Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics and Therapeutic Data of the Study Population
2.2. Overall Survival and Therapy Response
2.3. Association of Biomarkers with Mortality
2.4. Association of Biomarkers with Disease Progression and Disease Control Rate
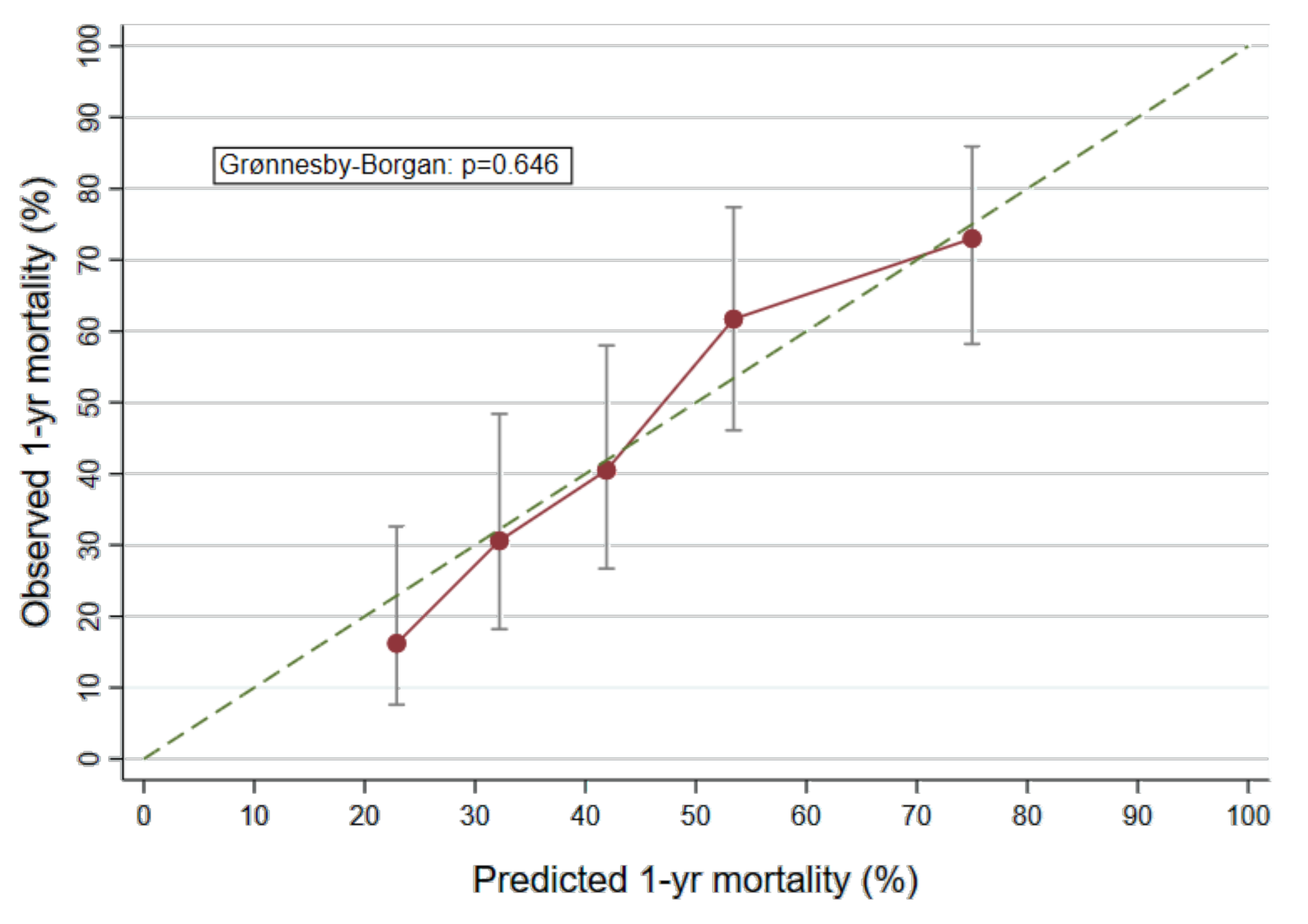
2.5. Derivation of a Biomarker Based Prognostic Model
3. Discussion
4. Materials and Methods
4.1. Study Design and Procedures
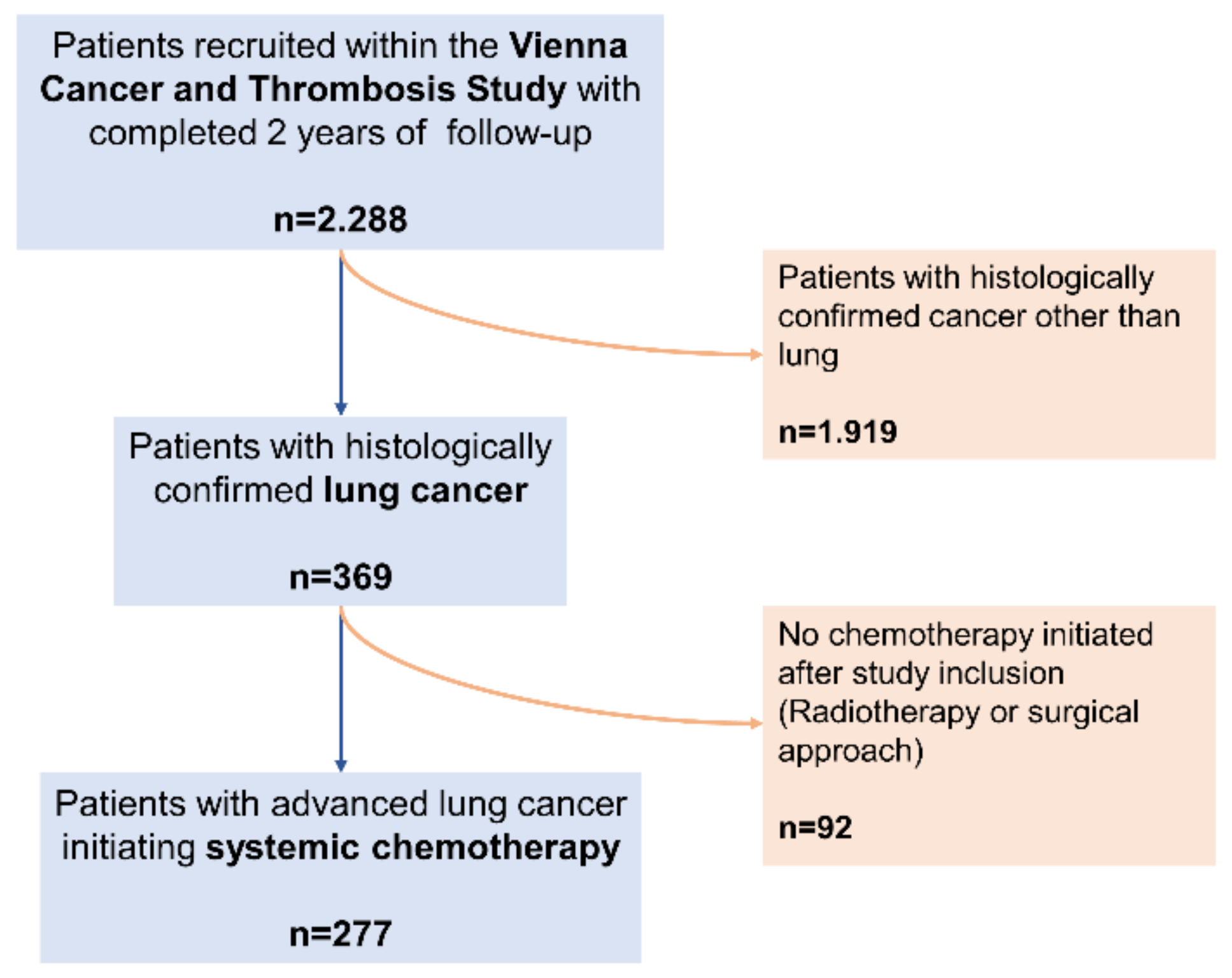
4.2. Derivation of Study Cohort
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Biomarker Description and Rationale
Appendix B
Vienna Cancer and Thrombosis Study (CATS): Design, in-/Exclusion
- patients with newly diagnosed cancer of the brain, breast, lung, upper or lower gastrointestinal tract, pancreas, kidney, prostate or gynaecologic system; sarcoma; hematologic malignancies (myeloma, high- and low-grade lymphoma); or progression of disease after complete or partial remission;
- histologic confirmation of diagnosis;
- age more than 18 years;
- willingness to participate;
- written informed consent.
