Cigarette Smoking Is Associated with Increased Risk of Malignant Gliomas: A Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Incidence Rates and Risk of Developing MG According to Smoking Status
2.3. Risk of Developing MG According to Smoking Amount and Duration
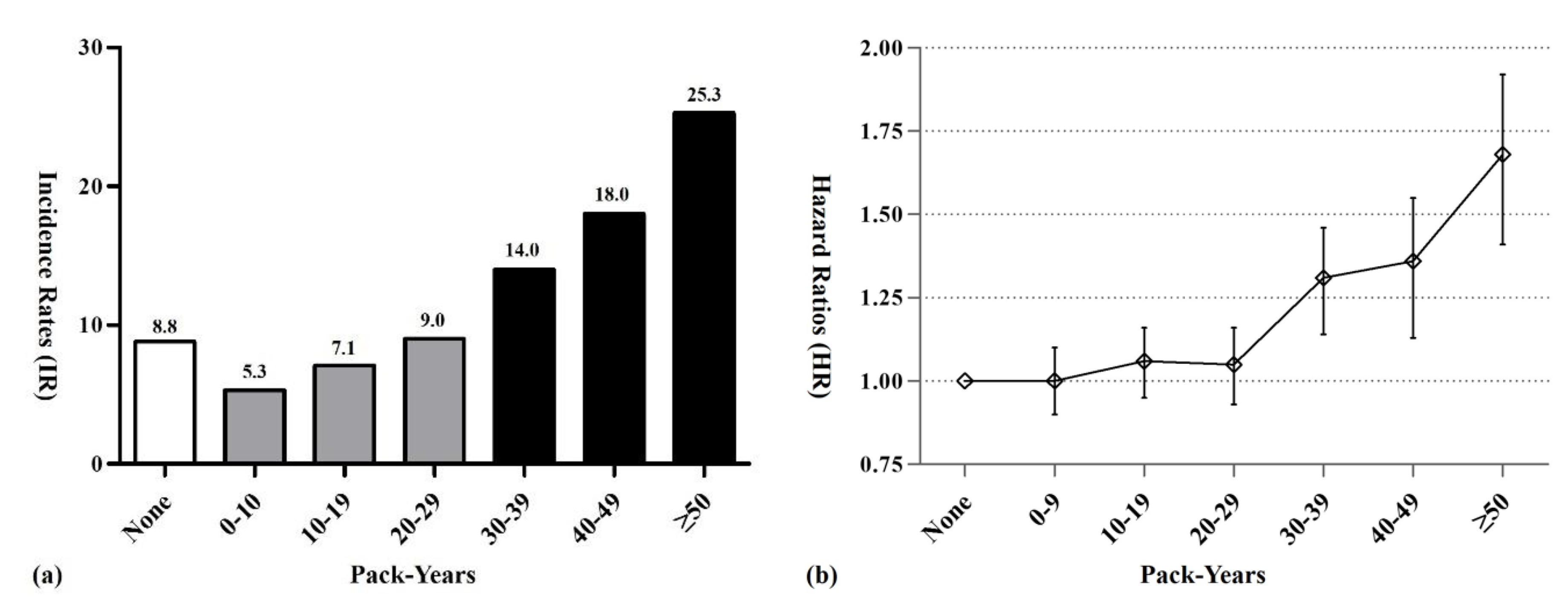
2.4. MG Risk According to Cumulative Lifetime Amount of Smoking
2.5. Subgroup Analyses According to Age and Sex
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Database Source
4.3. Study Population
4.4. Definitions
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733. [Google Scholar] [CrossRef]
- Jee, S.H.; Samet, J.M.; Ohrr, H.; Kim, J.H.; Kim, I.S. Smoking and cancer risk in Korean men and women. Cancer Causes Control 2004, 15, 341–348. [Google Scholar] [CrossRef]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M.; Comparative Risk Assessment Collaborating Group. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Peto, J. That the Effects of Smoking Should be Measured in Pack-Years: Misconceptions 4; Nature Publishing Group: Berlin, Germany, 2012. [Google Scholar]
- Similä, T.; Virtanen, J.I. Association between smoking intensity and duration and tooth loss among Finnish middle-aged adults: The Northern Finland Birth Cohort 1966 Project. BMC Public Health 2015, 15, 1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Fahmideh, M.A.; Cote, D.J.; Muskens, I.S.; Schraw, J.M.; Scheurer, M.E.; Bondy, M.L. Risk factors for childhood and adult primary brain tumors. Neuro-Oncology 2019, 21, 1357–1375. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, A.A.; Jameson, M.J.; Broaddus, W.C.; Lin, P.S.; Chung, T.D. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol. 2013, 30, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Tierney, W.; Hossain, M.; Puvenna, V.; Janigro, D.; Cucullo, L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: Expanding the awareness of smoking toxicity in an underappreciated area. Int. J. Environ. Res. Public Health 2010, 7, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhao, W.; Qi, Z.; He, J. Smoking and glioma risk: Evidence from a meta-analysis of 25 observational studies. Medicine 2016, 95, e2447. [Google Scholar] [CrossRef]
- Li, H.-X.; Peng, X.-X.; Zong, Q.; Zhang, K.; Wang, M.-X.; Liu, Y.-Z.; Han, G.-L. Cigarette smoking and risk of adult glioma: A meta-analysis of 24 observational studies involving more than 2.3 million individuals. OncoTargets Ther. 2016, 9, 3511. [Google Scholar]
