Real-World Data on Thromboprophylaxis in Active Cancer Patients: Where Are We? Are We Getting There?
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of Patients
2.2. Risk Assissment for CAT
2.3. Stratification of Patients According to the Khorana Score
2.4. Characteristics of Patients with Khorana Score ≤2
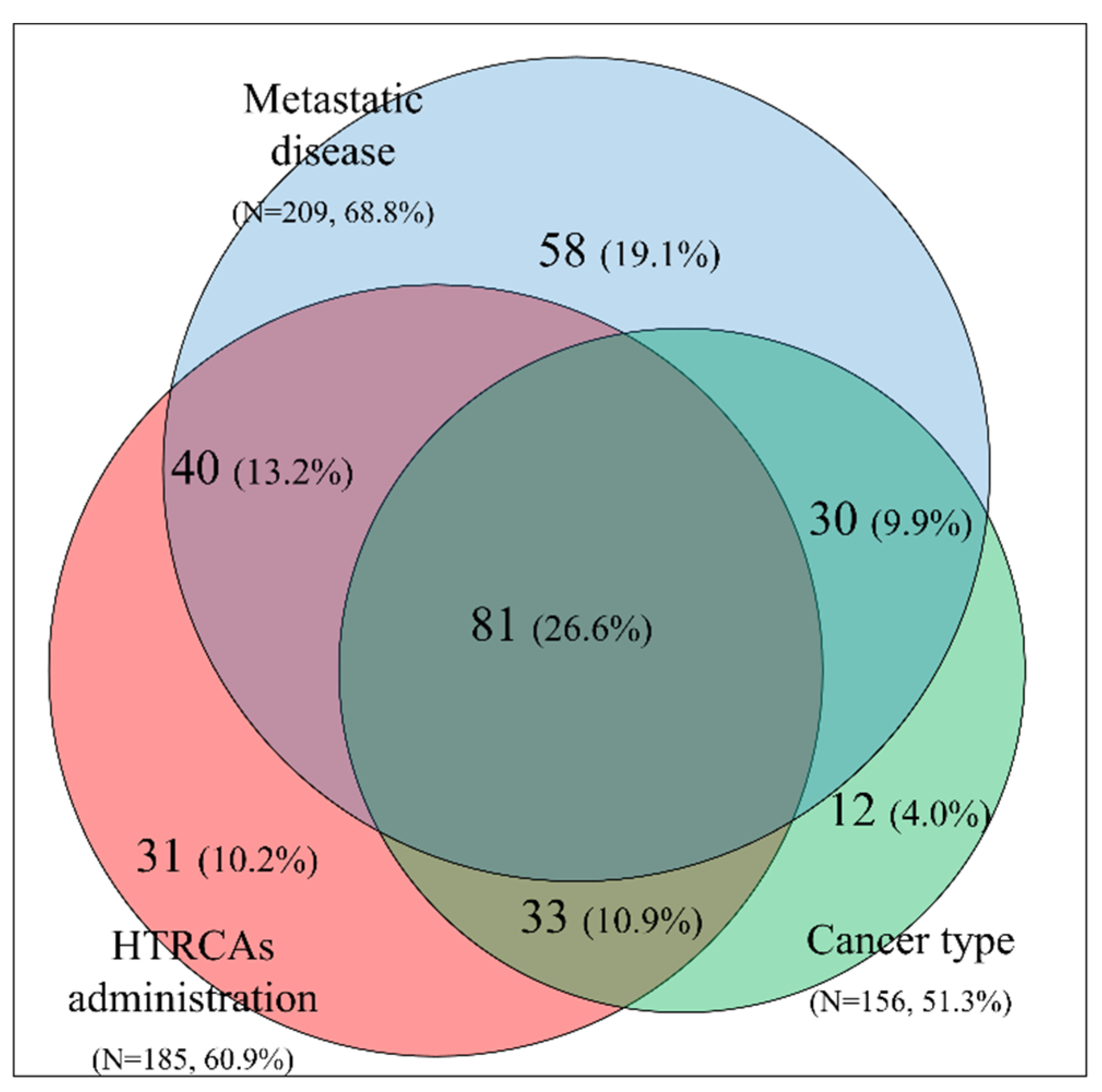
2.5. Clinical Features Influencing the Decision to Administer Thromboprophylaxis for Patients with Khorana Score ≤2
2.6. Thromboprophylaxis Regiments, Doses and Duration
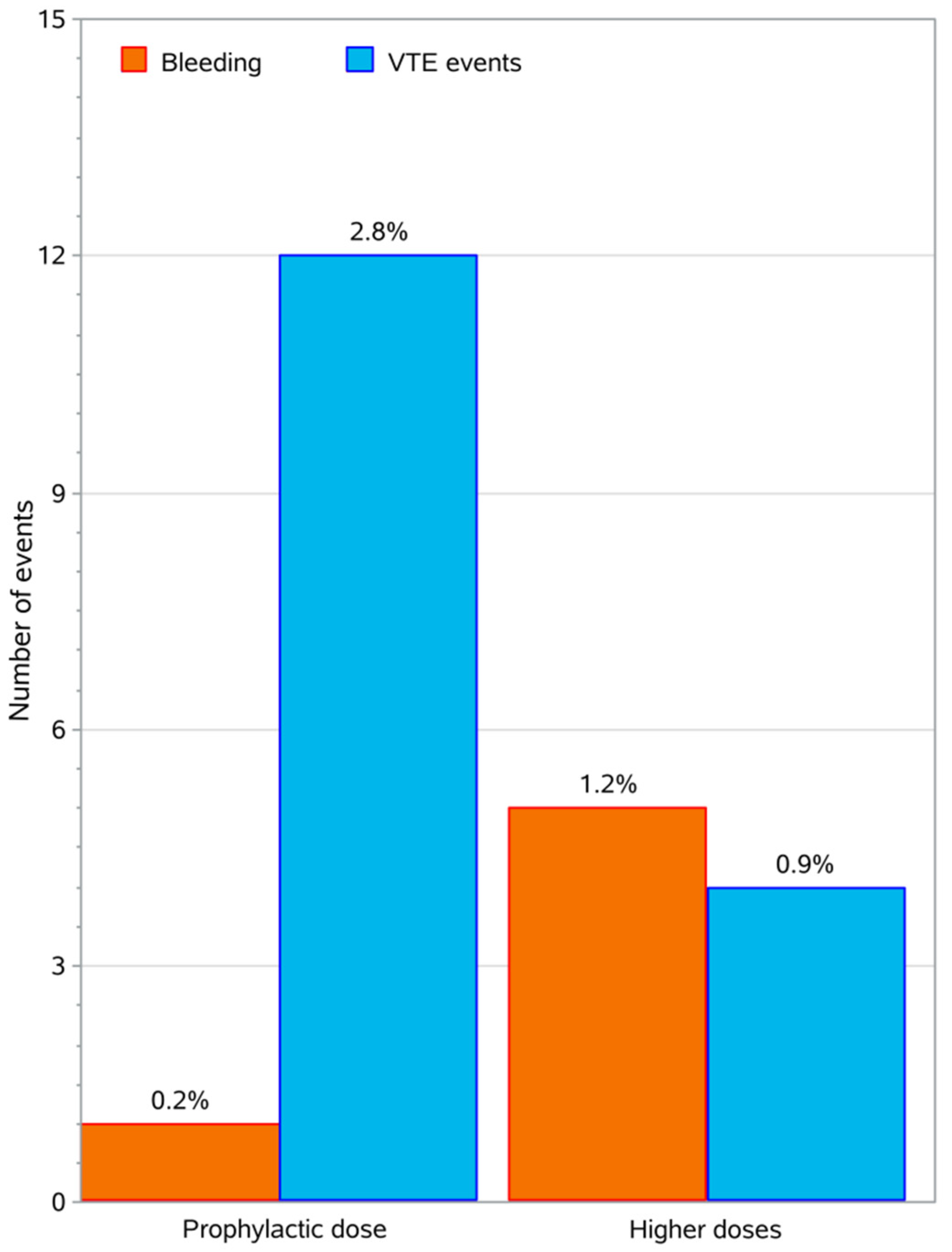
2.7. Thrombotic and Bleeding Events
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Patients Characteristics | Value |
|---|---|
| Number of patients | 304 |
| Male (%) | 50.7% |
| Age (mean ± SD) | 65.5 ± 11.3 |
| Lung | 72 (23.7%) |
| Breast | 37 (12.2%) |
| Colorectal | 30 (9.9%) |
| Ovarian | 25 (8.2%) |
| Pancreas | 24 (7.9%) |
| Stomach | 14 (4.6%) |
| Prostate | 14 (4.6%) |
| Bladder | 12 (4.0%) |
| Testis | 4 (1.3%) |
| Other* | 72 (23.7%) |
| Patients Characteristics | Value |
|---|---|
| BMI (mean ± SD) | 26.1 ± 4.8 |
| BMI ≥ 35 kg/m2 | 3.6% |
| Anemia (Hg <10 g/L) | 12.5% |
| PLT count ≥ 350 × 109/L | 20.6% |
| Leucocytes count > 11 × 109/L | 11.2% |
| Erythropoietin use | 15.6% |
| Metastatic disease | 68.8% |
| HTRCAs | 60.9% |
| Khorana score ≤ 1 | 47.0% |
| Khorana score = 2 | 53.0% |
| Parameter | p-Value | OR | LCL | UCL |
|---|---|---|---|---|
| Gender (Female vs. Male) | 0.5170 | 1.4 | 0.5 | 3.9 |
| Age (<65 years vs higher) | 0.2162 | 0.5 | 0.2 | 1.5 |
| BMI (<35 kg/m2 vs. higher) | 0.0018 | 5.6 | 1.7 | 18.8 |
| Smoking (No vs. Yes) | 0.2014 | 2.1 | 0.7 | 6.6 |
| History of thrombosis (No vs. Yes) | 0.1919 | 3.8 | 0.4 | 33.0 |
| History of trauma (No vs. Yes) | 0.0362 | 4.7 | 0.96 | 23.0 |
| History of surgery (No vs. Yes) | 0.3796 | 1.6 | 0.6 | 4.3 |
| History of comorbidities (No vs. Yes) | 0.7005 | 1.2 | 0.4 | 3.6 |
| Hospitalized Patient (No vs. Yes) | 0.0525 | 3.1 | 0.9 | 10.2 |
| Metastasis (No vs. Yes) | 0.7410 | 0.8 | 0.3 | 2.5 |
| Radiotherapy (No vs. Yes) | 0.3795 | 1.6 | 0.6 | 4.5 |
| HTRCAs (No vs. Yes) | 0.8886 | 1.1 | 0.4 | 3.1 |
| Agents for Erythropoiesis (No vs. Yes) | 0.0682 | Not applicable, one group had zero observations | ||
| Leukocyte Count (pre chemotherapy) (Low vs. High) | 0.4697 | 1.5 | 0.5 | 4.4 |
| Hemoglobin (High vs. Low) | 0.0403 | All thrombotic cases had high hemoglobin | ||