- overt bacterial or viral infection within the last 2 weeks;
- venous or arterial thromboembolism within the last 3 months;
- continuous anticoagulation with vitamin K antagonists or low molecular weight heparin (LMWH); patients were allowed to take aspirin, ticlopidine, or clopidogrel, and immobilized patients were treated with LMWH as thrombosis prophylaxis during their hospital stay;
- surgery or radiotherapy within the last 2 weeks;
- chemotherapy within the last 3 months.
Appendix C
Biomarker Measurement: Methods and Timepoints
- D-dimer: Quantitative latex assay (STA-LIAtest D-DI; Diagnostica-Stago, Asnieres, France) on an STA-R analyser (Diagnostica-Stago).
- F1+2: Enzyme-linked immunoassay (Enzygnost F1+2; Dade-Behring, Marburg, Germany).
- FVIII: Sysmex CA 7000 analyser using factor VIII-deficient plasma (Technoclone, Vienna, Austria) and APTT Actin-FS (Dade-Behring).
- Peak-TG: Technothrombin TGA kit, (Technoclone) on a fully automated, computer-controlled microplate reader (BioTek, FL ×800, Winooski, Vermont, USA) and a specially adapted software (Technothrombin TGA, Vienna, Austria) using the fluorogenic substrate Z-Gly-Gly-Arg-AMC (Bachem, Bubendorf, Switzerland); The reaction was triggered with the TGA RC low reagent, which contained 71.6 pM recombinant human tissue factor lipidated in 3.2 μmol/L phospholipid micelles (phosphatidylcholine [2.56 μmol/L] and phosphatidylserine [0.64 μmol/L]).
- sP-selectin: human sP-selectin Immunoassay (R&D Systems, Minneapolis, MN, USA)
- Fibrinogen: routinely measured in platelet poor plasma according to Clauss (STA Fibrinogen; Diagnostica-Stago).
Inflammatory Biomarkers and Blood Count Parameters:
- CRP: Immunoturbidimetry.
- Blood count parameters (leucocyte count, leucocyte subpopulations, thrombocyte count, haemoglobin): Sysmex XE-5000/XN-1000/XN-2000.
- NLR: Calculated ratio of absolute neutrophil count to absolute lymphocyte count.
- LMR: Calculated ratio of absolute lymphocyte count to absolute monocyte count.
- PLR: Calculated ratio of absolute platelet count to absolute lymphocyte count.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgensztern, D.; Ng, S.H.; Gao, F.; Govindan, R. Trends in Stage Distribution for Patients with Non–small Cell Lung Cancer: A National Cancer Database Survey. J. Thorac. Oncol. 2010, 5, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow–up. Ann. Oncol. 2019, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Fruh, M.; De Ruysscher, D.; Popat, S.; Crino, L.; Peters, S.; Felip, E. Small–cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow–up. Ann. Oncol. 2013, 24 (Suppl. 6), vi99–vi105. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- O’Callaghan, D.S.; O’Donnell, D.; O’Connell, F.; O’Byrne, K.J. The Role of Inflammation in the Pathogenesis of Non-small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 2024–2036. [Google Scholar] [CrossRef] [Green Version]
