Viral and Immunological Analytes are Poor Predictors of the Clinical Treatment Response in Kaposi’s Sarcoma Patients
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Subjects
2.2. Similar Viral Profiles in Responders and Non-Responders of KS Treatment
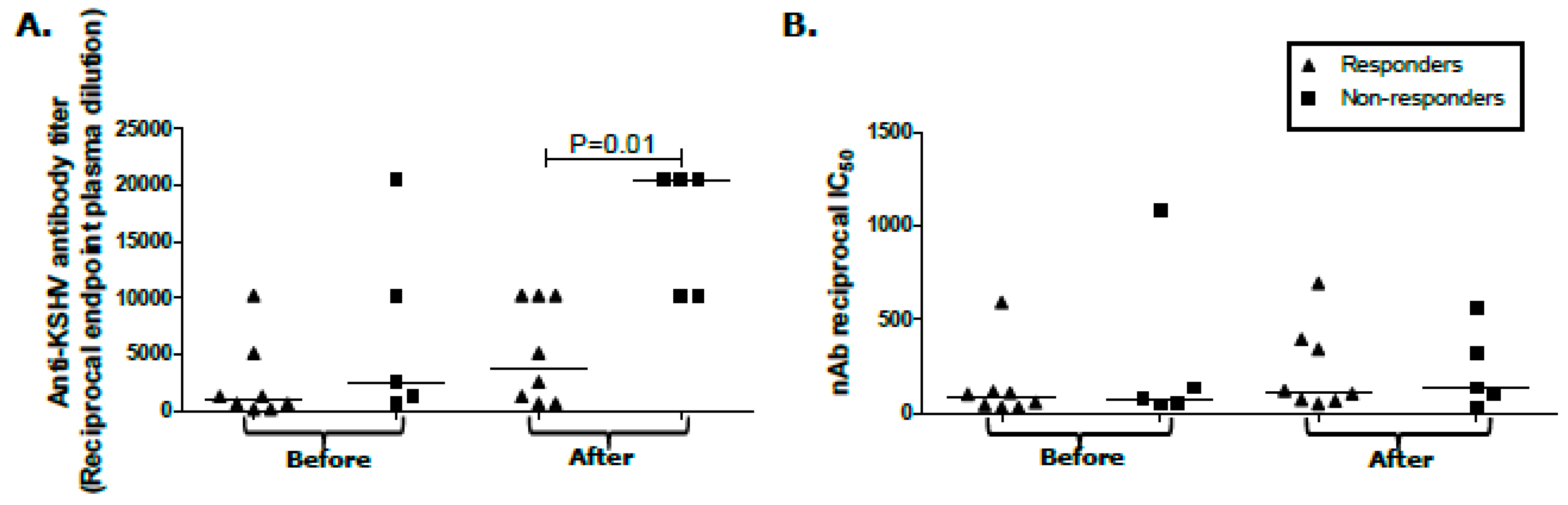
2.3. Humoral Response Differentials between KS Treatment Responders and Non-Responders
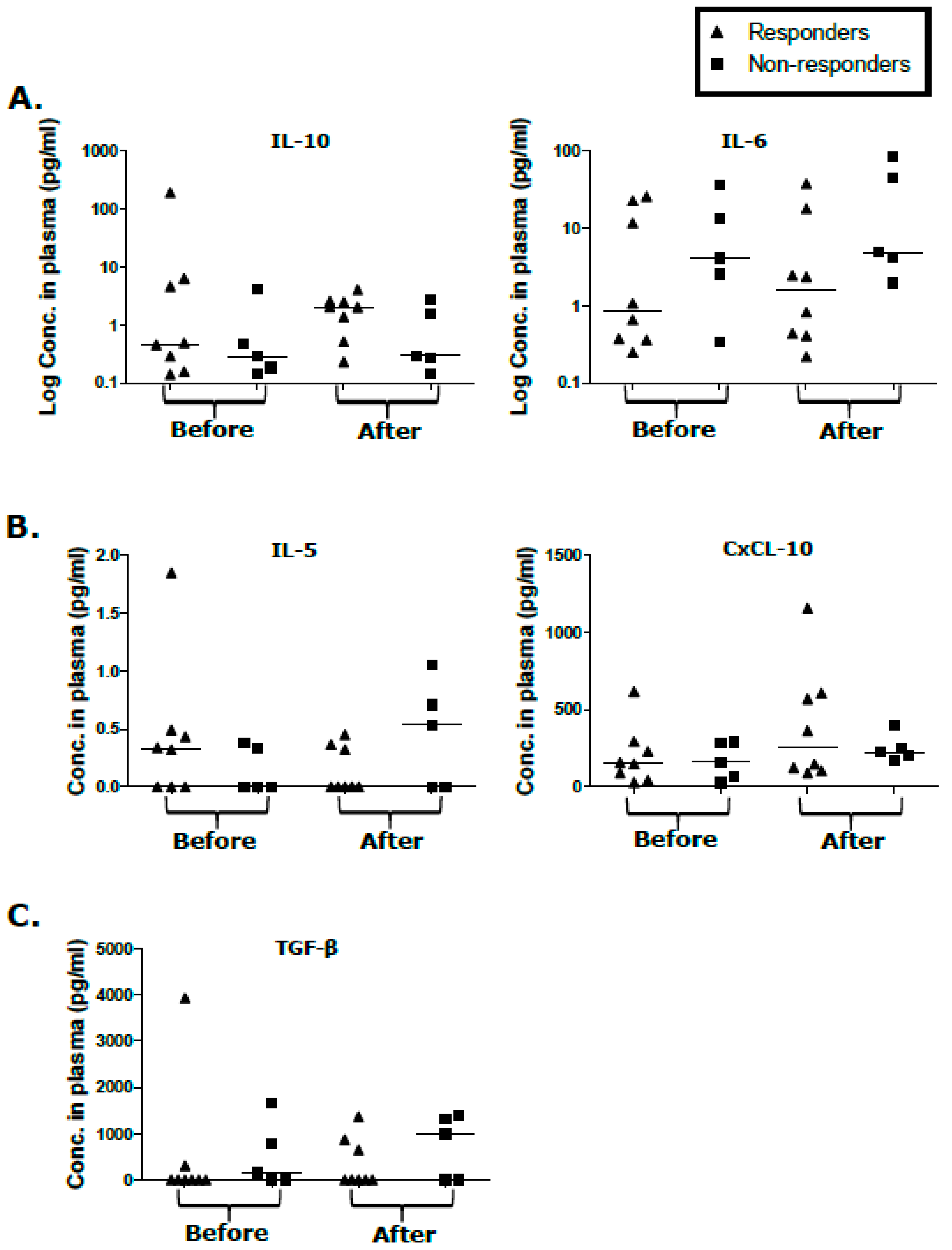
2.4. Cytokine/Chemokine Levels in Responders and Non-Responders Unaffected by KS Treatment
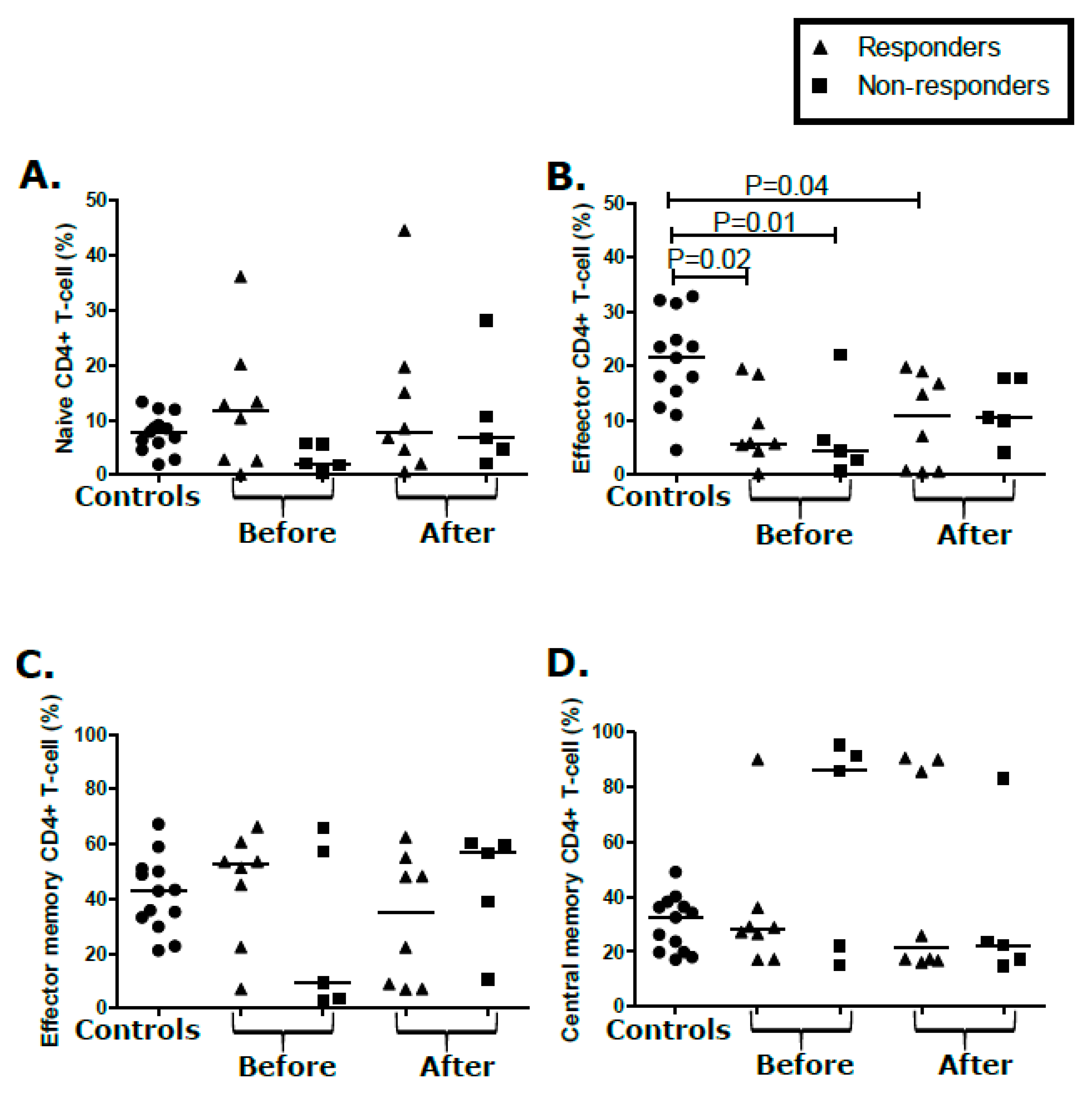
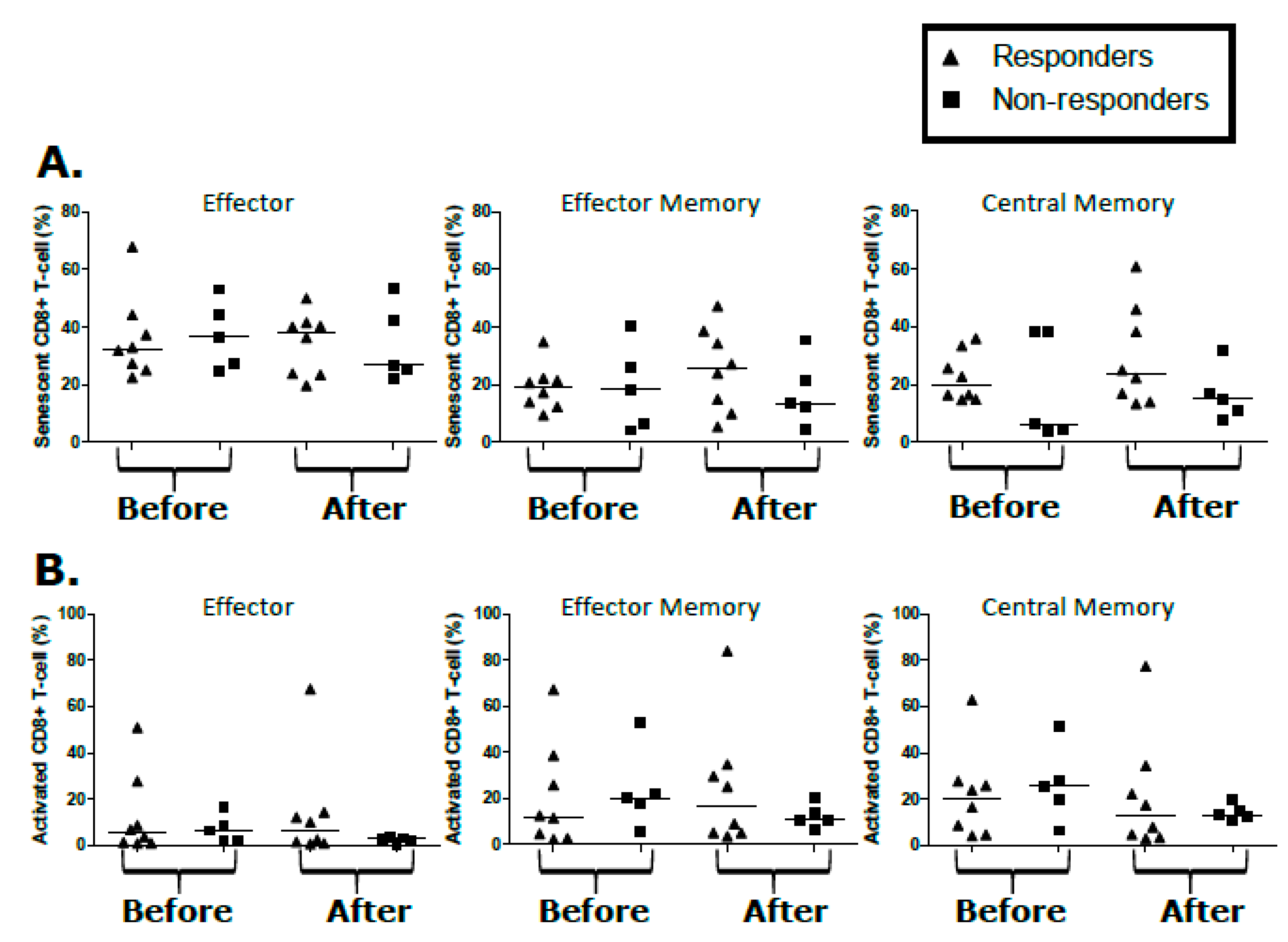
2.5. T-Cell Populations Are Not Differential between KS Treatment Responders and Non-Responders
3. Discussion
4. Materials and Methods
4.1. Study Design, Subjects, and Samples