- Hou, L.; Jiang, J.; Liu, B.; Han, W.; Wu, Y.; Zou, X.; Nasca, P.C.; Xue, F.; Chen, Y.; Zhang, B. Smoking and adult glioma: A population-based case-control study in China. Neuro-Oncology 2015, 18, 105–113. [Google Scholar] [CrossRef]
- Seliger, C.; Ricci, C.; Meier, C.R.; Bodmer, M.; Jick, S.S.; Bogdahn, U.; Hau, P.; Leitzmann, M.F. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro-Oncology 2015, 18, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Vida, S.; Richardson, L.; Cardis, E.; Krewski, D.; McBride, M.; Parent, M.-E.; Abrahamowicz, M.; Leffondré, K.; Siemiatycki, J. Brain tumours and cigarette smoking: Analysis of the INTERPHONE Canada case–control study. Environ. Health 2014, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Braganza, M.; Rajaraman, P.; Park, Y.; Inskip, P.; Freedman, N.; Hollenbeck, A.; De González, A.B.; Kitahara, C. Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP Diet and Health Study. Br. J. Cancer 2014, 110, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachance, D.H.; Yang, P.; Johnson, D.R.; Decker, P.A.; Kollmeyer, T.M.; McCoy, L.S.; Rice, T.; Xiao, Y.; Ali-Osman, F.; Wang, F. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. Am. J. Epidemiol. 2011, 174, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Cabaniols, C.; Giorgi, R.; Chinot, O.; Ferahta, N.; Spinelli, V.; Alla, P.; Barrie, M.; Lehucher-Michel, M.-P. Links between private habits, psychological stress and brain cancer: A case–control pilot study in France. J. Neuro-Oncol. 2011, 103, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Mandelzweig, L.; Novikov, I.; Sadetzki, S. Smoking and risk of glioma: A meta-analysis. Cancer Causes Control 2009, 20, 1927–1938. [Google Scholar] [CrossRef]
- Benson, V.S.; Pirie, K.; Green, J.; Casabonne, D.; Beral, V. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br. J. Cancer 2008, 99, 185. [Google Scholar] [CrossRef]
- Holick, C.N.; Giovannucci, E.L.; Rosner, B.; Stampfer, M.J.; Michaud, D.S. Prospective study of cigarette smoking and adult glioma: Dosage, duration, and latency. Neuro-Oncology 2007, 9, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Silvera, S.A.N.; Miller, A.B.; Rohan, T.E. Cigarette smoking and risk of glioma: A prospective cohort study. Int. J. Cancer 2006, 118, 1848–1851. [Google Scholar] [CrossRef]
- Efird, J.T.; Friedman, G.D.; Sidney, S.; Klatsky, A.; Habel, L.A.; Udaltsova, N.V.; Van Den Eeden, S.; Nelson, L.M. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: Cigarette smoking and other lifestyle behaviors. J. Neuro-Oncol. 2004, 68, 57–69. [Google Scholar] [CrossRef]