- Goldin-Lang, P.; Tran, Q.V.; Fichtner, I.; Eisenreich, A.; Antoniak, S.; Schulze, K.; Coupland, S.E.; Poller, W.; Schultheiss, H.-P. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol. Rep. 2008, 20, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Wang, S.; Long, G. C-reactive protein is a significant predictor of improved survival in patients with advanced non-small cell lung cancer. Medicine 2019, 98. [Google Scholar] [CrossRef]
- Machado, D.; Marques, C.; Dias, M.; Campainha, S.; Barroso, A. Inflammatory prognostic biomarkers in advanced non-small cell lung cancer. Pulmonology 2019, 25, 181–183. [Google Scholar] [CrossRef]
- Akinci Ozyurek, B.; Sahin Ozdemirel, T.; Buyukyaylaci Ozden, S.; Erdogan, Y.; Kaplan, B.; Kaplan, T. Prognostic Value of the Neutrophil to Lymphocyte Ratio (NLR) in Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2017, 18, 1417–1421. [Google Scholar]
- Cedres, S.; Torrejon, D.; Martinez, A.; Martinez, P.; Navarro, A.; Zamora, E.; Mulet-Margalef, N.; Felip, E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin. Transl. Oncol. 2012, 14, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Pang, Z.; Shen, H.; Ni, Y.; Du, J.; Liu, Q. The Prognostic Value of PLR in Lung Cancer, a Meta-analysis Based on Results from a Large Consecutive Cohort. Sci. Rep. 2016, 6, 34823. [Google Scholar] [CrossRef] [Green Version]
- Regina, S.; Valentin, J.B.; Lachot, S.; Lemarie, E.; Rollin, J.; Gruel, Y. Increased tissue factor expression is associated with reduced survival in non-small cell lung cancer and with mutations of TP53 and PTEN. Clin. Chem. 2009, 55, 1834–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Wang, C.; Lv, B.; Jiang, Y.; Wang, L. Proteinase-activated receptor-2 enhances Bcl2-like protein-12 expression in lung cancer cells to suppress p53 expression. Arch. Med. Sci. 2019, 15, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Miyake, S.; Ohdama, S.; Matsubara, O.; Masuda, S.; Yakumaru, K.; Yoshizawa, Y. Expression of tissue factor in non-small-cell lung cancers and its relationship to metastasis. Br. J. Cancer 1999, 79, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, D.; Xu, W.Y.; Wang, Y.W.; Che, G.W. Prognostic Value of Pretreatment Lymphocyte–to–Monocyte Ratio in Non-Small Cell Lung Cancer: A Meta–Analysis. Oncol. Res. Treat. 2019, 42, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, G.; Wu, Q.; Deng, Y.; Liu, Y.; Wang, J. Prognostic value of lymphocyte–to–monocyte ratio among Asian lung cancer patients: A systematic review and meta-analysis. Oncotarget 2017, 8, 110606–110613. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A.; Panova–Noeva, M.; Russo, L. Procoagulant mechanisms in tumour cells. Best Pract. Res. Clin. Haematol. 2009, 22, 49–60. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Potter, J.M.; Kaplan, L.S.; Talmage, K.; Jackson, D.G.; Degen, J.L. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002, 62, 6966–6972. [Google Scholar]
- Grafetstatter, M.; Husing, A.; Gonzalez Maldonado, S.; Sookthai, D.; Johnson, T.; Pletsch–Borba, L.; Katzske, V.A.; Hoffmeister, M.; Buger, P.; Kaaks, R. Plasma Fibrinogen and sP–Selectin are Associated with the Risk of Lung Cancer in a Prospective Study. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Buccheri, G.; Ferrigno, D.; Ginardi, C.; Zuliani, C. Haemostatic abnormalities in lung cancer: Prognostic implications. Eur. J. Cancer 1997, 33, 50–55. [Google Scholar] [CrossRef]
