Sex-Specific Differences in Primary CNS Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Inclusion and Clinical Data
2.2. MR Imaging Data
2.3. Digital Pathology Data
2.4. Cluster Analysis
2.5. Survival Analysis
2.6. Assessment of Intratumoral Heterogeneity
2.7. Data and Code Availability
3. Results
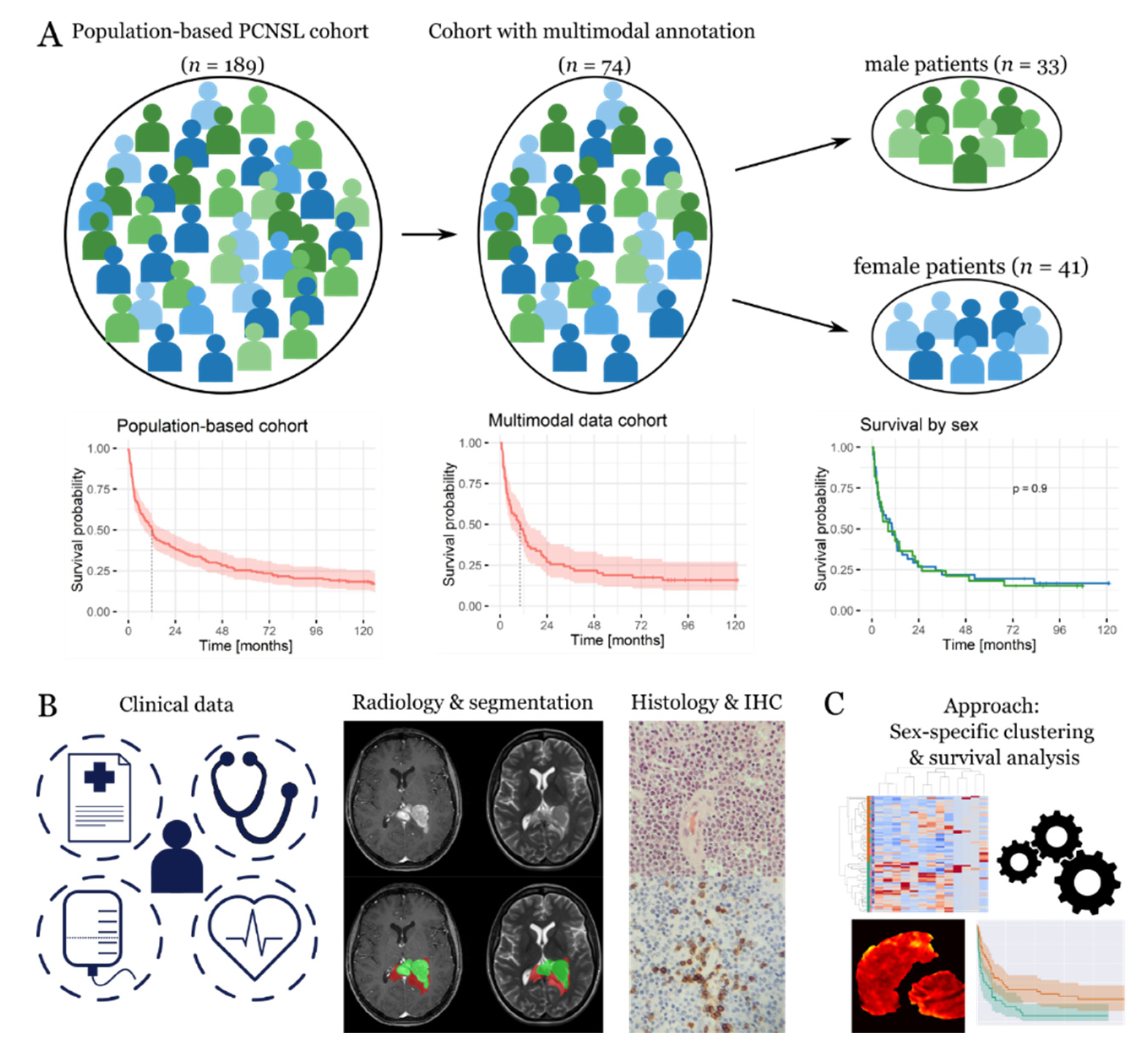
3.1. General Characterization of the Patient Cohort
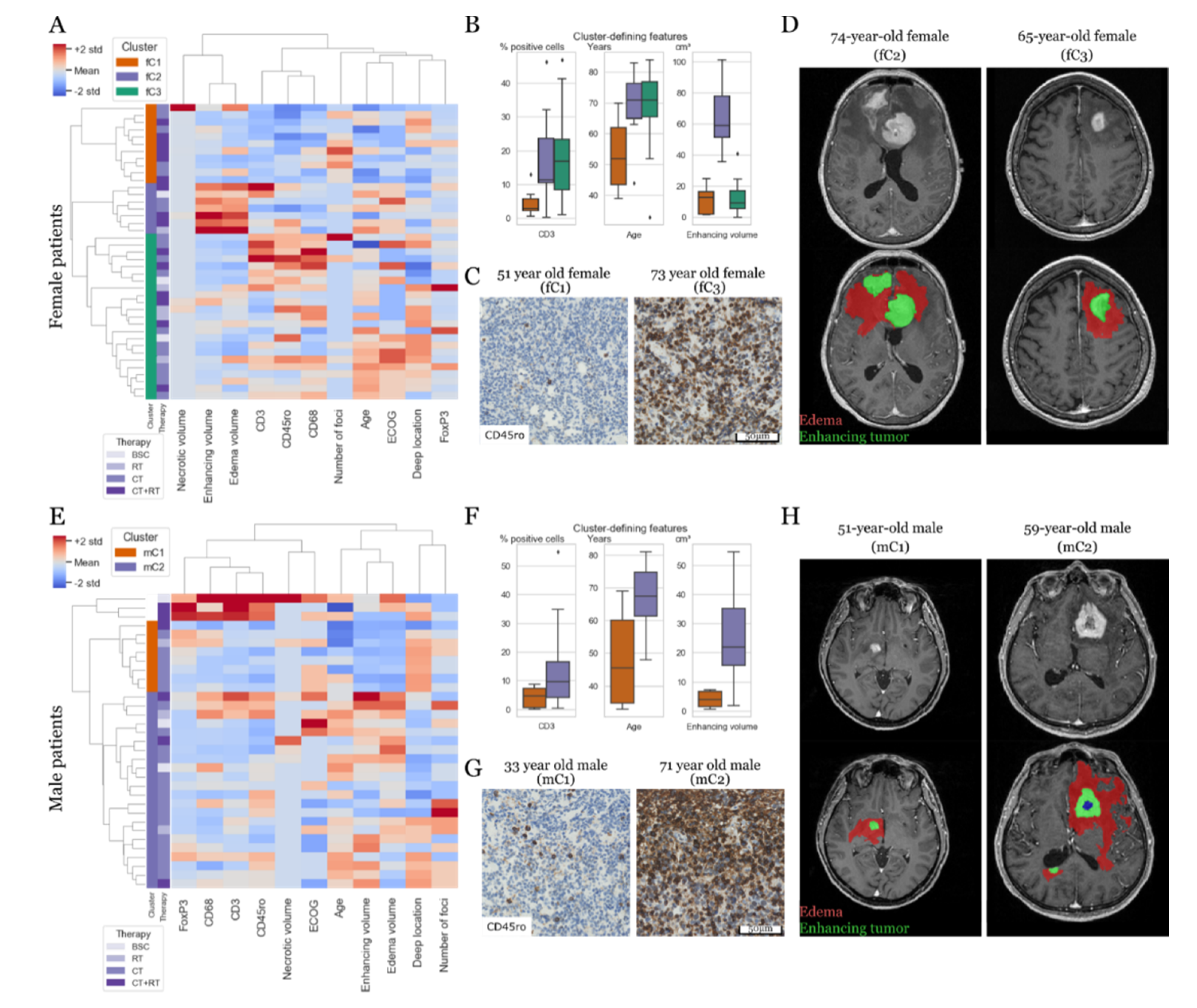
3.2. Prognostic Constellations Differ between Females and Males
3.3. Tumor-Associated Immune Response and MR Phenotypes Show Differential Prognostic Impact
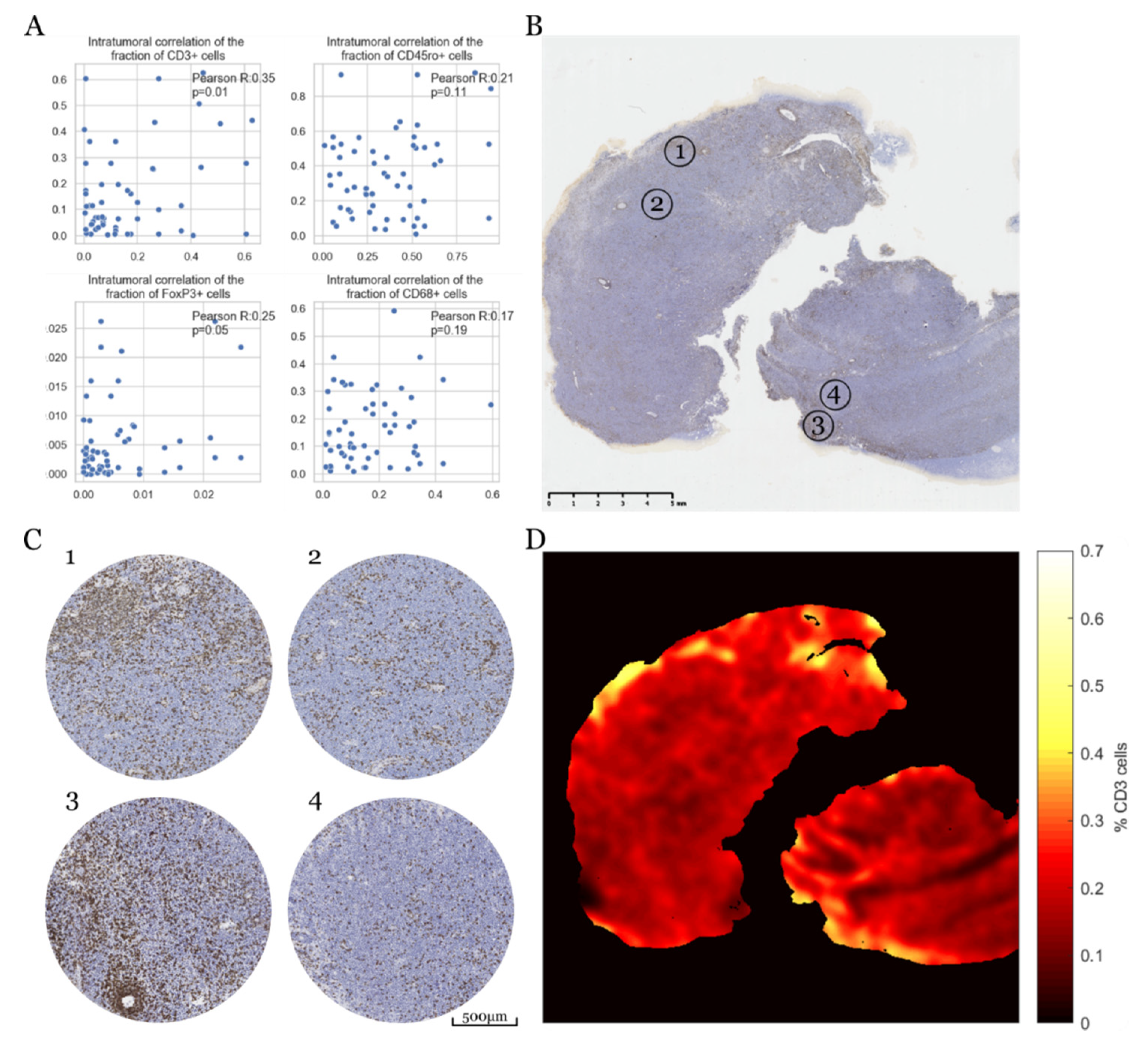
3.4. Intratumoral Heterogeneity of Immune Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ober, C.; Loisel, D.A.; Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008, 9, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.K.; Hurria, A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J. Clin. Oncol. 2010, 28, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Caetano, M.S.; Hassane, M.; Van, H.T.; Bugarin, E.; Cumpian, A.M.; McDowell, C.L.; Cavazos, C.G.; Zhang, H.; Deng, S.; Diao, L.; et al. Sex specific function of epithelial STAT3 signaling in pathogenesis of K-ras mutant lung cancer. Nat. Commun. 2018, 9, 4589. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019, 11, eaao5253. [Google Scholar] [CrossRef] [Green Version]
- Warrington, N.M.; Sun, T.; Luo, J.; McKinstry, R.C.; Parkin, P.C.; Ganzhorn, S.; Spoljaric, D.; Albers, A.C.; Merkelson, A.; Stewart, D.R.; et al. The cyclic AMP pathway is a sex-specific modifier of glioma risk in type I neurofibromatosis patients. Cancer Res. 2015, 75, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Ruda, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet. Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef] [Green Version]
