Abstract
Adolescents and young adults (AYAs) represent a distinct group of patients. The objectives of this study were: To compare adolescent prognosis to that of younger children; to compare the results achieved with the two consecutive protocols in both age groups; to analyze clinical characteristics of children and adolescents. Between 1996 and 2017, 1759 patients aged <18 years were evaluable for the study. Five hundred and sixty patients were treated with the MH’96 protocol and 1199 with the LH2004 protocol. Four hundred and eighty-two were adolescents aged ≥15 years. Patients in both age groups showed very favorable prognoses. In particular, OS improved with the LH2004 protocol, especially in the adolescent group and in the low risk group, where radiation therapy was spared. Adolescent characteristics differed significantly from the children’s according to sex, histology, and the presence of symptoms. Remarkable is the decrease both in mixed cellularity in the children and in low stages in both age groups in the LH2004 protocol with respect to MH’96 protocol. Based on our experience, adopting pediatric protocols for AYA does not compromise patient outcomes.
1. Introduction
Hodgkin’s lymphoma (HL) is the most common cancer diagnosed in adolescents and young adults (AYAs) between 15 and 24 years [1,2]. HLs are responsible for 16% of annual cancer diagnoses in AYAs, and studies suggest that lymphoma-related mortality is higher in AYAs than in younger children [3]. In fact, despite excellent outcomes registered, this age group recently gained attention due to SEER (Surveillance, Epidemiology, and End Results) data evidence of a lack of improvement in survival rates compared to both children and adults [4]. As it is in children, in this age group the major challenge is the balance between radiotherapy and chemotherapy and the general role and need for radiotherapy, especially in early stage disease. Delayed diagnosis, the relative lack of participation of these patients in clinical trials, decreased treatment adherence, and social environment have been pointed out as contributing factors, but no clear etiology has been defined [5,6,7]. It is also noteworthy that the incidence rate of HL in AYAs in Italy (64.6 cases per million) is the highest in Europe (29.7 per million) [8,9]. Research on the characteristics of this age group could help identify specific biological features of the disease, which, in turn, would help define optimal treatment.
Our aims for this study were:
- To evaluate the prognosis of adolescents in comparison with younger children when treated with the same protocol;
- to compare the results achieved in both age categories with the most recent Italian Association of Pediatric Hematology and Oncology (AIEOP) chemo-radiotherapeutic protocol (LH2004) and the previous one (MH’96); and
- to identify significant differences in clinical presentation of children and of adolescents, as well as an analysis of age as a prognostic factor that might impact the outcome of these categories of patients differently.
2. Materials and Methods
This particular analysis compares patients aged between 15 and 18 years to those younger than 15 years. All patients were registered at the 40 Italian Pediatric Onco-Hematology Centers applying the MH’96 AIEOP protocol from February 1996 to May 2004 and at the 35 Italian Pediatric Onco-Hematology Centers applying the LH2004 protocol from June 2004 to June 2017 (Table S1). The results of the MH’96 AIEOP Protocol have already been published, without a specific analysis on adolescents [10].
Diagnosis was histologically confirmed and centrally reviewed according to the WHO classification [11] by the same two pathologists (E.S.G.D.A, E.S.). Patients had a complete anamnesis, and underwent physical examination, routine laboratory tests, chest X-rays, abdomen ultrasound, a neck/chest/abdomen/pelvis contrast-enhanced computed tomography (CT) scan, and were staged according to the Ann Arbor classification. Bone marrow biopsy was performed in patients with stages III-IV or B symptoms. Mediastinal masses were evaluated at diagnosis and after induction therapy with 67Ga scan, progressively replaced with 18FDG-PET TC. Radiological surveillance after stop therapy included chest X-rays and abdomen ultrasound, and CT scan if necessary. 18FDG-PET TC was not suggested for screening during follow-up.
Patients were divided into three therapeutic groups, which were equivalent in both protocols. Group 1 (GR1) included stages IA and IIA with no mediastinal involvement or with mediastinal-thoracic ratio (M/T) < 0.33, less than four nodal regions, and no lung hilar adenopathy. Group 3 (GR3) included stages IIIB and IV, and patients with M/T ≥ 0.33, irrespective of the stage. Group 2 (GR2) consisted of patients not included in GR1 and GR3.
The “Children” group included patients aged <15 years and the “Adolescents” group those between 15 and 18 years.
2.1. Response Criteria
Response to treatment was evaluated according to the three therapeutic group’s schedules. Complete response (CR) was defined as the absence of clinical, radiological (ultrasound and CT scan evaluation) and radio-isotopic evidence of disease. Bulky mediastinal involvement was considered in CR with a reduction of ≥75% of the volume (greatest diameter × height × 0.52) and negative Ga scan or 18FDG-PET. Partial response (PR) was defined as tumor volumetric reduction ≥75%, <75%, >50%, ≤50%. Progressive disease (PD) was defined as disease progression during first-line chemotherapy or only transient response (CR or PR) during therapy or within three months from stop therapy. Criteria were either an increase in tumor size in previously involved sites and/or involvement of a new site. Relapse was defined as a pathologically confirmed recurrence of HL after three months from stop therapy [10].
2.2. Treatment
2.2.1. MH’96 Protocol
As previously published, GR1 patients received three ABVD. Patients with PR ≤ 50% after the first two courses of chemotherapy were shifted to intensified therapy with two IEP/OPPA/COPP. GR2 and GR3 patients received four and six COPP/ABV (cyclophosphamide, vincristine, procarbazine, prednisolone, adriamycin, bleomycin, vinblastine) respectively at 28-day intervals. Patients with PR ≤ 50% after the first two courses of chemotherapy were shifted to intensified therapy with IEP/OPPA/IEP/OPPA/IEP. Radiotherapy (RT) started four weeks after the completion of chemotherapy with involved fields (IF) RT, defined on anatomical boundaries, that include the entire lymph node regions containing the affected nodes. GR1 patients without initial mediastinal involvement in CR after the end of chemotherapy were not irradiated. All other patients in CR or PR ≥ 75% received low-dose irradiation (20 Gy at 1.8 Gy/fraction), while patients with PR < 75%, got a higher dose (36 Gy). The treatment exceeding 20 Gy was confined to residual mass. Patients with pulmonary or renal involvement persisting after the first two courses received 10 Gy on residual lesions. The liver dose was 12 Gy, and a boost dose up to 15 Gy was given to persistent foci at the end of chemotherapy (Table 1) [10].

Table 1.
MH’96 and LH2004 protocols.
2.2.2. LH2004 Protocol
GR1 patients received three ABVD, and no RT in CR patients or 25.2 Gy in PR patients. Patients with PR ≤ 50% after the first two courses of chemotherapy were treated with IEP/OPPA/COPP/IEP and RT (14.4 Gy if CR, 25.2Gy if PR ≥ 50%).
GR2 patients received four COPP/ABV, followed by 14.4 Gy RT in CR patients. Patients in PR after the end of chemotherapy received two further cycles of IEP followed by RT (14.4 Gy/25.2 Gy). Patients with PR ≤ 50% after the first two courses of chemotherapy were treated with IEP/OPPA/IEP/OPPA/IEP and RT (14.4 Gy/25.2 Gy).
GR 3 patients received six COPP/ABV followed by RT (14.4 Gy/25.2 Gy). Patients in PR after four cycles of chemotherapy received two cycles of IEP followed by 14.4 Gy IFRT if CR or two more COPP/ABV if PR, followed by RT (14.4 Gy/25.2 Gy). Patients with PR ≤ 50% after the first two courses of chemotherapy were treated with IEP/OPPA/IEP/OPPA/IEP and RT (14.4 Gy/25.2 Gy). RT started four weeks after the completion of chemotherapy with local fields, including the only lymph node affected area, and not the entire region. Partial responder patients received a boost up to 35 Gy to the residual volume larger than 50 cm3 (Table 1).