- Tas, F.; Kilic, L.; Serilmez, M.; Keskin, S.; Sen, F.; Duranyildiz, D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir. Med. 2013, 107, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buccheri, G.; Torchio, P.; Ferrigno, D. Plasma levels of D–dimer in lung carcinoma. Cancer 2003, 97, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Qian, Y.; Fang, S.; Wang, Y.; Tang, Y.; Gu, W. Prognostic Value of Plasma Fibrinogen in Lung Cancer Patients: A Meta-Analysis. J. Cancer 2018, 9, 3904–3911. [Google Scholar] [CrossRef] [Green Version]
- Ay, C.; Vormittag, R.; Dunkler, D.; Simanek, R.; Chiriac, A.-L.; Drach, J.; Quehenberger, P.; Wagner, O.; Zielinski, C. D-Dimer and Prothrombin Fragment 1 + 2 Predict Venous Thromboembolism in Patients With Cancer: Results From the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2009, 27, 4124–4129. [Google Scholar] [CrossRef]
- Ay, C.; Dunkler, D.; Pirker, R.; Thaler, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.; Pabinger, I. High D–dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012, 97, 1158. [Google Scholar] [CrossRef] [Green Version]
- Vormittag, R.; Simanek, R.; Ay, C.; Dunkler, D.; Quehenberger, P.; Marosi, C.; Zielinski, C.; Pabinger, I. High Factor VIII Levels Independently Predict Venous Thromboembolism in Cancer Patients. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2176–2181. [Google Scholar] [CrossRef] [Green Version]
- Ay, C.; Dunkler, D.; Simanek, R.; Thaler, J.; Koder, S.; Marosi, C.; Zielinski, C.; Pabinger, I. Prediction of Venous Thromboembolism in Patients With Cancer by Measuring Thrombin Generation: Results From the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2011, 29, 2099–2103. [Google Scholar] [CrossRef]
- Ay, C.; Simanek, R.; Vormittag, R.; Dunkler, D.; Alguel, G.; Koder, S.; Kornek, G.; Marosi, C.; Wagner, O.; Zielinski, C. High plasma levels of soluble P–selectin are predictive of venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008, 112, 2703. [Google Scholar] [CrossRef] [Green Version]
- Tiedje, V.; Dunkler, D.; Ay, C.; Horvath, B.; Quehenberger, P.; Pabinger, M.; Zielinski, C.; Pabinger, I.; Mannhalter, C. The role of fibrinogen plasma levels, the –455G>A fibrinogen and the factor XIII A subunit (FXIII–A) Val34Leu polymorphism in cancer-associated venous thrombosis. Thromb. Haemost. 2011, 106, 908–913. [Google Scholar] [CrossRef]
- Koster, T.; Rosendaal, F.R.; Reitsma, P.H.; van der Velden, P.A.; Briet, E.; Vandenbroucke, J.P. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA polymorphisms—The Leiden Thrombophilia Study (LETS). Thromb. Haemost. 1994, 71, 719–722. [Google Scholar] [PubMed]