| PLT Count High (pre chemotherapy) | 0.6221 | 0.8 | 0.3 | 2.2 |
| Khorana Score (≤2 vs. ≥3) | 0.7428 | 0.8 | 0.3 | 2.6 |
| Agent for anticoagulation (prophylactic vs. higher dose) | 0.0413 | 0.3 | 0.1 | 1.0 |
| Parameter | p-Value | OR | LCL | UCL |
|---|---|---|---|---|
| Gender (Femalevs. Male) | 0.1544 | 0.2 | 0.1 | 2.0 |
| Age (<65 years vs. higher) | 0.0353 | 7.3 | 0.8 | 62.9 |
| BMI (<35 kg/m2 vs. higher) | 0.2957 | 0.3 | 0.04 | 2.99 |
| Smoking (Novs. Yes) | 0.2332 | 0.3 | 0.03 | 1.39 |
| History of thrombosis (No vs. Yes) | 0.0075 | 0.1 | 0.01 | 0.9 |
| History of trauma (No vs. Yes) | 0.6481 | Not applicable, one group had zero observations | ||
| History of surgery (No vs. Yes) | 0.5461 | 1.7 | 0.3 | 9.3 |
| History of comorbidities (No vs. Yes) | 0.2027 | 0.4 | 0.1 | 1.8 |
| History of renal Insufficiency (No vs. Yes) | 0.1271 | 0.2 | 0.02 | 1.9 |
| History of bleeding (No vs. Yes) | 0.8655 | Not applicable, one group had zero observations | ||
| Hospitalized Patient (No vs. Yes) | 0.3804 | Not applicable, one group had zero observations | ||
| Metastasis (No vs. Yes) | 0.5452 | 0.5 | 0.1 | 4.5 |
| Radiotherapy (No vs. Yes) | 0.5383 | 1.9 | 0.2 | 16.8 |
| HTRCAs (No vs. Yes) | 0.3681 | 2.1 | 0.4 | 10.4 |
| Use of Agents for Erythropoiesis (No vs. Yes) | 0.2699 | Not applicable, one group had zero observations | ||
| Leukocyte Count (pre chemotherapy) (Low vs. High) | 0.0127 | 0.2 | 0.03 | 0.8 |
| Hemoglobin (High vs. Low) | 0.2147 | Not Applicable | ||
| PLT Count High (pre chemotherapy) | 0.1309 | 0.3 | 0.05 | 1.6 |
| Khorana Score (≤2 vs. ≥3) | 0.0028 | 0.1 | 0.01 | 0.7 |
| Agent for anticoagulation (prophylactic vs. higher dose) | 0.0248 | Not applicable, one group had zero observations | ||
References
- Trousseau, A. Phlegmatia Alba Dolens, 2nd ed.; J.-B. Baillière et fils: Paris, France, 1865; Volume 3. [Google Scholar]
- Agnelli, G.; Verso, M. Management of venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2011, 9, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-associated thrombosis: An overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Van Es, N.; Le Gal, G.; Otten, H.M.; Robin, P.; Piccioli, A.; Lecumberri, R.; Jara-Palomares, L.; Religa, P.; Rieu, V.; Rondina, M.; et al. Screening for Occult Cancer in Patients With Unprovoked Venous Thromboembolism: A Systematic Review and