As in the previous MH’96 protocol, patients with nodular lymphocyte predominance (nLP) histological subtype were treated like patients with common HL (cHL) until 2011, when some centers chose to participate in the European International protocol for LP, named EuroNet-PHL-LP1 (EudraCT 2007-004092-19) for stages I-IIA.
The study was approved by the ethics committee or the institutional review board of each participating institution (Table S2). Written informed consent was obtained from the parents or legal guardians of all patients. After completion of treatment, biannual monitoring was scheduled for at least five years. Patients’ follow-up was updated in September 2019.
2.3. Statistical Methods
All data were collected in a central database (AIEOP MH) and analyzed. Overall survival (OS) was calculated from the date of diagnosis to the date of either death due to any cause or last contact. Event free survival (EFS) is considered to be from the date of diagnosis to the date of either a first event (relapse, second malignant neoplasm—SMN or death), or the last contact for those who are still alive and free of disease. Freedom from progression (FFP) was defined as the interval from the date of diagnosis to that of relapse or PD, or last follow-up for patients without recurrent disease. SMN and death before recurrence were considered competing for events. The Kaplan–Meier method was used to estimate EFS, FFP, and OS probabilities [12], while differences between the groups were calculated using the log-rank test [13]. Results were expressed as a probability (%) and standard error (SE). All p values were two-sided, and values <0.05 were considered to be statistically significant.
3. Results
The MH’96 protocol enrolled 605 patients: 45 patients were excluded from the analysis because of previous therapy (8), age >18 years (7), lack of information on treatment response (6), or no data beyond registration (24), resulting in 560 patients (92.5%) considered evaluable.
The LH2004 protocol enrolled 1300 patients: 101 were excluded from the analysis because of previous therapy (6), age >18 years (15), wrong diagnosis (5), protocol error (3), immunodeficiency (2), lack of information on treatment response (48), or no data beyond registration (22), resulting in 1199 patients (92.2%) considered evaluable.
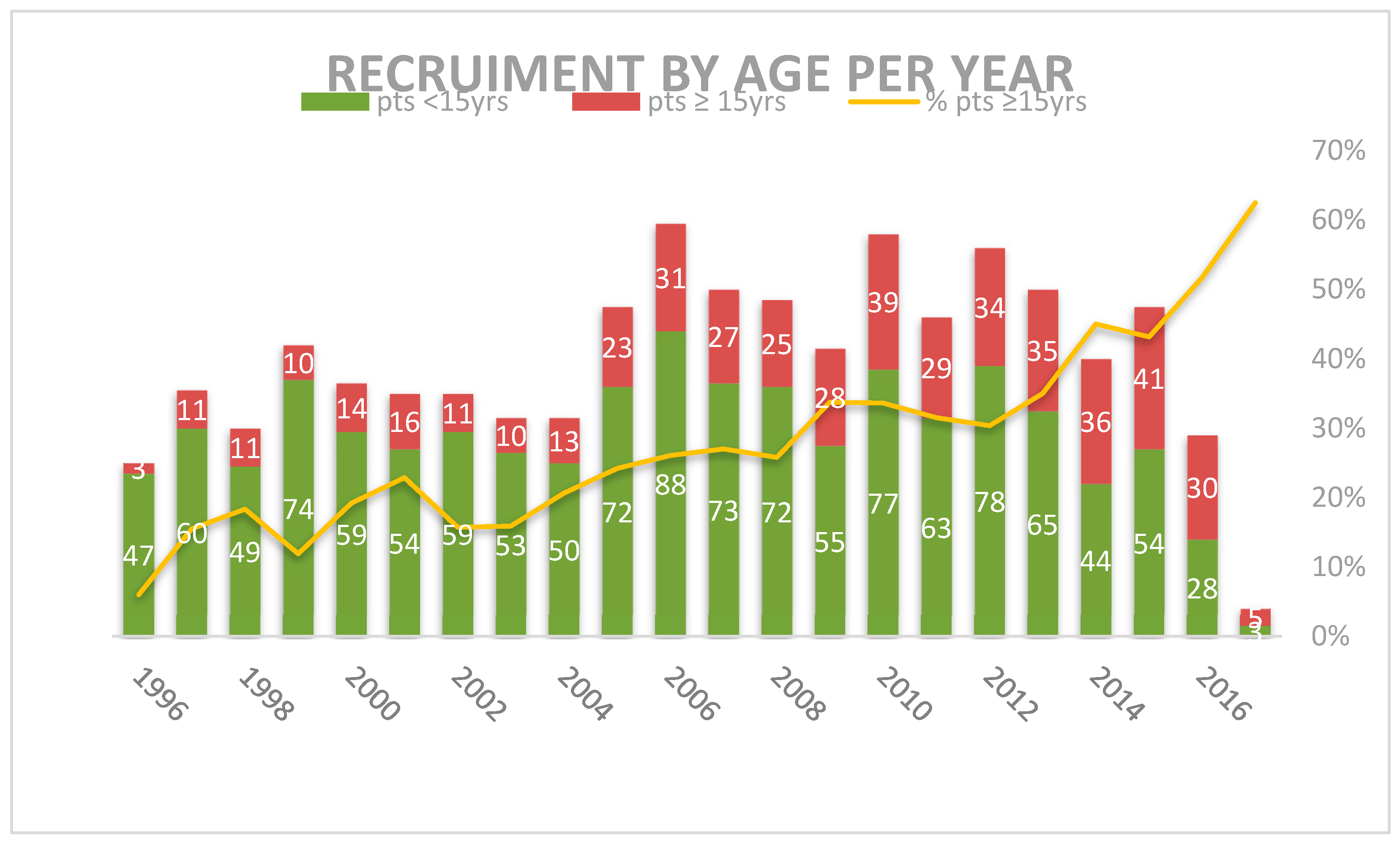
Over the 22 years between 1996 and 2017, the 1759 patients <18 years were enrolled and were considered evaluable for the study. There were 482 adolescents aged ≥15 years, representing the 27.4% of the total. Their recruitment progressively grew from 11 per year between 1997 and 1999 to 40 per year in 2015, representing 16.1% of MH’96 patients and the 32.9% of LH2004 patients (p = 0.000) (Figure 1 and Table 2).
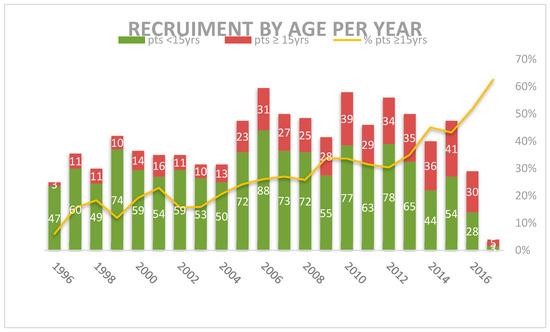
Figure 1.
Distribution of patients by group of age per year of recruitment.

Table 2.
Demographic and clinical characteristics of patients analyzed and comparison between the two protocols.
3.1. Patients Characteristics
The demographic and clinical characteristics of analyzed patients are reported in Table 2.
The relative frequency of patient characteristics in the two protocols (Table 2) and in the age categories (Table 3) were compared.

Table 3.
Comparison of demographic and clinical characteristics of patients between the two classes of age.