4.2. Kaposi’s Sarcoma Response Criteria
4.3. HIV-1 Status and HIV-1 and KSHV Quantification in Plasma and Tumor Tissues
4.4. rKSHV.219 Production, KSHV Serology, and Neutralization Assays
4.5. Multiplex Bead-Based Immunoassay
4.6. Flow Cytometry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, M.; Peter, M.; Damania, B.A.; Cesarman, E. Chapter 65 Kaposi’s Sarcoma—Associated Herpesvirus. In Fields Virology; American Society of Microbiology: Washington, DC, USA, 2013; Volume 6, pp. 1–72. [Google Scholar]
- Dollard, S.C.; Butler, L.M.; Jones, A.M.G.; Mermin, J.H.; Chidzonga, M.; Chipato, T.; Shiboski, C.H.; Brander, C.; Mosam, A.; Kiepiela, P.; et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: Insights on the origin of the “Kaposi’s sarcoma belt”. Int. J. Cancer 2010, 127, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Nalwoga, A.; Cose, S.; Wakeham, K.; Miley, W.; Ndibazza, J.; Drakeley, C.; Elliott, A.; Whitby, D.; Newton, R. Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop. Med. Int. Health 2015, 20, 665–672. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4390463/pdf/tmi0020-0665.pdf (accessed on 7 August 2018). [CrossRef] [PubMed]
- Newton, R.; Labo, N.; Wakeham, K.; Miley, W.; Asiki, G.; Johnston, W.T.; Whitby, D. Kaposi sarcoma–associated herpesvirus in a rural Ugandan cohort, 1992–2008. J. Infect. Dis. 2018, 217, 263–269. Available online: https://academic.oup.com/jid/article/217/2/263/4584515 (accessed on 7 August 2018). [CrossRef] [PubMed]
- Cook-Mozaffari, P.; Newton, R.; Beral, V.; Burkitt, D.P. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br. J. Cancer 1998, 78, 1521–1528. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2063225&tool=pmcentrez&rendertype=abstract (accessed on 7 August 2018). [CrossRef] [Green Version]
- McHardy, J.; Williams, E.H.; Geser, A.; De-Thé, G.; Beth, E.; Giraldo, G. Endemic Kaposi’s sarcoma: Incidence and risk factors in the west Nile district Of Uganda. Int. J. Cancer 1984, 33, 203–212. [Google Scholar] [CrossRef]
- Wabinga, H.R.; Parkin, D.M.; Wabwire-Mangen, F.; Nambooze, S. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br. J. Cancer 2000, 82, 1585–1592. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10789729 (accessed on 17 May 2017).