- Neuhauser, M.; Roetzer, T.; Oberndorfer, S.; Kitzwoegerer, M.; Payer, F.; Unterluggauer, J.J.; Haybaeck, J.; Stockhammer, G.; Iglseder, S.; Moser, P.; et al. Increasing use of immunotherapy and prolonged survival among younger patients with primary CNS lymphoma: A population-based study. Acta Oncol. 2019, 58, 967–976. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosee, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet. Haematol. 2017, 4, e510–e523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.E.C.; Issa, S.; Bakunina, K.; Minnema, M.C.; Seute, T.; Durian, M.; Cull, G.; Schouten, H.C.; Stevens, W.B.C.; Zijlstra, J.M.; et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): A randomised, open-label, phase 3 intergroup study. Lancet. Oncol. 2019, 20, 216–228. [Google Scholar] [CrossRef]
- Tun, H.W.; Johnston, P.B.; DeAngelis, L.M.; Atherton, P.J.; Pederson, L.D.; Koenig, P.A.; Reeder, C.B.; Omuro, A.M.P.; Schiff, D.; O’Neill, B.; et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood 2018, 132, 2240–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, J.L.; Geng, H.; Fraser, E.J.; Formaker, P.; Chen, L.; Sharma, J.; Killea, P.; Choi, K.; Ventura, J.; Kurhanewicz, J.; et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018, 2, 1595–1607. [Google Scholar] [CrossRef] [Green Version]
- Ghesquieres, H.; Houillier, C.; Chinot, O.; Choquet, S.; Molucon-Chabrot, C.; Beauchene, P.; Gressin, R.; Morschhauser, F.; Schmitt, A.; Gyan, E.; et al. Rituximab-Lenalidomide (REVRI) in Relapse or Refractory Primary Central Nervous System (PCNSL) or Vitreo Retinal Lymphoma (PVRL): Results of a “Proof of Concept” Phase II Study of the French LOC Network. Blood 2016, 128, 785. [Google Scholar] [CrossRef]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.; Zhou, X.; Guo, Z.; Huang, R.; Yue, C.; He, Y.; Li, M.; Chen, Y.; Liu, Y.; Chang, L.J.; et al. CD19 and CD70 Dual-Target Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Relapsed and Refractory Primary Central Nervous System Diffuse Large B-Cell Lymphoma. Front. Oncol. 2019, 9, 1350. [Google Scholar] [CrossRef] [Green Version]