The characteristics of the 482 adolescents analyzed were fairly uniform throughout the two decades, except for stage distribution and the more common extra-nodal disease in the recent study (Table 2). However, the adolescents’ characteristics differed significantly from the children’s according to sex, histology, and presence of symptoms in both protocols (Table 1). In the LH2004 protocol, the male-to-female ratio was inverted in children (M 58.2%, F 41.8%, M/F: 1.4) with respect to adolescents (M 48.2%, F 51.8%, M/F: 0.93). In both protocols nodular sclerosis was more frequent in adolescents than in children (80% and 64.7% vs. 80.9% and 73%), while both mixed cellularity (13.3% and 22.6% vs. 6.4% and 9.8%) and nodular lymphocyte predominance were less frequent (3.3% and 10.9% vs. 4.0% and 9.0%). An important decrease in mixed cellularity frequency in children through the two protocols (MH’96: 22.6%, LH2004: 9.8%) was observed, with a relative increase in nodular sclerosis (MH’96: 64.7%, LH2004: 73.0%). Adolescent patients presented with B-symptoms more frequently than children. Both children and adolescents in the LH2004 protocol were included in higher therapeutic groups than in MH’96.
3.2. Survival
The probabilities of OS, FFP, and EFS at 5 and 10 years registered in children and adolescents treated with MH’96 and LH 2004 protocols are reported in Figure 2 and Figure 3. Median observation time was 11.0 years (4 months–17.7 years) and 7.3 years (3 months–15.8 years) respectively.
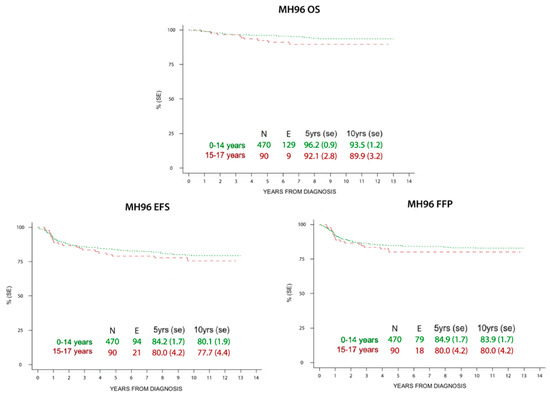
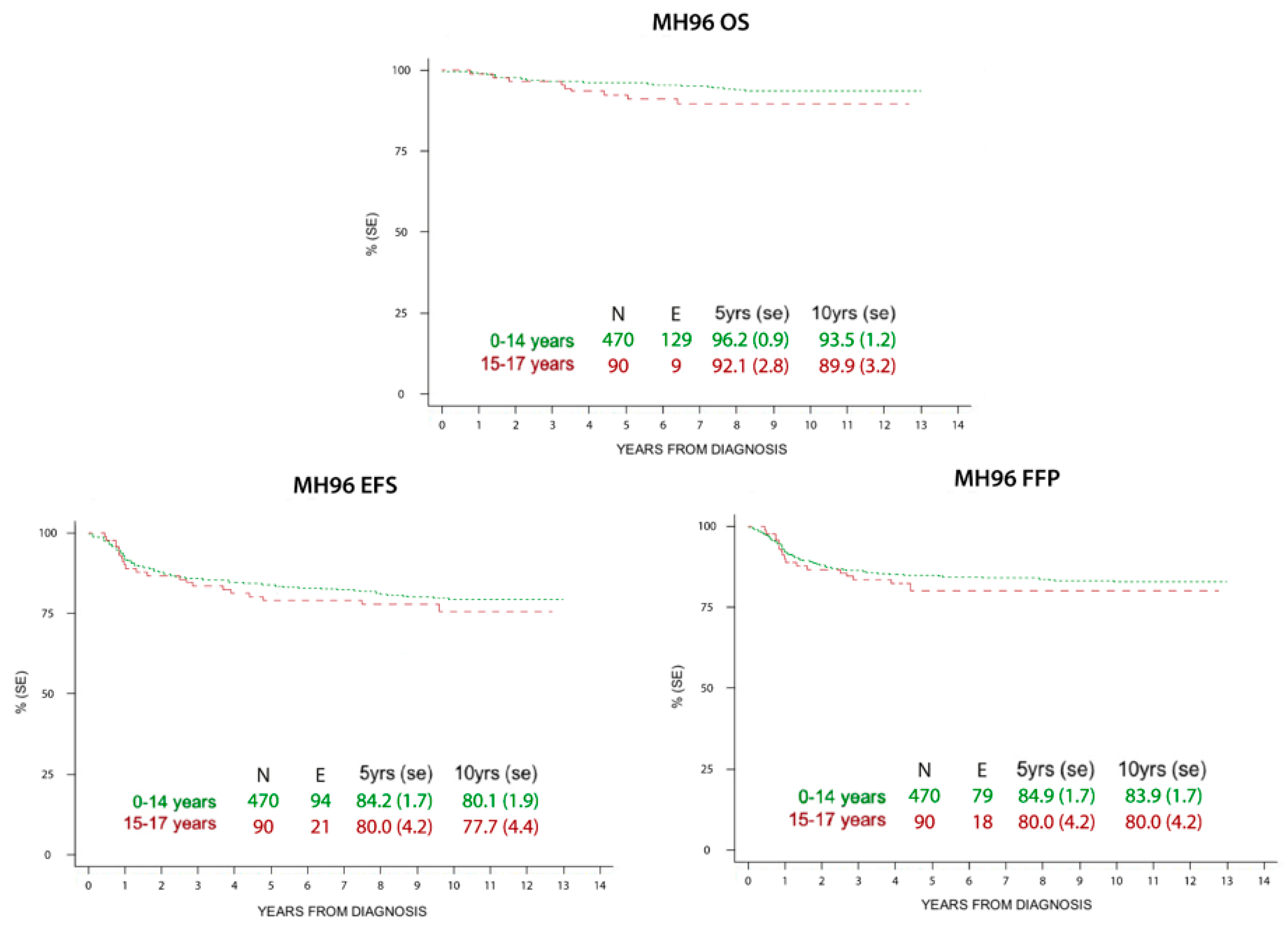
Figure 2.
Overall survival (OS), event free survival (EFS), and freedom from progression (FFP) at 5 and 10 years in children and adolescents treated with MH’96 protocol.