- Chokunonga, E.; Levy, L.M.; Bassett, M.T.; Mauchaza, B.G.; Thomas, D.B.; Parkin, D.M. Cancer incidence in the African population of Harare, Zimbabwe: Second results from the cancer registry 1993-1995. Int. J. Cancer 2000, 85, 54–59. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L29573301 (accessed on 3 December 2019). [CrossRef]
- Semeere, A.S.; Busakhala, N.; Martin, J.N. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Curr. Opin. Oncol. 2012, 24, 522–530. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22729153 (accessed on 26 September 2018). [CrossRef] [Green Version]
- Chan, J.; Kravcik, S.; Angel, J.B. Development of Kaposi’s sarcoma despite sustained suppression of HIV plasma viremia. J. Acquir. Immune Defic. Syndr. 2000, 23, 361. [Google Scholar]
- Maurer, T.; Ponte, M.; Leslie, K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N. Engl. J. Med. 2007, 357, 1352–1353. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F.; Hullsiek, K.H.; Ganesan, A. Is Kaposi’s sarcoma occurring at higher CD4 cell counts over the course of the HIV epidemic? AIDS 2010, 24, 2881–2883. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20827160 (accessed on 26 September 2018). [CrossRef] [PubMed] [Green Version]
- Palich, R.; Veyri, M.; Valantin, M.-A.; Marcelin, A.-G.; Guihot, A.; Pourcher, V.; Jary, A.; Solas, C.; Makinson, A.; Poizot-Martin, I.; et al. Recurrence and occurrence of Kaposi’s sarcoma in HIV-infected patients on antiretroviral therapy despite suppressed HIV viremia. Clin Infect Dis 2020, 70, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- El-Mallawany, N.K.; McAtee, C.; Campbell, L.R.; Kazembe, P.N. Pediatric Kaposi sarcoma in context of the HIV epidemic in sub-Saharan Africa: Current perspectives. Pediatr. Health Med. Ther. 2018, 9, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Irira, M.; Ngocho, J.S.; Youze, J. Prevalence and outcome of HIV-associated malignancies among HIV-infected children enrolled into care at Kilimanjaro Christian medical center 2006 to 2014: A hospital-based retrospective analytical study. J. Pediatr. Hematol. Oncol. 2018. [Google Scholar] [CrossRef]
- Makombe, S.D.; Harries, A.D.; Yu, J.K.L.; Mindy Hochgesang, M.; Mhango, E.; Weigel, R.; Pasulani, O.; Fitzgerald, M.; Schouten, E.J.; Libamba, E.; et al. Outcomes of patients with Kaposi’s sarcomawho start antiretroviral therapy under routine programme conditions in Malawi. Trop Doct. 2008, 38, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Chalya, P.L.; Mbunda, F.; Rambau, P.; Jaka, H.; Masalu, N.; Mirambo, M.M.; Mushi, M.F.; Kalluvya, S. Kaposi’s sarcoma: A 10-year experience with 248 patients at a single tertiary care hospital in Tanzania. BMC Res. Notes 2015, 8, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Estimated Cancer Incidence, Mortality and Prevalence in Tanzania 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed on 1 June 2017).
- Mwafongo, A.A.; Rosenberg, N.E.; Ng’Ambi, W.; Werner, A.B.; Garneau, W.M.; Gumulira, J.; Phiri, S.; Hosseinipour, M.C. Treatment outcomes of AIDS-associated Kaposi’s sarcoma under a routine antiretroviral therapy program in Lilongwe, Malawi: Bleomycin/vincristine compared to vincristine monotherapy. PLoS ONE 2014, 9, e91020. [Google Scholar] [CrossRef] [PubMed]
- Fardhdiani, V.; Molfino, L.; Zamudio, A.G.; Manuel, R.; Luciano, G.; Ciglenecki, I.; Rusch, B.; Toutous-Trellu, L.; Coldiron, M.E. HIV-associated Kaposi’s sarcoma in Maputo, Mozambique: Outcomes in a specialized treatment center, 2010–2015. Infect. Agents Cancer 2018, 13, 5. [Google Scholar] [CrossRef] [Green Version]
- Mtonga, W.; Mujajati, A.; Munkombwe, D.; Kalungia, A.C.; Muungo, L.T.; West, J.; Wood, C.; Ngalamika, O. Therapeutic outcomes in AIDS-associated Kaposi’s sarcoma patients on antiretroviral therapy treated with chemotherapy at two tertiary hospitals in Lusaka, Zambia. Curr. HIV Res. 2018, 16, 231–236. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29992888 (accessed on 31 December 2019). [CrossRef]