- Chaturvedi, A.K.; Caporaso, N.E.; Katki, H.A.; Wong, H.-L.; Chatterjee, N.; Pine, S.R.; Chanock, S.J.; Goedert, J.J.; Engels, E.A. C-reactive protein and risk of lung cancer. J. Clin. Oncol. 2010, 28, 2719–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Ganz, T. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wei, S.; Jiang, N.; Zhang, L.; Wang, S.; Cao, X.; Zhao, Y.; Wang, P. The prognostic impact of decreased pretreatment haemoglobin level on the survival of patients with lung cancer: A systematic review and meta-analysis. BMC Cancer 2018, 18, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, I.; Makino, S.; Kiyokawa, H.; Katoh, H.; Ebihara, Y.; Ohyashiki, K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer 2001, 92, 2399–2405. [Google Scholar] [CrossRef]
- Yu, D.; Liu, B.; Zhang, L.; Du, K. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp. Ther. Med. 2013, 5, 1351–1354. [Google Scholar] [CrossRef] [Green Version]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.; Wolfe, R.; Ball, D.; Conron, M.; Stirling, R.G.; Solomon, B.; MacManus, M.; Officer, A.; Karnam, S.; Burbury, K. Lung cancer prognostic index: A risk score to predict overall survival after the diagnosis of non-small-cell lung cancer. Br. J. Cancer 2017, 117, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’Reilly, D.S.J.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Hu, K.; Zhou, Y.; Li, W. Clinical utility of the modified Glasgow prognostic score in lung cancer: A meta-analysis. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the Yield of Medical Tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Gönen, M.; Heller, G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005, 92, 965–970. [Google Scholar] [CrossRef]
- Lee, E.T.; Wang, J.W. Statistical Methods for Survival Data Analysis, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Gronnesby, J.K.; Borgan, O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.; Kengne, A.P.; Woodward, M.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Grobbee, D.E. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012, 98, 683–690. [Google Scholar] [CrossRef] [Green Version]


| Variable | n (% Missing Values) | Median [IQR] (Range) or Count (%) |
|---|---|---|
| Demographics and clinical characteristics | ||
| Age (years) | 277 (0%) | 61 [56, 67] |
| Female Gender | 277 (0%) | 103 (37.2%) |
| BMI (kg/m2) | 275 (0.7%) | 24.6 [22.2, 28.1] |
| ECOG | 154 (44.4%) | 1 [1, 2] |
| History of smoking | 271 (2.1%) | 203 (74.9%) |
| History of VTE * | 277 (0%) | 9 (3.2%) |
| m-LCPI for NSCLC ** | 230 (0.4%) | - |
| Group 1 (≤8) | - | 9 (3.9%) |
| Group 2 (9–11) | - | 36 (15.7%) |
| Group 3 (12–14) | - | 162 (70.4%) |
| Group 4 (≥15) | - | 23 (10.0%) |
| Tumour specifics at inclusion | ||
| Histology | 277 (0%) | - |
| SCLC | - | 46 (16.6%) |
| NSCLC | - | 231 (83.4%) |
| Adenocarcinoma | - | 165 (59.6%) |
| SCC | - | 45 (16.2%) |
| LCNEC | - | 9 (3.2%) |
| Others | - | 12 (4.3%) |
| Stage | 277 (0%) | - |
| I | - | 1 (0.4%) |
| II | - | 4 (1.4%) |
| III | - | 76 (27.4%) |
| IV | - | 196 (70.8%) |
| Distant metastatic site | - | - |
| Cerebral | - | 51 (18.4%) |
| Bones | - | 63 (22.7%) |
| Lung contralateral | - | 64 (23.1) |
| Pleural | - | 38 (13.7%) |
| Adrenal | - | 36 (13.0%) |
| Liver | - | 24 (8.7%) |
| Histological grade | 196 (29.2%) | - |
| G1 (well differentiated, low grade) | - | 5 (1.8%) |