- Abramson, J.S.; McGree, B.; Noyes, S.; Plummer, S.; Wong, C.; Chen, Y.B.; Palmer, E.; Albertson, T.; Ferry, J.A.; Arrillaga-Romany, I.C. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 783–784. [Google Scholar] [CrossRef]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef]
- Mahale, P.; Shiels, M.S.; Lynch, C.F.; Engels, E.A. Incidence and outcomes of primary central nervous system lymphoma in solid organ transplant recipients. Am. J. Transplant. 2018, 18, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreisl, T.N.; Panageas, K.S.; Elkin, E.B.; Deangelis, L.M.; Abrey, L.E. Treatment patterns and prognosis in patients with human immunodeficiency virus and primary central system lymphoma. Leuk. Lymphoma 2008, 49, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Batchelor, T.T. Diagnosis and management of primary central nervous system lymphoma. Cancer 2017, 123, 4314–4324. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Blay, J.Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Jahr, G.; Broi, M.D.; Holte, H., Jr.; Beiske, K.; Meling, T.R. Evaluation of Memorial Sloan-Kettering Cancer Center and International Extranodal Lymphoma Study Group prognostic scoring systems to predict Overall Survival in intracranial Primary CNS lymphoma. Brain Behav. 2018, 8, e00928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meling, T.R.; Latysheva, A.; Da Broi, M.; Jahr, G.; Holte, H., Jr.; Beiske, K.; Emblem, K.E. Is deep brain involvement in intracranial primary central nervous system lymphoma of importance for penetration of chemotherapeutic agents? Neuroradiology 2018, 60, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Riihijarvi, S.; Fiskvik, I.; Taskinen, M.; Vajavaara, H.; Tikkala, M.; Yri, O.; Karjalainen-Lindsberg, M.L.; Delabie, J.; Smeland, E.; Holte, H.; et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: A correlative study from a Nordic phase II trial. Haematologica 2015, 100, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.C.; Liao, H.; Lin, S.X.; Xia, Y.; Wang, X.X.; Gao, Y.; Lin, Z.X.; Lu, J.B.; Huang, H.Q. High expression of tumor-infiltrating macrophages correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med. Oncol. 2012, 29, 2317–2322. [Google Scholar] [CrossRef]
- Nam, S.J.; Go, H.; Paik, J.H.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk. Lymphoma 2014, 55, 2466–2476. [Google Scholar] [CrossRef]
- Wada, N.; Zaki, M.A.; Hori, Y.; Hashimoto, K.; Tsukaguchi, M.; Tatsumi, Y.; Ishikawa, J.; Tominaga, N.; Sakoda, H.; Take, H.; et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: A study of the Osaka Lymphoma Study Group. Histopathology 2012, 60, 313–319. [Google Scholar] [CrossRef]
- Cho, H.; Kim, S.H.; Kim, S.J.; Chang, J.H.; Yang, W.I.; Suh, C.O.; Cheong, J.W.; Kim, Y.R.; Lee, J.Y.; Jang, J.E.; et al. The prognostic role of CD68 and FoxP3 expression in patients with primary central nervous system lymphoma. Ann. Hematol. 2017, 96, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, H.; Cho, H.; Kim, Y.R.; Lee, J.Y.; Jang, J.E.; Yundeok, K.; Cheong, J.W.; Min, Y.H.; Kim, J.S. The Prognostic Role of CD68 and FoxP3 Expression in Primary Central Nervous System Lymphoma. Blood 2015, 126, 1457. [Google Scholar] [CrossRef]
- Bashir, R.; Chamberlain, M.; Ruby, E.; Hochberg, F.H. T-cell infiltration of primary CNS lymphoma. Neurology 1996, 46, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lin, C.H.; Cheng, A.L.; Medeiros, L.J.; Chang, K.C. Primary central nervous system diffuse large B-cell lymphoma has poorer immune cell infiltration and prognosis than its peripheral counterpart. Histopathology 2015, 67, 625–635. [Google Scholar] [CrossRef]