- Zheng, T.; Cantor, K.P.; Zhang, Y.; Chiu, B.C.; Lynch, C.F. Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa. Cancer Epidemiol. Prev. Biomark. 2001, 10, 413–414. [Google Scholar]
- Park, S.; Chun, J.; Han, K.-D.; Soh, H.; Kang, E.A.; Lee, H.J.; Im, J.P.; Kim, J.S. Dose–response relationship between cigarette smoking and risk of ulcerative colitis: A nationwide population-based study. J. Gastroenterol. 2019, 54, 881–890. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, D.-H.; Park, Y.-G.; Kwon, D.-Y.; Choi, M.; Jung, J.-H.; Han, K. Cancer risk in patients with Parkinson’s disease in South Korea: A nationwide, population-based cohort study. Eur. J. Cancer 2019, 117, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Lee, J.H.; Lee, S.Y.; Lee, J.H.; Yu, D.S.; Han, K.D.; Park, Y.G. Association between smoking and Behçet’s disease: A nationwide population-based study in Korea. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chang, J.; Kim, K.; Park, S.M.; Lee, K. Effect of smoking cessation and reduction on the risk of cancer in Korean men: A population based study. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Do Han, K.; Han, J.H.; Lee, J.H. Smoking and risk of psoriasis: A nationwide cohort study. J. Am. Acad. Dermatol. 2017, 77, 573–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godtfredsen, N.S.; Prescott, E.; Osler, M. Effect of smoking reduction on lung cancer risk. Jama 2005, 294, 1505–1510. [Google Scholar] [CrossRef] [Green Version]
- Abbruscato, T.J.; Lopez, S.P.; Roder, K.; Paulson, J.R. Regulation of blood-brain barrier Na, K, 2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J. Pharmacol. Exp. Ther. 2004, 310, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Abbruscato, T.J.; Lopez, S.P.; Mark, K.S.; Hawkins, B.T.; Davis, T.P. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J. Pharm. Sci. 2002, 91, 2525–2538. [Google Scholar] [CrossRef]

| Characteristic Title | Never-Smokers | Former Smokers | Current Smokers | p-Value |
|---|---|---|---|---|
| n = 5,820,623 (59.32) | n = 1,400,124 (14.27) | n = 2,591,021 (26.41) | ||
| Mean age, years a | 48.47 ± 14.55 | 48.67 ± 13.03 | 42.66 ± 12.49 | <0.001 |
| 20–39, n (%) | 1,576,423 (27.08) | 364,065 (26.00) | 1,173,409 (45.29) | |
| 40–64, n (%) | 3,336,188 (57.32) | 854,543 (61.03) | 1,253,281 (48.37) | |
| ≥65, n (%) | 908,012 (15.6) | 181,516 (12.96) | 164,331 (6.34) | |
| Male | 1,634,168 (28.08) | 1,316,955 (94.06) | 2,437,271 (94.07) | <0.001 |
| Height, cm a | 160.03 ± 8.55 | 169.01 ± 6.71 | 169.98 ± 7.01 | <0.001 |