- Ramaswami, R.; Uldrick, T.S.; Polizzotto, M.N.; Wyvill, K.M.; Goncalves, P.; Widell, A.; Lurain, K.; Steinberg, S.M.; Figg, W.D.; Tosato, G.; et al. A pilot study of liposomal doxorubicin combined with bevacizumab followed by bevacizumab monotherapy in patients with advanced Kaposi sarcoma. Clin. Cancer Res. 2019, 25, 4238–4247. [Google Scholar] [CrossRef] [Green Version]
- Gantt, S.; Kakuru, A.; Wald, A.; Walusansa, V.; Corey, L.; Casper, C.; Orem, J. Clinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan children. Pediatr. Blood Cancer 2010, 54, 670–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Carbonero, L.; Barrios, A.; Saballs, P.; Sirera, G.; Santos, J.; Palacios, R.; Valencia, M.E.; Alegre, M.; Podzamczer, D.; Gonzalez-Lahoz, J.; et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi’s sarcoma. AIDS 2004, 18, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Lidenge, S.J.; Tso, F.Y.; Ngalamika, O.; Ngowi, J.R.; Mortazavi, Y.; Kwon, E.H.; Shea, D.M.; Minhas, V.; Mwaiselage, J.; Wood, C.; et al. Similar immunological profiles between african endemic and human immunodeficiency virus type 1–associated epidemic Kaposi Sarcoma (KS) patients reveal the primary role of KS-Associated Herpesvirus in KS pathogenesis. J. Infect. Dis. 2018, 219, 1318–1328. Available online: https://academic.oup.com/jid/article-abstract/219/8/1318/5191126 (accessed on 11 May 2020). [CrossRef] [PubMed]
- Xu, Q.; Katakura, Y.; Yamashita, M.; Fang, S.; Tamura, T.; Matsumoto, S.-E.; Aiba, Y.; Teruya, K.; Osada, K.; Nishikawa, R.; et al. IL-10 augments antibody production in in vitro immunized lymphocytes by inducing a Th2-type response and B cell maturation. Biosci. Biotechnol. Biochem. 2004, 68, 2279–2284. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15564665 (accessed on 15 March 2020). [CrossRef] [Green Version]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. Available online: https://academic.oup.com/intimm/article-abstract/21/12/1303/686327 (accessed on 14 August 2018). [CrossRef] [Green Version]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 2014, 74, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kuwa, N.Y.; Minhas, V.; Marimo, C.; Shea, D.M.; Kankasa, C.; Wood, C. Higher levels of neutralizing antibodies against KSHV in KS patients compared to asymptomatic individuals from Zambia. PLoS ONE 2013, 8, e71254. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3743886&tool=pmcentrez&rendertype=abstract (accessed on 2 December 2015). [CrossRef]
- Weninger, W.; Manjunath, N.; Von Andrian, U.H. Migration and differentiation of CD8+ T cells. Immunol. Rev. 2002, 186, 221–233. [Google Scholar] [CrossRef]
- Marelli-Berg, F.; Cannella, L.; Dazzi, F.; Mirenda, V. The highway code of T cell trafficking. J. Pathol. 2008, 214, 179–189. [Google Scholar] [CrossRef]
- Radu, O.; Pantanowitz, L. Kaposi Sarcoma. Arch. Pathol. Lab. Med. 2013, 137, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borok, M.Z.; Fiorillo, S.; Gudza, I.; Putnam, B.; Ndemera, B.; White, I.E.; Gwanzura, L.; Schooley, R.T.; Campbell, T.B. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clin. Infect. Dis. 2010, 51, 342–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, I.-U.; Pria, A.D.; Papanastasopoulos, P.; Stegmann, K.; Bradshaw, D.; Nelson, M.; Bower, M. The clinical application of plasma Kaposi sarcoma herpesvirus viral load as a tumour biomarker: Results from 704 patients. HIV Med. 2015, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lee, J.Y.; Kaplan, L.D.; Dezube, B.J.; Noy, A.; Krown, S.E.; Levine, A.M.; Yu, Y.; Hayward, G.S.; Ambinder, R.F.; et al. Effects of chemotherapy in AIDS-associated non-Hodgkin’s lymphoma on Kaposi’s sarcoma Herpesvirus DNA in blood. J. Clin. Oncol. 2009, 27, 2496–2502. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19349542 (accessed on 7 January 2020). [CrossRef] [Green Version]
