- With available formalin-fixed tumor tissue sample for IHC testing.
4.3.2. Data Sources
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guerin |
| CIS | Cancer in situ |
| EORTC | European Organization for Research and Treatment of Cancer |
| GATA3 | Transcription factor encoded by GATA3 gene |
| HE | Histological examination |
| IC+ | Positively anti-PD-L1-stained immune cells |
| ICP | Immune cells population |
| IHC | Immunohistochemistry |
| KRT5/6 | Keratin 5 and 6 |
| MIBC | Muscular-invasive bladder cancer |
| NMIBC | Non-muscular invasive bladder cancer |
| PD1 | Programmed death receptor 1 |
| PD-L1 | Programmed death receptor ligand 1 |
| RFS | Relapse-free survival |
| TCP | Tumor cells population |
| TUR | Transurethral resection |
| WHO | World Health Organization |
Appendix A


Appendix B

References
- Eifler, J.B.; Scarpato, K.R.; Clark, P.E. Management of noninvasive bladder cancers. Curr. Opin. Oncol. 2015, 27, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdel, D.E.; Zhang, S.; et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine 2016, 12, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Chen, S.; Yang, L.; Li, Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J. Hematol. Oncol. 2013, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brower, V. Anti-PD-L1 inhibitor durvalumab in bladder cancer. Lancet Oncol. 2016, 17, e275. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zheng, F.F.; Mao, Y.L.; Ye, L.F.; Bian, J.; Lai, D.H.; Ye, Y.L.; Dai, Y.P. Effects of programmed death-ligand 1 expression on OK-432 immunotherapy following transurethral resection in non-muscle invasive bladder cancer. Oncol. Lett. 2017, 13, 4818–4824. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef]
- Xylinas, E.; Robinson, B.D.; Kluth, L.A.; Volkmer, B.G.; Hautmann, R.; Kufer, R.; Zerbib, M.; Kwon, E.; Thompson, R.H.; Boorjian, S.A.; et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur. J. Surg. Oncol. 2014, 40, 121–127. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Sheinin, Y.; Crispen, P.L.; Farmer, S.A.; Lohse, C.M.; Kuntz, S.M. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin. Cancer Res. 2008, 14, 4800–4808. [Google Scholar] [CrossRef] [Green Version]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Worst, T.S.; Stoehr, R.; Eckstein, M.; Denzinger, S.; Burger, M.; Hartmann, A. High PDL1 mRNA expression predicts better survival of stage pT1 non-muscle-invasive bladder cancer (NMIBC) patients. Cancer Immunol. Immunother. 2018, 67, 403–412. [Google Scholar] [CrossRef]
- Kawahara, T.; Ishiguro, Y.; Ohtake, S.; Kato, I.; Ito, Y.; Ito, H.; Makiyama, K.; Kondo, K.; Miyoshi, Y.; Yumura, Y.; et al. PD-1 and PD-L1 are more highly expressed in high-grade bladder cancer than in low-grade cases: PD-L1 might function as a mediator of stage progression in bladder cancer. BMC Urol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can. Urol. Assoc. J. 2009, 3, S193–S198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukup, V.; Capoun, O.; Cohen, D.; Hernandez, V.; Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Lam, T.; MacLennan, S.; et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non-muscle-invasive bladder cancer: A European association of urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur. Urol. 2017, 72, 801–813. [Google Scholar] [PubMed] [Green Version]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Descamps-Dudez, O. Heterogeneity in PD-L1 expression and CD8+ infiltrates in low grade versus high grade serous ovarian carcinomas. Personal communication, 2016. [Google Scholar] [CrossRef]
- Ventana PD-L1 (SP263) Assay Staining in Urothelial Carcinoma. Interpretation Guide. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160046c.pdf (accessed on 26 December 2019).