- Wohrer, A.; Waldhor, T.; Heinzl, H.; Hackl, M.; Feichtinger, J.; Gruber-Mosenbacher, U.; Kiefer, A.; Maier, H.; Motz, R.; Reiner-Concin, A.; et al. The Austrian Brain Tumour Registry: A cooperative way to establish a population-based brain tumour registry. J. Neuro Oncol. 2009, 95, 401–411. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Keuken, M.C.; Bazin, P.L.; Crown, L.; Hootsmans, J.; Laufer, A.; Muller-Axt, C.; Sier, R.; van der Putten, E.J.; Schafer, A.; Turner, R.; et al. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. Neuroimage 2014, 94, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef] [Green Version]
- Klughammer, J.; Kiesel, B.; Roetzer, T.; Fortelny, N.; Nemc, A.; Nenning, K.H.; Furtner, J.; Sheffield, N.C.; Datlinger, P.; Peter, N.; et al. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. Nat. Med. 2018, 24, 1611–1624. [Google Scholar] [CrossRef]
- Roetzer, T.; Leskovar, K.; Peter, N.; Furtner, J.; Muck, M.; Augustin, M.; Lichtenegger, A.; Nowosielski, M.; Hainfellner, J.A.; Baumann, B.; et al. Evaluating cellularity and structural connectivity on whole brain slides using a custom-made digital pathology pipeline. J. Neurosc Method 2019, 311, 215–221. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Phansalkar, N.; More, S.; Sabale, A.; Joshi, M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Kerala, India, 10–12 February 2011; pp. 218–220. [Google Scholar]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F. Topographic distance and watershed lines. Signal. Process. 1994, 38, 113–125. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Walt, S.V.d.; Colbert, S.C.; Varoquaux, G. The NumPy array: A structure for efficient numerical computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Method 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Biecek, A.K.M.K.P. Survminer: Drawing Survival Curves using ‘ggplot2’. Available online: https://CRAN.R-project.org/package=survminer (accessed on 6 June 2020).