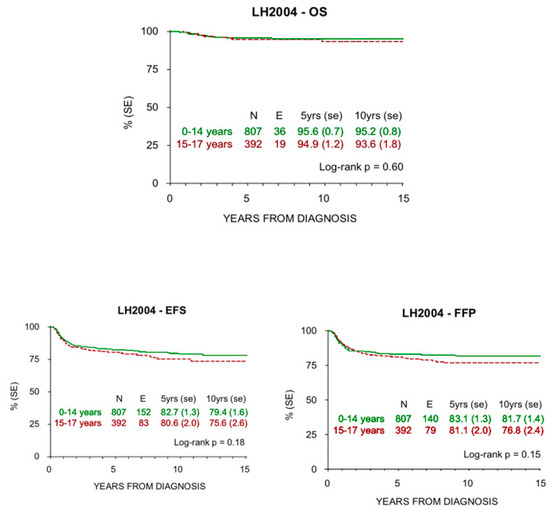
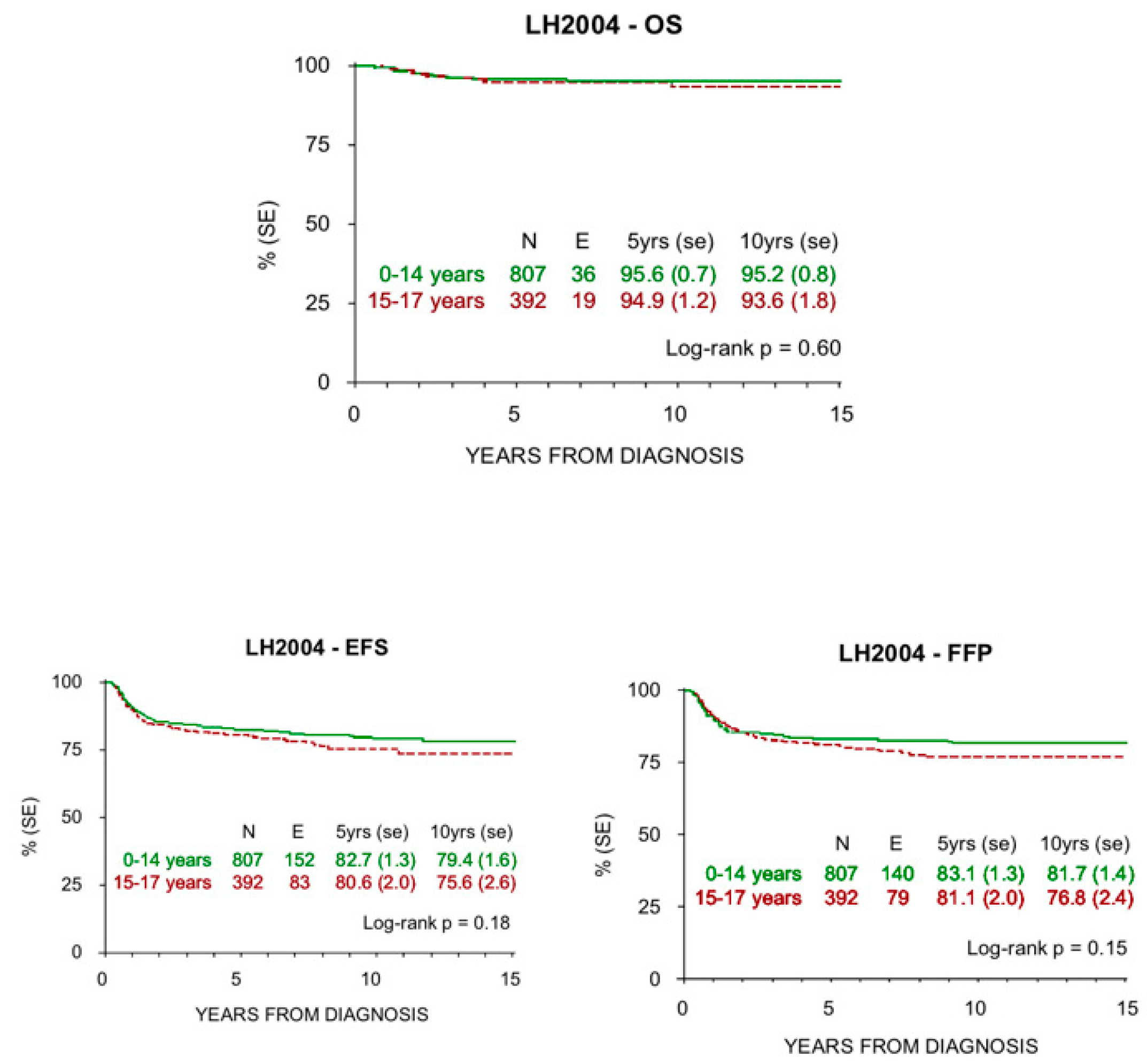
Figure 3.
OS, EFS, and FFP at 5 and 10 years in children and adolescents treated with LH2004 protocol.
There was no statistically significant difference in the outcomes of both groups.
The historical comparison between the two protocols showed an improvement of OS with the most recent one (LH2004), especially in low risk patients and in the adolescent group (Table 4). At the same time, EFS and FFP rates were superimposable in both the studies, but with smaller RT volumes and lower doses in the LH2004 protocol.

Table 4.
Overall survival in children and adolescent patients related to group of treatment and era protocol.
Patients with nLP HL presented a 10-year OS = 100% in both protocols [10] (Figures S1 and S2). In any case, the histotype was not associated with any favorable impact on EFS, neither in the MH’96 protocol [10] nor in the LH2004 (Figures S3 and S4), and there was no statistical difference between children and adolescents with nPL.
Second malignant neoplasms were the only long-term side effects considered in this analysis. In the MH’96 protocol 18 SMNs were solid tumors (thyroid carcinoma: 14, breast cancer: 1, lung cancer: 1, leiomyosarcoma: 1, osteosarcoma: 1), while 5 were hematological malignancies (acute myeloblastic leukemia AML: 2, non-Hodgkin lymphoma NHL: 2, mycosis fungoides: 1). A third malignancy (soft tissue sarcoma) occurred after AML. Eighteen were registered in the children group and 5 in the adolescent group. All but one case of solid tumors occurred within the irradiation field after a median time of 7.9 years (2.3–10.2 years). Hematological malignancies occurred at a median time of 4.2 years (11 months–7.4 years) [10]. In the LH2004 protocol, 13 SMNs were solid tumors (thyroid carcinoma: 10, mucoepidermoid carcinoma: 2, Ewing’s sarcoma: 1) and 5 were primary mediastinal large B-cell lymphomas (PMLBCL). Fourteen were diagnosed in the children group and 4 in the adolescent group. Solid tumors occurred at a median time of 6.8 years (4.3–11.6 years). The PMLBCL occurred at a median time of 12.6 months (4–22 months)
4. Discussion
In Italy, before 1996 the upper age limit for inclusion in a pediatric HL protocol was 15 years. This was extended up to 18 years for the MH’96 and LH2004 protocols. This study, therefore, includes all adolescents treated in an Italian pediatric protocol from 1996 to 2016, when Italy entered the international EuroNet-PHL-C2 protocol. Comparison with other studies which include older patients up to 21–25 years old might be limited, but those were eliminated by our analysis because of the eligibility, even though treated in some centers. Over the 20 years, adolescent enrollment in AIEOP centers progressively grew from 16.1% of MH’96 patients to 32.9% of LH2004 patients (p = 0.000). This reflected a similar increase reported in AYAs with lymphoma and other cancers treated with AIEOP protocols in Italy in the same time span [14,15,16]. This trend was also seen in North America, with 16–21 AYAs: from 28% in POG-9426 between 1996 and 2005, to 38% in COG-AHOD0431 between 2006 and 2009 [17]. Moreover, a recent study from the Dutch pediatric centers recorded an even greater increase in registration of adolescents, from 27% in 2004 to 81% in 2015 [18]. This evidence alone does not suggest an increased incidence of the disease in the overall population, yet it does indicate the progressive referral of adolescents to pediatrics centers.
The characteristics of the 482 adolescents analyzed were uniform throughout the two decades, but differed from those of children in sex, histology, and clinical presentation.
It is well known that the male gender is related to most cancers, and it is strongly associated with each type of lymphoma, including HL. This suggests a role of gender itself in hematologic tumors connected to genetic and immune-related factors, rather than hormonal variations, which better relate to bone tumors and germ-cell tumors (GCTs) [19]. Our patient population is predominantly male, except for adolescents in the most recent protocol, where the male-to-female ratio showed an increase in girls with age, similarly to other publications [18,20,21,22,23].