- Tedeschi, R.; Bidoli, E.; Bortolin, M.T.; Schioppa, O.; Vaccher, E.; De Paoli, P. Plasma biomarkers of clinical response during chemotherapy plus combination antiretroviral therapy (cART) in HIV+ patients with advanced Kaposi sarcoma. Oncotarget 2015, 6, 30334–30342. [Google Scholar] [CrossRef] [Green Version]
- Bower, M.; Stebbing, J.; Tuthill, M.; Campbell, V.; Krell, J.; Holmes, P.; Ozzard, A.; Nelson, M.; Gazzard, B.; Powles, T.; et al. Immunologic recovery in survivors following chemotherapy for AIDS-related non-Hodgkin lymphoma. Blood 2008, 111, 3986–3990. [Google Scholar] [CrossRef] [Green Version]
- Bonomi, M.; Ahmed, T.; Addo, S.; Kooshki, M.; Palmieri, D.; Levine, B.J.; Ruiz, J.; Grant, S.; Petty, W.J.; Triozzi, P.L.; et al. Circulating immune biomarkers as predictors of the response to pembrolizumab and weekly low dose carboplatin and paclitaxel in NSCLC and poor PS: An interim analysis. Oncol. Lett. 2018, 17, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2017, 75, 689–713. [Google Scholar] [CrossRef] [Green Version]
- Uldrick, T.S.; Wang, V.; O’Mahony, D.; Aleman, K.; Wyvill, K.M.; Marshall, V.; Steinberg, S.M.; Pittaluga, S.; Maric, I.; Whitby, D.; et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin. Infect. Dis. 2010, 51, 350–358. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2946207&tool=pmcentrez&rendertype=abstract (accessed on 7 January 2020). [CrossRef] [Green Version]
- Host, K.M.; Jacobs, S.R.; West, J.A.; Zhang, Z.; Costantini, L.M.; Stopford, C.M.; Dittmer, D.P.; Damania, B. Kaposi’s Sarcoma-associated Herpesvirus increases PD-L1 and Proinflammatory Cytokine expression in human monocytes. mBio 2017, 8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5635685/pdf/mBio.00917-17.pdf (accessed on 8 August 2018). [CrossRef] [Green Version]
- Krown, S.E.; Roy, D.; Lee, J.Y.; Dezube, B.J.; Reid, E.G.; Venkataramanan, R.; Han, K.; Cesarman, E.; Dittmer, D.P. Rapamycin With Antiretroviral Therapy in AIDS-Associated Kaposi Sarcoma: An AIDS malignancy consortium study. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 59, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koon, H.B.; Krown, S.E.; Lee, J.Y.; Honda, K.; Rapisuwon, S.; Wang, Z.; Aboulafia, D.; Reid, E.G.; Rudek, M.A.; Dezube, B.J.; et al. Phase II trial of imatinib in AIDS-associated Kaposi’s Sarcoma: AIDS malignancy consortium protocol 042. J. Clin. Oncol. 2014, 32, 402–408. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24378417 (accessed on 15 March 2020). [CrossRef] [PubMed] [Green Version]
- Coghill, A.E.; Hildesheim, A. Epstein-barr virus antibodies and the risk of associated malignancies: Review of the literature. Am. J. Epidemiol. 2014, 180, 687–695. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25167864 (accessed on 19 October 2018). [CrossRef] [PubMed] [Green Version]
- Wakeham, K.; Johnston, W.T.; Nalwoga, A.; Webb, E.L.; Mayanja, B.N.; Miley, W.; Elliott, A.; Whitby, D.; Newton, R. Trends in Kaposi’s sarcoma-associated Herpesvirus antibodies prior to the development of HIV-associated Kaposi’s sarcoma: A nested case-control study. Int. J. Cancer 2014, 136, 2822–2830. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25395177 (accessed on 19 October 2018). [CrossRef] [PubMed]
- Roshan, R.; Labo, N.; Trivett, M.; Miley, W.; Marshall, V.; Coren, L.; Castro, E.M.C.; Perez, H.; Holdridge, B.; Davis, E.; et al. T-cell responses to KSHV infection: A systematic approach. Oncotarget 2017, 8, 109402–109416. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29312617 (accessed on 8 January 2020). [CrossRef] [PubMed]