- Davick, J.J.; Frierson, H.F.; Smolkin, M.; Gru, A.A. PD-L1 expression in tumor cells and the immunologic milieu of bladder carcinomas: A pathologic review of 165 cases. Hum. Pathol. 2018, 81, 184–191. [Google Scholar] [CrossRef]
- Blinova, E.; Roshchin, D.; Kogan, E.; Samishina, E.; Demura, T.; Deryabina, O.; Suslova, I.; Blinov, D.; Zhdanov, P.; Osmanov, U.; et al. Patient-derived non-muscular invasive bladder cancer xenografts of main molecular subtypes of the tumor for anti-Pd-l1 treatment assessment. Cells 2019, 8, 526. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ho, S.; Chang, C.C.; Zhang, Q.Y.; Vasilescu, E.R.; Vlad, G.; Suciu-Foca, N. Molecular and cellular characterization of human CD8 T suppressor cells. Front. Immunol. 2016, 7, 549. [Google Scholar] [CrossRef] [Green Version]
- Wankowicz, S.A.M.; Werner, L.; Orsola, A.; Novak, J.; Bowden, M.; Choueiri, T.K.; de Torres, I.; Morote, J.; Freeman, G.J.; Signoretti, S.; et al. Differential expression of PD-L1 in high grade T1 vs. muscle invasive bladder carcinoma and its prognostic implications. J. Urol. 2017, 198, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Jimeno, A. Atezolizumab: A novel PD-L1 inhibitor in cancer therapy with a focus in bladder and non-small cell lung cancers. Drugs Today (Barc.) 2017, 53, 217–237. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Slodkowska, E.; Jungbluth, A.; Liu, S.K.; Vesprini, D.; Enepekides, D.; Higgins, K.; Katabi, N.; Xu, B.; Downes, M.R. PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am. J. Surg. Pathol. 2018, 42, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Heidegger, I.; Fritz, J.; Danzl, M.; Sprung, S.; Zelger, B.; Brunner, A.; Pircher, A. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget 2017, 8, 66849–66864. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Tsai, Y.C.; Jeng, Y.M. Biological significance of GATA3, cytokeratin 20, cytokeratin 5/6 and p53 expression in muscle-invasive bladder cancer. PLoS ONE 2019, 14, e0221785. [Google Scholar] [CrossRef] [Green Version]
- Lerner, S.P.; McConkey, D.J.; Hoadley, K.A.; Chan, K.S.; Kim, W.Y.; Radvanyi, F.; Höglund, M.; Real, F.X. Bladder cancer molecular taxonomy: summary from a consensus meeting. Bladder Cancer 2016, 2, 37–47. [Google Scholar] [CrossRef] [Green Version]





| Relapse | Subgroups | HR | 95% CI | p-Value |
|---|---|---|---|---|
| First relapse, chemotherapy- and immunotherapy-naive settings | Luminal NMIBC | 0.29 | 0.10–0.83 | 0.022 |
| Basal NMIBC | 3.59 | 0.98–13.14 | 0.053 | |
| Double-negative NMIBC | - | - | - | |
| First relapse, mitomycin-treated settings | Luminal NMIBC | - | - | - |
| Basal NMIBC | - | - | - | |
| Double-negative NMIBC | - | - | - | |
| First relapse, BCG-treated settings | Luminal NMIBC | 0.04 | 0.006–0.37 | 0.004 |
| Basal NMIBC | - | - | - | |
| Double-negative NMIBC | 1.44 | 0.49–4.17 | 0.50 |
| Study Group | Tumor Stage, Grade, n | Gender (n) | Age, Mean ± SD | Tumor Histology |
|---|---|---|---|---|
| Primary tumors, n = 20 in each group | ||||
| Luminal NMIBC | T1, HG, n = 8 | M (3), F (5) | 54.2 ± 4.1 | Urothelial papillary carcinoma, micropapillary carcinoma |
| T1, LG, n = 12 | M (8), F (4) | 57.6 ± 3.8 | ||
| Basal NMIBC | T1, HG, n = 6 | M (3), F (3) | 61.4 ± 5.3 | |