Histologic subtype distributions present modifications according to age: nodular sclerosis was more frequent in adolescents than in children, while mixed cellularity and lymphocyte predominance was less frequent. This trend registered in our experience was also recorded by other recent researches [22,23,24,25,26]. A peculiarity that emerged in our study was the critical decrease in mixed cellularity frequency in children in the second protocol (22.6% vs. 9.8%). Conversely, there was an increase in nodular sclerosis and not otherwise specified (NOS) cHL, as written in the report on HL incidence in Europe in 1978–1997 [21]. A cause for this variation is not very easily identified. The slightly superior mean and median age in the more recent protocol might be a hypothesis. As for the increase in cHL-NOS, we would suggest two possible explanations. The first one could relate to the long span of time considered in this case series, and the subsequent changes in the available immunohistochemical markers and in the general approach to the diagnosis. Secondly, the increasing use of a needle-biopsy approach in recent decades has certainly limited the precise definition of histological subcategories in daily practice in this diagnostic field, as in many others [27].This could be investigated in future, as children have classically been reported with an incidence of mixed cellularity >30% [28]. This histological type has frequently been associated with Epstein–Barr virus [29], although the infection is more typical of adolescents. In addition, it has been connected to different immune statuses in different age groups [30].
B-symptoms were more frequent among adolescents than children [22]. This characteristic also emerged when comparing adolescents with adults [31], which suggests a direct connection with age rather than with disease stage, which was not more advanced in adolescents than in children.
Another peculiarity of our study, which lasted over an extended period, is that in the LH2004 protocol both children and adolescents were less frequently in low-stage disease, in contrast with other reports [18,22,25], while there was no difference between children and adolescents in stage distribution. Extra-nodal involvement increased, but only in adolescents enrolled in the LH2004 protocol. This trend can be explained by the introduction of PET-CT in clinical practice, with its high sensitivity in detecting active disease [23,32], when compared to CT alone and bone marrow biopsy [33]. Therefore, patients in the LH2004 protocol were less frequently placed in GR1. This could mean that patients with a more recent diagnosis underwent better staging and more appropriate treatment. The use of PET-CT for evaluating response to therapy allows for better identification of residual masses after chemotherapy, while neither CT nor MRI can easily distinguish the fibrotic or vital nature of residual tissues [34]. The use of this response-adapted strategy allows for a de-escalation of treatment intensity, with a reduced dose of radiotherapy, even in patients initially placed in higher-risk groups. We calculated that radiotherapy in LH2004 was avoided in 70% of the GR1 patients compared to the 57% in MH’96, (where only those without an initial mediastinal involvement were considered). In LH2004, 77% of GR2 and 67% of GR3 patients received a low-dose (14.4Gy) irradiation, while 71% of GR2 and only 62% of GR3 patients in the MH’96 trial were given the same.
HL survival progressively increased in the last century, from 10% to >85% 5-year survival rate in all ages since the 1960s. In addition, HL is one of the most curable malignancies nowadays [35]. Pediatric patients have shown highly favorable prognoses in recent reports, which is confirmed in our study for both children and adolescents. In particular, the older patients presented an increased survival rate in the LH2004 protocol (94.9% at 5 years and 93.6% at 10 years) compared to the MH’96 protocol (92.1% at 5 years and 89.9% at 10 years), whereas there was not this difference among the children. This improvement is more evident in low risk patients, where radiation therapy was spared, but is also present in the other therapeutic groups where doses and radiation fields were reduced. These results may reflect better treatment group stratification and tailored therapy due to the introduction of PET-CT.
AYAs gained priority attention following evidence of worse survival in comparison with both children and adults. Adult protocols were utilized in many international studies with different results. Adolescents aged 15–18 treated in the United Kingdom between 1969 and 1998 years showed OS rates lower than those of young adults, aged 20–25 [36,37]. It is not very easy to make a comparison with these studies, because of the old therapeutic regimens applied, together with old staging systems such as laparotomy and splenectomy, which are no longer considered appropriated [38]. In the period 1978–2003, a Greek study showed a progressive improvement in the OS of the 16–23-year-old AYAs treated with three consecutive adult chemotherapeutic regimens [39] and the more recent studies performed between 1979–2013 in Europe and Canada registered 5-year OS rates even higher than 90% [23,40,41]. However, AYA groups have been treated in both pediatric and adult centers without specific criteria, and current research aims to identify the best strategies for this unique group through several studies. Hungarian research between 1990 and 2004 showed less favorable results in 14–18-year-old patients treated with adult protocols, compared to patients aged >18-years and to adolescents treated in pediatric centers [42]. Similarly, a North American study between 1999 and 2006 showed the OS of patients aged 17–21 treated with an adult trial was lower than the OS of young adults (aged 22–44) enrolled in the same trial. It was also inferior to the OS of adolescents aged 17–21, treated with the pediatric COG protocol in 2002–2009 [43] (Table 5).

Table 5.
Outcome of adolescents compared with children and adults in international studies.
Unfortunately, there is no published data regarding adolescents treated with adult protocols in Italy, but recent studies conducted elsewhere in Europe showed no significant differences between the two approaches, even if a widespread use of RT and anthracyclines is generally observed in adult trials [24,44]. Data from the Canadian IMPACT (Initiative to Maximize Progress in Adolescent and Young Adult Cancer) study, comparing the outcome of 15–21 year-old AYAs treated in pediatric or adult centers between 1992 and 2012, showed no differences in OS and EFS. Patients treated in the pediatric centers received more frequent radiation treatment, but a lower dose, than adults and showed a higher rate of SMNs. In adult centers, patients received greater cumulative doses of anthracyclines and a higher rate of cardiovascular events was registered, even though not statistically significant [45]. A similar use of RT with more frequency and lower dose in pediatric centers compared to adults was reported in USA [43] (Table 5).
Studies in Europe [18,20,22,42,46,47,48,49,50] and North America [23,43,45], evaluating AYAs included in pediatric protocols show 5-year OS higher than 90%, with no differences compared to children. On the other hand, EFS was lower in adolescents aged >13 years than in younger patients included in the POG 8725 protocol [51]. Similarly, the 13–18-year-old patients registered in the GPOH-HD-2002 protocol showed lower EFS than children [48]. The same disadvantage was observed in adolescents treated in northern Tunisia, who showed a lower EFS than young adults [52]. However, our study, carried out in pediatric oncology centers, showed no significant differences between the two age groups in OS, EFS, and FFP rates, as reported in other studies [18,20,23,46,47,48,50] (Table 5).
Given the very favorable survival rates achieved in the last decade in AYAs affected by HL, future studies should focus not only on treatment efficacy but also on identifying the best approaches, in terms of a better quality of life during therapy and reduced long-term negative side effects [53,54,55,56]. The choice of OS as the only parameter defining outcome excludes relapses or long-term harmful effects that profoundly affect the quality of life [57]. One useful end-point could be EFS, which includes second tumors, frequently registered in AYAs affected by HL [58,59].
There were fewer SMNs registered in the LH2004 than in MH’96. Even though the median observation time in LH2004 was 4 years shorter, SMNs occurrence had already reached the median time of the MH’96 protocol. The reduction of radiation treatment in terms of doses and fields could be responsible for this improvement, but it is well known that a plateau for solid tumors is not reached even at 30 years from treatment [60,61,62]. Very bizarre is the occurrence of 5 cases of PMLBCLs after a confirmed diagnosis of HL, even if secondary NHL are rising as SMNs, which were markedly higher in patients treated for HL [63] and the most frequent subtype of secondary NHL is diffuse large B-cell lymphoma (DLBCL) which has been reported in 78% of cases [64].