- Van Bogaert, L.-J. Clinicopathological proficiency in the diagnosis of Kaposi’s Sarcoma. ISRN AIDS 2012, 2012, 1–7. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24052878 (accessed on 17 October 2018). [CrossRef] [Green Version]
- Amerson, E.H.; Woodruff, C.M.; Forrestel, A.; Wenger, M.; McCalmont, T.; LeBoit, P.; Maurer, T.; Laker-Oketta, M.; Muyindike, W.; Bwana, M.; et al. Accuracy of clinical suspicion and pathologic diagnosis of kaposi sarcoma in East Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 71, 295–301. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26452066 (accessed on 17 October 2018). [CrossRef]
- Krown, S.E.; Testa, M.A.; Huang, J. Aids-related Kaposi’s sarcoma: Prospective validation of the AIDS clinical trials group staging classification. J. Clin. Oncol. Am. Soc. Clin. Oncol. 1997, 15, 3085–3092. [Google Scholar] [CrossRef]
- Vieira, J.; O’Hearn, P.M. Use of the red fluorescent protein as a marker of Kaposi’s sarcoma-associated herpesvirus lytic gene expression. Virology 2004, 325, 225–240. Available online: http://ac.els-cdn.com/S0042682204002284/1-s2.0-S0042682204002284-main.pdf?_tid=f331a708-47dc-11e7-852d-00000aab0f6c&acdnat=1496440175_4714b3413ecfb09f17ec515735c3da12 (accessed on 2 June 2017). [CrossRef] [Green Version]

| Variables | Kaposi’s Sarcoma-Associated Herpes Virus (KSHV) Infected Asymptomatic Controls | Participants with Kaposi’s Sarcoma (KS) | p-Values * | ||
|---|---|---|---|---|---|
| HIV-1− (n = 6) | HIV-1+ (n = 7) | KS Responders (n = 8) | KS Non-Responders (n = 5) | ||
| Median age-years (Range) | 49 (33–55) | 36 (34–48) | 38 (26–57) | 44 (39–58) | 0.05 |
| Male gender (%) | 4 (67) | 3 (43) | 4 (50) | 5 (100) | 0.07 |
| Nodular KS lesions (%) | NA | NA | 3 (37.5) | 4 (80) | 0.15 |
| KS duration ** (Range) | NA | NA | 12 (1–96) | 6 (3–36) | 0.68 |
| Median CD4 count (Range) | 620 (303–1398) | 316 (124–675) | 377.5 (130–887) | 150 (91–150) ^ | 0.09 |
| Plasma HIV-1 load (copies/mL) | NA | BDL | 3.5E4 (BDL-1.5E5) | 1.0E4 (BDL-1.5E4) | 0.80 |
| Anti-retroviral therapy (ART) duration *** (Range) | NA | 12 (1–36) | 8 (1–36) | 24 (0.25–72) | 0.52 |
| ID | KSHV Virion in Plasma (PCR) (Copies/mL) | CD4 Count (Cells/µL) | HIV-1 Plasma Viral Copies/mL | |||||
|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | |
| 21147 | - | - | NA | NA | 887 | 523 | NA | NA |
| 21182 | + | - | 1300 | NA | 854 | 1154 | NA | NA |
| 21117 | + | - | BDL | NA | 130 | 262 | 3450 | BDL |
| 21145 | - | - | NA | NA | 422 | 370 | BDL | BDL |
| 21161 | - | - | NA | NA | 710 | 306 | 148,000 | 369,000 |
| 21186 | + | + | 2100 | BDL | 143 | 211 | 34,700 | BDL |
| 21220 | - | - | NA | NA | 167 | NR | BDL | BDL |
| 21228 | - | - | NA | NA | 333 | 353 | BDL | BDL |
| 21119 | + | - | BDL | NA | 221 | 244 | BDL | BDL |
| 21185 | - | - | NA | NA | 178 | 112 | BDL | BDL |
| 21120 | + | - | BDL | NA | 91 | 482 | 14,500 | BDL |
| 21121 | - | - | NA | NA | 98 | 314 | 6310 | BDL |
| 21204 | + | - | BDL | NA | 150 | 152 | BDL | BDL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidenge, S.J.; Tso, F.Y.; Mortazavi, Y.; Ngowi, J.R.; Shea, D.M.; Mwaiselage, J.; Wood, C.; West, J.T. Viral and Immunological Analytes are Poor Predictors of the Clinical Treatment Response in Kaposi’s Sarcoma Patients. Cancers 2020, 12, 1594. https://doi.org/10.3390/cancers12061594
Lidenge SJ, Tso FY, Mortazavi Y, Ngowi JR, Shea DM, Mwaiselage J, Wood C, West JT. Viral and Immunological Analytes are Poor Predictors of the Clinical Treatment Response in Kaposi’s Sarcoma Patients. Cancers. 2020; 12(6):1594. https://doi.org/10.3390/cancers12061594
Chicago/Turabian StyleLidenge, Salum J., For Yue Tso, Yasaman Mortazavi, John R. Ngowi, Danielle M. Shea, Julius Mwaiselage, Charles Wood, and John T. West. 2020. "Viral and Immunological Analytes are Poor Predictors of the Clinical Treatment Response in Kaposi’s Sarcoma Patients" Cancers 12, no. 6: 1594. https://doi.org/10.3390/cancers12061594