| Weight, cm a | 60.26 ± 10.52 | 69.62 ± 10.22 | 69.25 ± 11.33 | <0.001 |
| Waist circumference, cm a | 78.27 ± 9.16 | 83.95 ± 7.87 | 82.61 ± 8.24 | <0.001 |
| BMI, kg/m2 a | 23.47 ± 3.25 | 24.32 ± 2.91 | 23.9 ± 3.21 | <0.001 |
| Urban residence, n (%) | 3,123,516 (53.66) | 739,082 (52.79) | 1,434,412 (55.36) | <0.001 |
| Heavy drinker b, n (%) | 117,516 (2.02) | 161,722 (11.55) | 399,344 (15.41) | <0.001 |
| Regular exercise c, n (%) | 2,705,616 (46.48) | 905,008 (64.64) | 1,436,112 (55.43) | <0.001 |
| Diabetes mellitus, n (%) | 469,259 (8.06) | 154,318 (11.02) | 221,970 (8.57) | <0.001 |
| Hypertension, n (%) | 1,507,526 (25.9) | 440,210 (31.44) | 560,252 (21.62) | <0.001 |
| Dyslipidemia, n (%) | 1,102,507 (18.94) | 281,650 (20.12) | 403,142 (15.56) | <0.001 |
| Previous ischemic heart disease, n (%) | 112,688 (3.12) | 45,101 (4.78) | 35,888 (2.19) | <0.001 |
| Previous cerebral stroke, n (%) | 50,943 (1.41) | 26,027 (2.76) | 22,864 (1.39) | <0.001 |
| Mean amounts of cigarette smoked per day | 0 | 16.0 ± 9.1 | 16.0 ± 7.3 | |
| Mean smoking duration | 0 | 16.2 ± 10.6 | 18.8 ± 11.1 | |
| Mean pack-years | 0 | 14.6 ± 14.6 | 16.0 ± 13.2 | |
| Hemoglobin, g/dL | 13.33 ± 1.49 | 14.69 ± 1.25 | 14.97 ± 1.26 | <0.001 |
| Smoking Status | Total, n | MG Events, n | Person-Years | MG Incidence Rate * | Crude HR (95% CI) | a Model 1 HR (95% CI) | b Model 2 HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Never-smokers | 5,820,623 | 3733 | 42,405,897 | 8.80 | 1 (reference) | 1 (reference) | 1 (reference) |
| Former smokers | 1,400,124 | 904 | 10,143,343 | 8.91 | 1.01 (0.94–1.09) | 0.98 (0.90–1.07) | 1.00 (0.92–1.09) |
| Current smokers | 2,591,021 | 1463 | 18,731,140 | 7.81 | 0.89 (0.84–0.94) | 1.19 (1.10–1.28) | 1.22 (1.13–1.32) |
| Smoking Status | Total Number, n (% of Proportion) | MG Events, n | Person-Years | MG Incidence Rate * | Crude HR (95% CI) | a Model 1 HR (95% CI) | b Model 2 HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Never-smokers | 5,820,623 (59.3) | 3,733 | 42,513,670 | 8.80 | 1 (reference) | 1 (reference) | 1 (reference) |
| Former smokers | |||||||
| Cigarettes smoked per day | |||||||
| <10 | 364,712 (3.7) | 155 | 2,620,568 | 5.72 | 0.84 (0.71–1.00) | 0.95 (0.80–1.12) | 0.97 (0.81–1.14) |
| 10–19 | 818,051 (8.3) | 467 | 5,949,887 | 7.85 | 0.88 (0.78–0.99) | 0.92 (0.83–1.03) | 0.94 (0.85–1.05) |
| ≥20 | 217,361 (2.2) | 282 | 1,528,462 | 18.45 | 1.20 (1.10–1.32) | 1.12 (0.98–1.28) | 1.15 (1.00–1.30) |
| Smoking duration (years) | |||||||
| <10 | 251,965 (2.6) | 136 | 1,832,908 | 5.72 | 0.66 (0.56–0.78) | 0.91 (0.77–1.09) | 0.93 (0.78–1.11) |