| T1, LG, n = 14 | M (9), F (5) | 60.2 ± 4.4 | ||
| Double-negative | CIS, HG, n = 1 | M (0), F (1) | 72 | |
| T1, HG, n = 7 | M (4), F (3) | 58.3 ± 2.9 | ||
| T1, LG, n = 12 | M (7), F (5) | 55.9 ± 3.7 | ||
| Total | LG (41), HG (19) | M (34), F (26) | Average 57.7 ± 3.6 | |
| Immunotherapy/chemotherapy-naive relapsed tumors, n = 20 in each group | ||||
| Luminal NMIBC | T1, HG, n = 9 | M (6), F (3) | 63.7 ± 4.1 | Urothelial papillary carcinoma |
| T1, LG, n = 11 | M (8), F (3) | 58.7 ± 4.6 | ||
| Basal NMIBC | T1, HG, n = 7 | M (4), F (3) | 64.3 ± 3.3 | |
| T1, LG, n = 13 | M (8), F (5) | 51.5 ± 4.5 | ||
| Double-negative | T1, HG, n = 5 | M (3), F (2) | 53.1 ± 5.4 | |
| T1, LG, n = 15 | M (7), F (8) | 54.6 ± 2.8 | ||
| Total | LG (39), HG (21) | M (36), F (24) | Average 55.4 ± 3.8 | |
| First relapse after prior intravesical Mitomycin treatment, n = 20 in each group | ||||
| Luminal NMIBC | T1, HG, n = 8 | M (4), F (4) | 50.2 ± 3.2 | Urothelial papillary carcinoma |
| T1, LG, n = 12 | M (7), F (5) | 54.2 ± 2.7 | ||
| Basal NMIBC | T1, HG, n = 6 | M (2), F (4) | 64.0 ± 3.6 | |
| T1, LG, n = 14 | M (11), F (3) | 59.4 ± 3.5 | ||
| Double-negative | T1, HG, n = 9 | M (3), F (6) | 55.6 ± 4.8 | |
| T1, LG, n = 11 | M (5), F (6) | 54.7 ± 3.5 | ||
| Total | LG (37), HG (23) | M (32), F (28) | Average 56.4 ± 3.5 | |
| First relapse after prior intravesical BCG treatment, n = 20 in each group | ||||
| Luminal NMIBC | CIS, HG, n = 2 | M (2), F (0) | 56; 67 | Urothelial papillary carcinoma, micropapillary carcinoma, squamous cancer |
| T1, HG, n = 7 | M (4), F (3) | 61.1 ± 3.2 | ||
| T1, LG, n = 11 | M (7), F (4) | 58.9 ± 2.6 | ||
| Basal NMIBC | T1, HG, n = 3 | M (2), F (1) | 48.4 ± 4.5 | |
| T1, LG, n = 17 | M (9), F (8) | 53.2 ± 2.9 | ||
| Double-negative | T1, HG, n = 4 | M (3), F (1) | 55.9 ± 3.8 | |
| T1, LG, n = 16 | M (9), F (7) | 57.5 ± 3.0 | ||
| Total | LG (46), HG (14) | M (36), F (24) | Average 53.8 ± 3.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinova, E.; Enikeev, D.; Roshchin, D.; Samyshina, E.; Deryabina, O.; Tertychnyy, A.; Blinov, D.; Kogan, E.; Dudina, M.; Barakat, H.; et al. Relapse-Free Survival and PD-L1 Expression in First High- and Low-Grade Relapsed Luminal, Basal and Double-Negative P53-Mutant Non-Muscular Invasive Bladder Cancer Depending on Previous Chemo- and Immunotherapy. Cancers 2020, 12, 1316. https://doi.org/10.3390/cancers12051316
Blinova E, Enikeev D, Roshchin D, Samyshina E, Deryabina O, Tertychnyy A, Blinov D, Kogan E, Dudina M, Barakat H, et al. Relapse-Free Survival and PD-L1 Expression in First High- and Low-Grade Relapsed Luminal, Basal and Double-Negative P53-Mutant Non-Muscular Invasive Bladder Cancer Depending on Previous Chemo- and Immunotherapy. Cancers. 2020; 12(5):1316. https://doi.org/10.3390/cancers12051316
Chicago/Turabian StyleBlinova, Ekaterina, Dmitry Enikeev, Dmitry Roshchin, Elena Samyshina, Olga Deryabina, Aleksander Tertychnyy, Dmitry Blinov, Evgenia Kogan, Marina Dudina, Haydar Barakat, and et al. 2020. "Relapse-Free Survival and PD-L1 Expression in First High- and Low-Grade Relapsed Luminal, Basal and Double-Negative P53-Mutant Non-Muscular Invasive Bladder Cancer Depending on Previous Chemo- and Immunotherapy" Cancers 12, no. 5: 1316. https://doi.org/10.3390/cancers12051316