| G2 (moderately differentiated, intermediate grade) | - | 80 (40.8%) |
| G3 (poorly differentiated, high grade) | - | 101 (51.5%) |
| G4 (undifferentiated, high grade) | - | 10 (5.1%) |
| Therapeutic management | ||
| Chemotherapy | 277 (0%) | 277 (100%) |
| Palliative intent | - | 249 (88.8%) |
| Neoadjuvant intent | - | 31 (11.2%) |
| Surgery (Primary) | 277 (0%) | 45 (16.2%) |
| Secondary metastasectomy | - | 13 (3.5%) |
| Radiotherapy | 277 (0%) | 119 (43.0%) |
| Cumulative dose | - | 60 [30, 90] |
| Chemotherapy regimen | 275 (0.7%) | - |
| Platin-Vinorelbine | - | 74 (26.9%) |
| Platin-Gemcitabine | - | 52 (18.9%) |
| Platin-Pemetrexed | - | 52 (18.9%) |
| Platin-Etoposid | - | 49 (17.8%) |
| Anti-VEGF-therapy | - | 6 (2.2%) |
| EGFR-TKI | - | 10 (3.6%) |
| Number of chemotherapy cycles | 271 (0.7%) | 4 [2, 5] |
| 2nd line chemotherapy | - | 112 (40.4%) |
| Checkpoint-inhibitor therapy after chemotherapy | - | 6 (2.2%) |
| Levels of biomarkers prior to initiation of therapy (median [IQR]) | ||
| Factor VIII (% activity) | 264 (4.7%) | 191 [156, 248] |
| sP-selectin (ng/mL) | 275 (0.7%) | 42.8 [33.4, 54.4] |
| D-dimer (µg/mL) | 247 (10.8%) | 0.88 [0.55, 1.90] |
| Prothrombin fragment 1+2 (pmol/L) | 273 (1.4%) | 225 [165, 348] |
| Fibrinogen (mg/dL) | 275 (0.7%) | 485 [376, 590] |
| Peak thrombin generation (nmol/L) | 271 (2.2%) | 363 [228, 509] |
| Platelet count (G/L) | 275 (0.7%) | 294 [241, 352] |
| Leucocyte count (G/L) | 275 (0.7%) | 8.34 [6.82–10.37] |
| Haemoglobin (mg/dl) | 275 (0.7%) | 12.9 [11.8, 14.0] |
| Neutrophil granulocytes (G/L) | 233 (15.9%) | 5.8 [4.5, 7.9] |
| Lymphocytes (G/L) | 232 (16.2%) | 1.2 [0.9, 1.6] |
| Monocytes (G/L) | 231 (16.6%) | 0.6 [0.4, 0.8] |
| Neutrophil-to-Lymphocyte Ratio (NLR) | 232 (16.2%) | 4.8 [3.3, 7.3] |
| Lymphocyte-to-Monocyte Ratio (LMR) | 230 (17.0%) | 2.0 [1.4, 3.2] |
| Platelet-to-Lymphocyte Ratio (PLR) | 232 (16.2%) | 246.5 [163.3, 337.2] |
| C-reactive protein (CRP) | 250 (9.7%) | 1.6 [0.6, 4.5] |
| Biomarker | HR for Death (Mortality) | HR for Disease Progression (PFS) | OR for Therapy Response (DCR) | |||
|---|---|---|---|---|---|---|
| Uni-Variable | Multi-Variable * | Uni-Variable | Multi-Variable * | Uni-Variable | Multi-Variable * | |
| Haemostatic Biomarkers | ||||||
| D-dimer | 1.58 [1.38–1.81] p < 0.001 | 1.50 [1.29–1.75] p < 0.001 | 1.41 [1.23–1.61] p < 0.001 | 1.34 [1.16–1.53] p < 0.001 | 0.69 [0.50–0.94] p = 0.020 | 0.73 [0.52–1.04] p = 0.083 |
| F1+2 | 1.24 [1.06–1.46] p = 0.008 | 1.15 [0.97–1.36] p = 0.098 | 1.25 [1.07–1.46] p = 0.006 | 1.22 [1.04–1.44] p = 0.013 | 0.69 [0.50–0.97] p = 0.031 | 0.71 [0.50–1.02] p = 0.063 |
| sP-selectin | 1.42 [1.11–1.83] p = 0.006 | 1.42 [1.09–1.83] p = 0.008 | 1.18 [0.94–1.48] p = 0.144 | 1.03 [0.81–1.30] p = 0.785 | 0.72 [0.47–1.11] p = 0.136 | 0.84 [0.53–1.32] p = 0.444 |
| Fibrinogen | 1.31 [1.02–1.96] p = 0.040 | 1.38 [0.98–1.93] p = 0.064 | 1.24 [0.94–1.65] p = 0.133 | 1.27 [0.95–1.71] p = 0.110 | 0.56 [0.31–1.00] p = 0.050 | 0.62 [0.33–1.14] p = 0.124 |