A different relapse time of patients younger than 15 years has emerged in our study. In LH2004, children developed relapses after a mean time of 17.9 months, which is earlier than children in MH’96 (22.3 months). They also showed an only slightly shorter median time of relapse. The use of more sensible imaging techniques in the recent protocol, like CT or PET-CT, might justify an earlier detection of relapses, but it does not happen in adolescents, whose relapse interval was superimposable. Another hypothesis of the cause of the earlier relapses could be the change of the disease in children, as the different distribution of the histologic subtypes would suggest, but it should be confirmed in the future studies with large patient populations recruited over a long period.
5. Conclusions
In Italy, as in other countries, the enrollment of adolescents in pediatric protocols increased along with referral to pediatric centers during the last decade. Specific training for the staff, increased attention for late effects like fertility impairment and secondary cancers, and the creation of specific “Teen areas”, (which already exist in some centers) could improve the quality of treatment of this particular group of patients. In our experience, adopting pediatric protocols did not compromise the adolescents’ outcome, as other international groups have demonstrated. Extending the pediatric oncologists’ philosophy of treatment, which entails reducing the amount of therapy and modeling strategy wherever possible to avoid long-term side effects, to adolescents may be the main road to having a population of long-term survivors with a good quality of life. Remarkable aspects of our study are the reduction of both the mixed cellularity variant and the remission period in children, as well as the reduction of low stages in both categories of age. It may be argued that these phenomena could be due to differences in the disease over time or to modification of the staging system and histologic analysis.
6. Strengths and Limitations
This study offers a complete overview of the HL adolescent patients treated with a national pediatric protocol in Italy, as the protocols include all adolescents aged 15–18 years old. The greatest limitation of this study is the unavailability of data to compare adolescents treated with adult protocols.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/6/1620/s1: Figure S1: Overall Survival: comparison between nodular Lymphocyte Predominance and other histotypes in children. Figure S2: Overall Survival: comparison between nodular Lymphocyte Predominance and other histotypes in adolescents. Figure S3: Event-Free Survival: comparison between nodular Lymphocyte Predominance and other histotypes in children. Figure S4: Event-Free Survival: comparison between nodular Lymphocyte Predominance and other histotypes in adolescents. Table S1: Participating Institutions, Table S2: Ethics Committees.
Author Contributions
Conceptualization, R.B. and G.F.; methodology, R.R.; validation, M.M.; formal analysis, R.R.; investigation, R.B., G.F., R.R.; acquisition and analysis of data, R.B., M.P. (Marta Pillon), A.S., A.G., S.B. (Salvatore Buffardi), M.B., L.V., M.Z., P.M., M.P. (Massimo Provenzi), P.F., F.R., S.D., E.F., S.B. (Sayla Bernasconi), R.D.S., T.C., F.P., I.D., R.M., F.V., A.S., S.C., K.P., M.C., P.B., D.S., R.P., D.G., A.C., M.M, E.S.G.D.A, E.S; data curation, R.B., G.F., R.R., M.M., writing—original draft preparation R.B., G.F.; writing—review and editing, R.B., G.F., M.M., M.P. (Marta Pillon), A.S., A.G., E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by “Federide onlus for the research on HL in adolescents”.
Acknowledgments
We acknowledge the Italian Association “Federide onlus for the research on HL in adolescents” for the economic support in developing the data banking. We recognize Eleonora Volta for her contribution as a data manager and Joanne Nora Maloney for her linguistic revision. We acknowledge all participating institutions, all pediatric-oncologists, and nurses and others who help in the treatment of our Hodgkin lymphoma patients, but above all thank patients and their families.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Birch, J.M.; Pang, D.; Alston, R.D.; Rowan, S.; Geraci, M.; Moran, A.; Eden, T.O.B. Survival from cancer in teenagers and young adults in England, 1979–2003. Br. J. Cancer 2008, 99, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Crombie, J.L.; LaCasce, A.S. Current considerations in AYA Hodgkin lymphoma. Br. J. Haematol. 2019, 184, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.; O’Leary, M.; Barr, R. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000; NIH Pub. No. 06-5767; National Cancer Institute: Bethesda, MD, USA, 2006.
- Bleyer, A.; Budd, T.; Montello, M. Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer 2006, 107, 1645–1655. [Google Scholar] [CrossRef]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Taddeo, D.; Egedy, M.; Frappier, J.-Y. Adherence to treatment in adolescents. Paediatr. Child Health 2008, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mobley, E.M.; Charlton, M.E.; Ward, M.M.; Lynch, C.F. Nonmetropolitan residence and other factors affecting clinical trial enrollment for adolescents and young adults with cancer in a US population-based study. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- AIRTUM Working Group; CCM; AIEOP Working Group. Italian cancer figures, report 2012: Cancer in children and adolescents. Epidemiol. Prev. 2013, 37, 1–225. [Google Scholar]
- Stiller, C.A. International patterns of cancer incidence in adolescents. Cancer Treat. Rev. 2007, 33, 631–645. [Google Scholar] [CrossRef]
- Burnelli, R.; Rinieri, S.; Rondelli, R.; Todesco, A.; Bianchi, M.; Garaventa, A.; Zecca, M.; Indolfi, P.; Conter, V.; Santoro, N.; et al. Long-term results of the AIEOP MH’96 childhood Hodgkin’s lymphoma trial and focus on significance of response to chemotherapy and its implication in low risk patients to avoid radiotherapy. Leuk. Lymphoma 2018, 1–10. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley & Sons, Inc.: New York, NY, USA, 1980; 321p, ISBN 0-471-05519-0. [Google Scholar] [CrossRef]
- Ferrari, A.; Dama, E.; Pession, A.; Rondelli, R.; Pascucci, C.; Locatelli, F.; Ferrari, S.; Mascarin, M.; Merletti, F.; Masera, G.; et al. Adolescents with cancer in Italy: Entry into the national cooperative paediatric oncology group AIEOP trials. Eur. J. Cancer 2009, 45, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Rondelli, R.; Pession, A.; Mascarin, M.; Buzzoni, C.; Mosso, M.L.; Maule, M.; Barisone, E.; Bertolotti, M.; Clerici, C.A.; et al. Adolescents with Cancer in Italy: Improving Access to National Cooperative Pediatric Oncology Group (AIEOP) Centers. Pediatr. Blood Cancer 2016, 63, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Quarello, P.; Mascarin, M.; Milano, G.M.; Tornesello, A.; Bertolotti, M.; Spinelli, M.; Ballotta, P.; Read Borghi, M.; Maule, M.; et al. Evolving Services for Adolescents with Cancer in Italy: Access to Pediatric Oncology Centers and Dedicated Projects. J. Adolesc. Young Adult Oncol. 2019. [Google Scholar] [CrossRef]