- Therneau, T.; Atkinson, B. Rpart: Recursive Partitioning and Regression Trees. Available online: https://CRAN.R-project.org/package=rpart (accessed on 6 June 2020).
- Therneau, T.M. A Package for Survival Analysis in S. Available online: https://CRAN.R-project.org/package=survival (accessed on 6 June 2020).
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Sankaran-Walters, S.; Macal, M.; Grishina, I.; Nagy, L.; Goulart, L.; Coolidge, K.; Li, J.; Fenton, A.; Williams, T.; Miller, M.K.; et al. Sex differences matter in the gut: Effect on mucosal immune activation and inflammation. Biol. Sex Differ. 2013, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Prieto, G.A.; Rosenstein, Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 2006, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Green, E.S.; Care, A.S.; Moldenhauer, L.M.; Prins, J.R.; Hull, M.L.; Barry, S.C.; Dekker, G. Therapeutic Potential of Regulatory T Cells in Preeclampsia-Opportunities and Challenges. Front. Immunol. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cowley, L.A.; Liu, X.S. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, O.; Ishii, G.; Kubota, K.; Murata, Y.; Naito, Y.; Mizuno, T.; Aokage, K.; Saijo, N.; Nishiwaki, Y.; Gemma, A.; et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008, 113, 1387–1395. [Google Scholar] [CrossRef]
- Menon, A.G.; Janssen-van Rhijn, C.M.; Morreau, H.; Putter, H.; Tollenaar, R.A.; van de Velde, C.J.; Fleuren, G.J.; Kuppen, P.J. Immune system and prognosis in colorectal cancer: A detailed immunohistochemical analysis. Lab. Investig. 2004, 84, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Clemente, C.G.; Mihm, M.C., Jr.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77, 1303–1310. [Google Scholar] [CrossRef]
- Ahmadi, R.; Dictus, C.; Hartmann, C.; Zurn, O.; Edler, L.; Hartmann, M.; Combs, S.; Herold-Mende, C.; Wirtz, C.R.; Unterberg, A. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir. 2009, 151, 1359–1365. [Google Scholar] [CrossRef]
- Lamborn, K.R.; Chang, S.M.; Prados, M.D. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004, 6, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Carroll, K.T.; Bryant, A.K.; Hirshman, B.; Alattar, A.A.; Joshi, R.; Gabel, B.; Carter, B.S.; Harismendy, O.; Vaida, F.; Chen, C.C. Interaction Between the Contributions of Tumor Location, Tumor Grade, and Patient Age to the Survival Benefit Associated with Gross Total Resection. World Neurosurg. 2018, 111, e790–e798. [Google Scholar] [CrossRef]
- Tejada Neyra, M.A.; Neuberger, U.; Reinhardt, A.; Brugnara, G.; Bonekamp, D.; Sill, M.; Wick, A.; Jones, D.T.W.; Radbruch, A.; Unterberg, A.; et al. Voxel-wise radiogenomic mapping of tumor location with key molecular alterations in patients with glioma. Neuro Oncol. 2018, 20, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Houillier, C.; Martin-Duverneuil, N.; Blonski, M.; Soussain, C.; Ghesquieres, H.; Houot, R.; Larrieu, D.; Soubeyran, P.; Gressin, R.; et al. Patterns of response and relapse in primary CNS lymphomas after first-line chemotherapy: Imaging analysis of the ANOCEF-GOELAMS prospective randomized trial. Neuro Oncol. 2017, 19, 422–429. [Google Scholar] [CrossRef] [Green Version]