| FVIII | 1.55 [1.17–2.06] p = 0.002 | 1.46 [1.08–1.98] p = 0.013 | 1.22 [0.94–1.57] p = 0.131 | 1.16 [0.88–1.52] p = 0.305 | 0.93 [0.57–1.51] p = 0.769 | 1.02 [0.60–1.72] p = 0.954 |
| Peak-TG | 1.09 [0.93–1.28] p = 0.267 | 1.06 [0.91–1.24] p = 0.467 | 1.12 [0.97–1.28] p = 0.124 | 1.06 [0.93–1.22] p = 0.374 | 0.82 [0.62–1.08] p = 0.160 | 0.86 [0.65–1.14] p = 0.295 |
| Inflammatory and blood count parameter | ||||||
| Platelet count | 1.25 [0.95–1.67] p = 0.110 | 1.28 [0.95–1.73] p = 0.102 | 1.14 [0.89–1.45] p = 0.304 | 1.13 [0.87–1.47] p = 0.355 | 0.66 [0.40–1.11] p = 0.116 | 0.75 [0.45–1.27] p = 0.285 |
| Leucocytes | 1.17 [0.86–1.59] p = 0.311 | 1.08 [0.79–1.46] p = 0.639 | 1.22 [0.91–1.64] p = 0.180 | 1.11 [0.83–1.49] p = 0.496 | 0.71 [0.41–1.22] p = 0.218 | 0.85 [0.48–1.52] p = 0.592 |
| Haemoglobin | 0.12 [0.05–0.27] p < 0.001 | 0.13 [0.06–0.30] p < 0.001 | 0.27 [0.13–0.54] p < 0.001 | 0.28 [0.14–0.59] p = 0.001 | 11.32 [2.61–48.97] p = 0.001 | 10.94 [2.26–53.1] p = 0.003 |
| Neutrophil granulocytes | 1.07 [0.80–1.42] p = 0.661 | 1.05 [0.78–1.41] p = 0.760 | 1.24 [0.95–1.62] p = 0.108 | 1.16 [0.89–1.53] p = 0.275 | 0.74 [0.44–1.24] p = 0.257 | 0.87 [0.50–1.51] p = 0.612 |
| Lymphocytes | 0.65 [0.52–0.81] p < 0.001 | 0.72 [0.57–0.91] p = 0.007 | 0.65 [0.52–0.79] p < 0.001 | 0.76 [0.61–0.93] p = 0.010 | 1.37 [0.88–2.12] p = 0.155 | 1.18 [0.74–1.88] p = 0.486 |
| Monocytes | 1.19 [0.99–1.43] p = 0.066 | 1.17 [0.98–1.40] p = 0.086 | 1.05 [0.89–1.24] p = 0.553 | 1.08 [0.93–1.27] p = 0.311 | 1.02 [0.75–1.38] p = 0.920 | 1.06 [0.78–1.46] p = 0.700 |
| NLR | 1.33 [1.12–1.59] p = 0.001 | 1.24 [1.03–1.49] p = 0.024 | 1.39 [1.19–1.61] p < 0.001 | 1.24 [1.05–1.46] p = 0.009 | 0.74 [0.53–1.03] p = 0.071 | 0.85 [0.60–1.21] p = 0.378 |
| LMR | 0.52 [0.39–0.69] p < 0.001 | 0.60 [0.45–0.80] p = 0.001 | 0.67 [0.52–0.86] p = 0.001 | 0.74 [0.58–0.94] p = 0.013 | 1.10 [0.72–1.70] p = 0.658 | 0.94 [0.60–1.47] p = 0.786 |
| PLR | 1.41 [1.17–1.72] p < 0.001 | 1.31 [1.08–1.61] p = 0.008 | 1.38 [1.16–1.65] p < 0.001 | 1.24 [1.03–1.48] p = 0.021 | 0.71 [0.50–1.01] p = 0.056 | 0.80 [0.56–1.16] p = 0.239 |
| CRP | 1.51 [1.35–1.71] p < 0.001 | 1.45 [1.27–1.65] p < 0.001 | 1.31 [1.18–1.47] p < 0.001 | 1.25 [1.11–1.40] p < 0.001 | 0.64 [0.50–0.81] p < 0.001 | 0.69 [0.53–0.89] p = 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moik, F.; Zöchbauer-Müller, S.; Posch, F.; Pabinger, I.; Ay, C. Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer. Cancers 2020, 12, 1619. https://doi.org/10.3390/cancers12061619
Moik F, Zöchbauer-Müller S, Posch F, Pabinger I, Ay C. Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer. Cancers. 2020; 12(6):1619. https://doi.org/10.3390/cancers12061619
Chicago/Turabian StyleMoik, Florian, Sabine Zöchbauer-Müller, Florian Posch, Ingrid Pabinger, and Cihan Ay. 2020. "Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer" Cancers 12, no. 6: 1619. https://doi.org/10.3390/cancers12061619
APA StyleMoik, F., Zöchbauer-Müller, S., Posch, F., Pabinger, I., & Ay, C. (2020). Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer. Cancers, 12(6), 1619. https://doi.org/10.3390/cancers12061619