- Pieters, R.S.; Wagner, H.; Baker, S.; Morano, K.; Ulin, K.; Cicchetti, M.G.; Bishop-Jodoin, M.; FitzGerald, T.J. The impact of protocol assignment for older adolescents with hodgkin lymphoma. Front. Oncol. 2014, 4, 317. [Google Scholar] [CrossRef]
- Reedijk, A.M.J.; Zijtregtop, E.A.M.; Coebergh, J.W.W.; Meyer-Wentrup, F.A.G.; Hebeda, K.M.; Zwaan, C.M.; Janssens, G.O.R.; Pieters, R.; Plattel, W.J.; Dinmohamed, A.G.; et al. Improved survival for adolescents and young adults with Hodgkin lymphoma and continued high survival for children in the Netherlands: A population-based study during 1990-2015. Br. J. Haematol. 2020. [Google Scholar] [CrossRef]
- Williams, L.A.; Richardson, M.; Marcotte, E.L.; Poynter, J.N.; Spector, L.G. Sex ratio among childhood cancers by single year of age. Pediatr. Blood Cancer 2019, e27620. [Google Scholar] [CrossRef]
- Landman-Parker, J.; Pacquement, H.; Leblanc, T.; Habrand, J.L.; Terrier-Lacombe, M.J.; Bertrand, Y.; Perel, Y.; Robert, A.; Coze, C.; Thuret, I.; et al. Localized Childhood Hodgkin’s Disease: Response-Adapted Chemotherapy with Etoposide, Bleomycin, Vinblastine, and Prednisone Before Low-Dose Radiation Therapy—Results of the French Society of Pediatric Oncology Study MDH90. J. Clin. Oncol. 2000, 18, 1500–1507. [Google Scholar] [CrossRef]
- Clavel, J.; Steliarova-Foucher, E.; Berger, C.; Danon, S.; Valerianova, Z. Hodgkin’s disease incidence and survival in European children and adolescents (1978-1997): Report from the Automated Cancer Information System project. Eur. J. Cancer Oxf. Engl. 1990 2006, 42, 2037–2049. [Google Scholar] [CrossRef]
- Englund, A.; Glimelius, I.; Rostgaard, K.; Smedby, K.E.; Eloranta, S.; Molin, D.; Kuusk, T.; de Brown, P.N.; Kamper, P.; Hjalgrim, H.; et al. Hodgkin lymphoma in children, adolescents and young adults—A comparative study of clinical presentation and treatment outcome. Acta Oncol. Stockh. Swed. 2017, 1–7. [Google Scholar] [CrossRef]
- Fernández, K.S.; Schwartz, C.L.; Chen, L.; Constine, L.S.; Chauvenet, A.; de Alarcón, P.A. Outcome of Adolescents and Young Adults Compared to Children with Hodgkin Lymphoma Treated with Response-Based Chemotherapy on Pediatric Protocols: A Children’s Oncology Group Report. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Gragera, R.; Solans, M.; Galceran, J.; Fernández-Delgado, R.; Fernández-Teijeiro, A.; Mateos, A.; Quirós-Garcia, J.R.; Fuster-Camarena, N.; De Castro, V.; Sánchez, M.J.; et al. Childhood and adolescent lymphoma in Spain: Incidence and survival trends over 20 years. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2018. [Google Scholar] [CrossRef]
- Bigenwald, C.; Galimard, J.-E.; Quero, L.; Cabannes-Hamy, A.; Thieblemont, C.; Boissel, N.; Brice, P. Hodgkin lymphoma in adolescent and young adults: Insights from an adult tertiary single-center cohort of 349 patients. Oncotarget 2017, 8, 80073–80082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pettit, T.; Sue, L.; Waugh, V.; Ballantine, K.; Gardner, K.; Bremer, L.; Pemberton, L.; Allison, L.; Adams, S.; Chou, E.; et al. An Age Stratified Analysis of the Access to Care Continuum Across Three Tumor Groups: Are There Delays for AYA? J. Adolesc. Young Adult Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.L.; Clarke, C.A.; Keegan, T.H.M.; Chang, E.T.; Weisenburger, D.D. Time Trends in Rates of Hodgkin Lymphoma Histologic Subtypes: True Incidence Changes or Evolving Diagnostic Practice? Cancer Epidemiol. Prev. Biomark. 2015, 24, 1474–1488. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.L. The management of Hodgkin disease in the young child. Curr. Opin. Pediatr. 2003, 15, 10. [Google Scholar] [CrossRef]
- Young, L.S.; Murray, P.G. Epstein-Barr virus and oncogenesis: From latent genes to tumours. Oncogene 2003, 22, 5108–5121. [Google Scholar] [CrossRef]
- Jessica, H.; Waxman, I.M.; Kelly, K.M.; Erin, M.; Cairo, M.S. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: State of the science. Br. J. Haematol. 2008, 144, 24–40. [Google Scholar] [CrossRef]
- Blum, K.A.; Keller, F.G.; Castellino, S.; Phan, A.; Flowers, C.R. Incidence and outcomes of lymphoid malignancies in adolescent and young adult patients in the United States. Br. J. Haematol. 2018, 183, 385–399. [Google Scholar] [CrossRef]
- Zaucha, J.M.; Chauvie, S.; Zaucha, R.; Biggii, A.; Gallamini, A. The role of PET/CT in the modern treatment of Hodgkin lymphoma. Cancer Treat. Rev. 2019, 77, 44–56. [Google Scholar] [CrossRef]
- Cistaro, A.; Cassalia, L.; Ferrara, C.; Quartuccio, N.; Evangelista, L.; Bianchi, M.; Fagioli, F.; Bisi, G.; Baldari, S.; Zanella, A.; et al. Italian Multicenter Study on Accuracy of 18F-FDG PET/CT in Assessing Bone Marrow Involvement in Pediatric Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18, e267–e273. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Mascarin, M.; Piccardo, A.; Castello, A.; Elia, C.; Guerra, L.; Borsatti, E.; Sala, A.; Todesco, A.; Zucchetta, P.; et al. FDG PET in response evaluation of bulky masses in paediatric Hodgkin’s lymphoma (HL) patients enrolled in the Italian AIEOP-LH2004 trial. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Satish, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef]
- Yung, L.; Smith, P.; Hancock, B.W.; Hoskin, P.; Gilson, D.; Vernon, C.; Linch, D.C. Long Term Outcome in Adolescents with Hodgkin’s Lymphoma: Poor Results using Regimens Designed for Adults. Leuk. Lymphoma 2004, 45, 1579–1585. [Google Scholar] [CrossRef]
- Herbertson, R.A.; Evans, L.S.; Hutchinson, J.; Horsman, J.; Hancock, B.W. Poor outcome in adolescents with high-risk Hodgkin lymphoma. Int. J. Oncol. 2008, 33, 145–151. [Google Scholar] [CrossRef]
- Rodriguez, L.; Research Fellow, Division of Haematology/Oncology, Department of Paediatrics, SickKids Hospital and University of Toronto; Punnett, A.; Fellowship Program Training Director, Division of Haematology/Oncology, Department of Paediatrics, SickKids Hospital and University of Toronto. Canada Hodgkin’s Lymphoma in Adolescents and Young Adults. Oncol. Hematol. Rev. US 2012, 8, 116. [Google Scholar] [CrossRef]
- Koumarianou, A.; Xiros, N.; Papageorgiou, E.; Pectasides, D.; Economopoulos, T. Survival improvement of young patients, aged 16-23, with Hodgkin lymphoma (HL) during the last three decades. Anticancer Res. 2007, 27, 1191–1197. [Google Scholar]
- Eichenauer, D.A.; Bredenfeld, H.; Haverkamp, H.; Müller, H.; Franklin, J.; Fuchs, M.; Borchmann, P.; Müller-Hermelink, H.-K.; Eich, H.T.; Müller, R.-P.; et al. Hodgkin’s lymphoma in adolescents treated with adult protocols: A report from the German Hodgkin study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 6079–6085. [Google Scholar] [CrossRef]
- Foltz, L.M.; Song, K.W.; Connors, J.M. Hodgkin’s lymphoma in adolescents. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 2520–2526. [Google Scholar] [CrossRef]