| 10–29 | 534,103 (5.4) | 300 | 3,880,899 | 7.85 | 0.88 (0.81–0.98) | 0.93 (0.82–1.05) | 0.95 (0.84–1.08) |
| ≥30 | 614,056 (6.3) | 468 | 4,429,536 | 18.45 | 2.10 (1.86–2.37) | 1.05 (0.94–1.17) | 1.07 (0.96–1.19) |
| Current smokers | |||||||
| Cigarettes smoked per day | |||||||
| <10 | 426,590 (4.3) | 129 | 3,051,709 | 4.03 | 0.97 (0.84–1.12) | 1.02 (0.85–1.23) | 1.05 (0.87–1.26) |
| 10–19 | 1,631,714 (16.6) | 604 | 11,868,42 | 5.09 | 0.75 (0.58–0.82) | 0.98 (0.88–1.08) | 1.00 (0.90–1.10) |
| ≥20 | 532,717 (5.4) | 730 | 3,747,653 | 19.48 | 0.99 (0.92–1.08) | 1.45 (1.32–1.59) | 1.50 (1.37–1.65) |
| Smoking duration (years) | |||||||
| <10 | 323,511 (3.3) | 199 | 2,331,871 | 4.03 | 0.47 (0.40–0.56) | 1.21 (1.04–1.40) | 1.25 (1.07–1.44) |
| 10–29 | 1,089,332 (11.1) | 518 | 7,895,617 | 5.09 | 0.58 (0.53–0.63) | 1.10 (0.99–1.21) | 1.13 (1.02–1.25) |
| ≥30 | 1,178,178 (12.0) | 746 | 8,503,651 | 19.48 | 2.22 (2.05–2.40) | 1.26 (1.15–1.38) | 1.29 (1.18–1.42) |
| Smoking Status | Age | Sex | |||
|---|---|---|---|---|---|
| 20–39 | 40–64 | ≥65 | Male | Female | |
| Never-smokers | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Former smokers | 1.20 (0.93–1.55) | 1.03 (0.92–1.17) | 0.92 (0.78–1.06) | 1.03 (0.94–1.13) | 0.85 (0.60–1.21) |
| Current smokers | 1.08 (0.88–1.32) | 1.27 (1.14–1.41) | 1.37 (1.20–1.50) | 1.30 (1.12–1.41) | 1.06 (0.84–1.32) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.; Han, K.-D.; Park, Y.-M.; Bae, J.M.; Kim, S.U.; Jeun, S.-S.; Yang, S.H. Cigarette Smoking Is Associated with Increased Risk of Malignant Gliomas: A Nationwide Population-Based Cohort Study. Cancers 2020, 12, 1343. https://doi.org/10.3390/cancers12051343
Ahn S, Han K-D, Park Y-M, Bae JM, Kim SU, Jeun S-S, Yang SH. Cigarette Smoking Is Associated with Increased Risk of Malignant Gliomas: A Nationwide Population-Based Cohort Study. Cancers. 2020; 12(5):1343. https://doi.org/10.3390/cancers12051343
Chicago/Turabian StyleAhn, Stephen, Kyung-Do Han, Yong-Moon Park, Jung Min Bae, Sang Uk Kim, Sin-Soo Jeun, and Seung Ho Yang. 2020. "Cigarette Smoking Is Associated with Increased Risk of Malignant Gliomas: A Nationwide Population-Based Cohort Study" Cancers 12, no. 5: 1343. https://doi.org/10.3390/cancers12051343
APA StyleAhn, S., Han, K.-D., Park, Y.-M., Bae, J. M., Kim, S. U., Jeun, S.-S., & Yang, S. H. (2020). Cigarette Smoking Is Associated with Increased Risk of Malignant Gliomas: A Nationwide Population-Based Cohort Study. Cancers, 12(5), 1343. https://doi.org/10.3390/cancers12051343