| Variable Type | Variable | Female | Male | p-value |
|---|---|---|---|---|
| Clinical prognostic/therapeutic (all as part of first line) | Age | 64.6 ± 13.2 yrs | 61.7 ± 14.6 yrs | 0.39 |
| ECOG score | 1 (IQR 0–2) | 1 (IQR 1–2) | 0.5 | |
| Immunodeficiency | 3 (7.3%) | 4 (12.1%) | 0.69 | |
| Combined radio-chemotherapy | 15 (36.6%) | 6 (18.2%) | 0.12 | |
| Chemotherapy only | 18 (43.9%) | 21 (63.6%) | 0.11 | |
| Radiotherapy only | 5 (12.2%) | 3 (9.1%) | 0.73 | |
| Best supportive care | 3 (7.3%) | 3 (9.1%) | 1 | |
| Methotrexate-Tx | 29 (70.7%) | 27 (81.8%) | 0.29 | |
| HD-Methotrexate-Tx | 27 (65.9%) | 23 (69.7%) | 0.81 | |
| Rituximab-Tx | 13 (31.7%) | 3 (9.1%) | 0.02 | |
| Poly-chemotherapy | 15 (36.6%) | 10 (30.3%) | 0.63 | |
| MR imaging | Enhancing volume | 20.7 ± 23.5 cm3 | 18.5 ± 13.9 cm3 | 0.65 |
| Necrotic volume | 0.4 ± 2.1 cm3 | 0.5 ± 1.8 cm3 | 0.8 | |
| Edema volume | 101.4 ± 82.3 cm3 | 92.0 ± 66.0 cm3 | 0.6 | |
| Left location | 47.0 ± 40.7% | 44.0 ± 40.7% | 0.75 | |
| Frontal location | 18.4 ± 27.1% | 25.1 ± 31.4% | 0.34 | |
| Temporal location | 11.7 ± 22.6% | 9.3 ±21.9% | 0.65 | |
| Parietal location | 7.0 ± 11.2% | 5.5 ± 17.3% | 0.66 | |
| Occipital location | 3.1 ± 9.6% | 2.2 ± 6.5% | 0.64 | |
| Deep location | 59.7 ± 29.3% | 57.9 ± 34.5% | 0.81 | |
| Number of foci | 1 (IQR 1–2) | 2 (IQR 1-3) | <0.01 | |
| Digital pathology | CD3+ TIL | 14.6 ± 12.4% | 16.0 ± 19.1% | 0.7 |
| CD45ro+ TIL | 40.2 ± 21.4% | 35.0 ± 23.9% | 0.34 | |
| CD68+ TAM | 19.6 ± 12.4% | 17.2 ± 12.3% | 0.41 | |
| FoxP3+ TIL | 0.9 ± 1.5% | 1.0 ± 1.5% | 0.73 |
| Female patients | |||
| Variable | HR | 95% CI | p |
| Age > 60 years | 4.37 | 1.8–10.6 | 0.001 |
| FoxP3 TIL | 1.65 | 1.04–2.63 | 0.035 |
| Male patients | |||
| Variable | HR | 95% CI | p |
| ECOG > 1 | 4.62 | 1.48–14.4 | 0.008 |
| Enhancing volume | 1.05 | 1.01–1.09 | 0.006 |
| Frontal location | 0.986 | 0.97–1 | 0.091 |
| FoxP3 TIL | 0.697 | 0.497–0.978 | 0.037 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roetzer, T.; Furtner, J.; Gesperger, J.; Seebrecht, L.; Bandke, D.; Brada, M.; Brandner-Kokalj, T.; Grams, A.; Haybaeck, J.; Kitzwoegerer, M.; et al. Sex-Specific Differences in Primary CNS Lymphoma. Cancers 2020, 12, 1593. https://doi.org/10.3390/cancers12061593
Roetzer T, Furtner J, Gesperger J, Seebrecht L, Bandke D, Brada M, Brandner-Kokalj T, Grams A, Haybaeck J, Kitzwoegerer M, et al. Sex-Specific Differences in Primary CNS Lymphoma. Cancers. 2020; 12(6):1593. https://doi.org/10.3390/cancers12061593
Chicago/Turabian StyleRoetzer, Thomas, Julia Furtner, Johanna Gesperger, Lukas Seebrecht, Dave Bandke, Martina Brada, Tanisa Brandner-Kokalj, Astrid Grams, Johannes Haybaeck, Melitta Kitzwoegerer, and et al. 2020. "Sex-Specific Differences in Primary CNS Lymphoma" Cancers 12, no. 6: 1593. https://doi.org/10.3390/cancers12061593