- Müller, J.; Illés, A.; Molnár, Z.; Rosta, A.; Váróczy, L.; Kovács, G. Adolescent hodgkin lymphoma: Are treatment results more favorable with pediatric than with adult regimens? J. Pediatr. Hematol. Oncol. 2011, 33, e60–e63. [Google Scholar] [CrossRef]
- Henderson, T.O.; Parsons, S.K.; Wroblewski, K.E.; Chen, L.; Hong, F.; Smith, S.M.; McNeer, J.L.; Advani, R.H.; Gascoyne, R.D.; Constine, L.S.; et al. Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: An adult intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) comparative analysis. Cancer Cytopathol. 2018, 124, 136–144. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, T.J.; Bishop-Jodoin, M. Hodgkin Lymphoma: Differences in Treatment Between Europe and the United States/North America: Evolving Trends in Protocol Therapy. Clin. Med. Insights Oncol. 2018, 12, 1179554918754885. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Baxter, N.N.; Hodgson, D.; Punnett, A.; Sutradhar, R.; Pole, J.D.; Nagamuthu, C.; Lau, C.; Nathan, P.C. Treatment patterns and outcomes in adolescents and young adults with Hodgkin lymphoma in pediatric versus adult centers: An IMPACT Cohort Study. Cancer Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P.; Andrieu, J.M. Hodgkin’s disease in childhood and adolescence: Results of chemotherapy-radiotherapy in clinical stages IA-IIB. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1985, 3, 1495–1502. [Google Scholar] [CrossRef]
- Schellong, G.; Pötter, R.; Brämswig, J.; Wagner, W.; Prott, F.J.; Dörffel, W.; Körholz, D.; Mann, G.; Rath, B.; Reiter, A.; et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin’s disease: The German-Austrian multicenter trial DAL-HD-90. The German-Austrian Pediatric Hodgkin’s Disease Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 3736–3744. [Google Scholar] [CrossRef]
- Mauz-Körholz, C.; Hasenclever, D.; Dörffel, W.; Ruschke, K.; Pelz, T.; Voigt, A.; Stiefel, M.; Winkler, M.; Vilser, C.; Dieckmann, K.; et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: The GPOH-HD-2002 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3680–3686. [Google Scholar] [CrossRef]
- Raze, T.; Lacour, B.; Cowppli-Bony, A.; Delafosse, P.; Velten, M.; Trétarre, B.; Defossez, G.; Hammas, K.; Woronoff, A.-S.; Ganry, O.; et al. Cancer Among Adolescents and Young Adults Between 2000 and 2016 in France: Incidence and Improved Survival. J. Adolesc. Young Adult Oncol. 2020. [Google Scholar] [CrossRef]
- Dony, A.; Belhabri, A.; Bertrand, Y.; Sebban, C.; Cony-Makhoul, P.; Sobh, M.; Rogasik, M.; Salles, G.; Anglaret, B.; Freycon, C.; et al. Pattern of Care and Outcomes of Adolescent and Young Adults with Lymphoma Treated in the Rhône-Alpes Region. J. Adolesc. Young Adult Oncol. 2019, 8, 684–696. [Google Scholar] [CrossRef]
- Weiner, M.A.; Leventhal, B.; Brecher, M.L.; Marcus, R.B.; Cantor, A.; Gieser, P.W.; Ternberg, J.L.; Behm, F.G.; Wharam, M.D.; Chauvenet, A.R. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients: A Pediatric Oncology Group study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 2769–2779. [Google Scholar] [CrossRef]
- Zawati, I.; Adouni, O.; Finetti, P.; Manai, M.; Manai, M.; Gamoudi, A.; Birnbaum, D.; Bertucci, F.; Mezlini, A. Adolescents and young adults with classical Hodgkin lymphoma in North Tunisia: Insights from an adult single-institutional study. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2020. [Google Scholar] [CrossRef]
- Anderson, C.; Lund, J.L.; Weaver, M.A.; Wood, W.A.; Olshan, A.F.; Nichols, H.B. Noncancer mortality among adolescents and young adults with cancer. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Hahn, E.E.; Wu, Y.-L.; Munoz-Plaza, C.E.; Garcia Delgadillo, J.; Cooper, R.M.; Chao, C.R. Use of recommended posttreatment services for adolescent and young adult survivors of Hodgkin lymphoma. Cancer 2019, 125. [Google Scholar] [CrossRef] [PubMed]
- Cepelova, M.; Kruseova, J.; Luks, A.; Capek, V.; Cepela, P.; Potockova, J.; Kraml, P. Accelerated atherosclerosis, hyperlipoproteinemia and insulin resistance in long-term survivors of Hodgkin lymphoma during childhood and adolescence. Neoplasma 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, I.; Englund, A.; Rostgaard, K.; Smedby, K.E.; Eloranta, S.; de Nully Brown, P.; Johansen, C.; Kamper, P.; Ljungman, G.; Hjalgrim, L.L.; et al. Distribution of hospital care among pediatric and young adult Hodgkin lymphoma survivors-A population-based cohort study from Sweden and Denmark. Cancer Med. 2019. [Google Scholar] [CrossRef]
- Jachimowicz, R.D.; Engert, A. The Challenging Aspects of Managing Adolescents and Young Adults with Hodgkin’s Lymphoma. Acta Haematol. 2014, 132, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Aleman, B.M.P.; van den Belt-Dusebout, A.W.; Klokman, W.J.; Van’t Veer, M.B.; Bartelink, H.; van Leeuwen, F.E. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3431–3439. [Google Scholar] [CrossRef]
- Xavier, A.C.; Epperla, N.; Taub, J.W.; Costa, L.J. Excess mortality among 10-year survivors of classical Hodgkin lymphoma in adolescents and young adults. Am. J. Hematol. 2018, 93, 238–245. [Google Scholar] [CrossRef]
- Reulen, R.C.; Frobisher, C.; Winter, D.L.; Kelly, J.; Lancashire, E.R.; Stiller, C.A.; Pritchard-Jones, K.; Jenkinson, H.C.; Hawkins, M.M.; British Childhood Cancer Survivor Study Steering Group. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 2011, 305, 2311–2319. [Google Scholar] [CrossRef]
- Van Leeuwen, F.E.; Ng, A.K. Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematology 2016, 2016, 323–330. [Google Scholar] [CrossRef]
- O’Brien, M.M.; Donaldson, S.S.; Balise, R.R.; Whittemore, A.S.; Link, M.P. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1232–1239. [Google Scholar] [CrossRef]
- Krishnan, B.; Morgan, G.J. Non-Hodgkin lymphoma secondary to cancer chemotherapy. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2007, 16, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Rueffer, U.; Josting, A.; Franklin, J.; May, M.; Sieber, M.; Breuer, K.; Engert, A.; Diehl, V.; German Hodgkin’s Lymphoma Study Group. Non-Hodgkin’s lymphoma after primary Hodgkin’s disease in the German Hodgkin’s Lymphoma Study Group: Incidence, treatment, and prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.M.; Ries, L.A.G.; Barr, R.D.; Geiger, A.M.; Dahlke, D.V.; Pollock, B.H.; Bleyer, W.A.; National Cancer Institute Next Steps for Adolescent and Young Adult Oncology Epidemiology Working Group. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer 2016, 122, 1009–1016. [Google Scholar] [CrossRef]
- Karim-Kos, H.E.; Hackl, M.; Mann, G.; Urban, C.; Woehrer, A.; Slavc, I.; Ladenstein, R. Trends in incidence, survival and mortality of childhood and adolescent cancer in Austria, 1994–2011. Cancer Epidemiol. 2016, 42, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.; Bowen, D.; Dunwoodie, E.; Feltbower, R.; Johnson, R.; Moran, A.; Stiller, C.; O’Hara, C. Survival patterns in teenagers and young adults with cancer in the United Kingdom: Comparisons with younger and older age groups. Eur. J. Cancer Oxf. Engl. 2015, 51, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).