Meta-analysis of Individual Patient Data. Ann. Intern. Med. 2017, 167, 410–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, M.; Lazo-Langner, A.; Shivakumar, S.; Tagalakis, V.; Zarychanski, R.; Solymoss, S.; Routhier, N.; Douketis, J.; Danovitch, K.; Lee, A.Y.; et al. Screening for Occult Cancer in Unprovoked Venous Thromboembolism. N. Engl. J. Med. 2015, 373, 697–704. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M.; Russo, L. The mechanisms of cancer-associated thrombosis. Thromb. Res. 2015, 135, S8–S11. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol./Hematol. 2017, 118, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef] [Green Version]
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011, 9, 120–138. [Google Scholar] [CrossRef]
- Lowe, G.D. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 2008, 140, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-induced thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, G.; Ridker, P.M. Is venous thromboembolism a chronic inflammatory disease? Clin. Chem. 2015, 61, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, C.N.; Hilt, Z.T.; Pariser, D.N.; Maurya, P. PAD4 and von Willebrand Factor Link Inflammation and Thrombosis. Circ. Res. 2019, 125, 520–522. [Google Scholar] [CrossRef]
- Chen, J.; Chung, D.W. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018, 132, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Mandala, M.; Reni, M.; Cascinu, S.; Barni, S.; Floriani, I.; Cereda, S.; Berardi, R.; Mosconi, S.; Torri, V.; Labianca, R. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. ESMO 2007, 18, 1660–1665. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [Green Version]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Oppelt, P.; Betbadal, A.; Nayak, L. Approach to chemotherapy-associated thrombosis. Vasc. Med. 2015, 20, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.X.; Lin, F.; Sun, Y.J.; Tang, L.N.; Shen, Z.; Yao, Y. Risk of venous and arterial thromboembolic events in cancer patients treated with gemcitabine: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2013, 76, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.; Falanga, A.; Khorana, A.A. A Validated Risk Score for Venous Thromboembolism Is Predictive of Cancer Progression and Mortality. Oncologist 2016, 21, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ay, C.; Dunkler, D.; Marosi, C.; Chiriac, A.L.; Vormittag, R.; Simanek, R.; Quehenberger, P.; Zielinski, C.; Pabinger, I. Prediction of venous thromboembolism in cancer patients. Blood 2010, 116, 5377–5382. [Google Scholar] [CrossRef]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Buller, H.R.; van Es, N. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- Van Es, N.; Franke, V.F.; Middeldorp, S.; Wilmink, J.W.; Buller, H.R. The Khorana score for the prediction of venous thromboembolism in patients with pancreatic cancer. Thromb. Res. 2017, 150, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Elalamy, I.; Canon, J.L.; Bols, A.; Lybaert, W.; Duck, L.; Jochmans, K.; Bosquée, L.; Peeters, M.; Awada, A.H.; Clement, P.; et al. Thrombo-Embolic Events in Cancer Patients with Impaired Renal Function. J. Blood Disord. Transf. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Maraveyas, A.; Waters, J.; Roy, R.; Fyfe, D.; Propper, D.; Lofts, F.; Sgouros, J.; Gardiner, E.; Wedgwood, K.; Ettelaie, C.; et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur. J. Cancer 2012, 48, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, U.; Opitz, B.; Deutschinoff, G.; Stauch, M.; Reitzig, P.C.; Hahnfeld, S.; Muller, L.; Grunewald, M.; Stieler, J.M.; Sinn, M.; et al. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2028–2034. [Google Scholar] [CrossRef]
- Thein, K.Z.; Yeung, S.J.; Oo, T.H. Primary thromboprophylaxis (PTP) in ambulatory patients with lung cancer receiving chemotherapy: A systematic review and meta-analysis of randomized controlled trials (RCTs). Asia-Pac. J. Clin. Oncol. 2018, 14, 210–216. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Kuderer, N.M.; Carrier, M.; Ortel, T.L.; Wun, T.; Rubens, D.; Hobbs, S.; Iyer, R.; Peterson, D.; et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb. Res. 2017, 151, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; George, D.J.; Kakkar, A.K.; Fisher, W.; Lassen, M.R.; Mismetti, P.; Mouret, P.; Chaudhari, U.; Lawson, F.; Turpie, A.G.; et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N. Engl. J. Med. 2012, 366, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barni, S.; Labianca, R.; Agnelli, G.; Bonizzoni, E.; Verso, M.; Mandala, M.; Brighenti, M.; Petrelli, F.; Bianchini, C.; Perrone, T.; et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: Results of a retrospective analysis of the PROTECHT study. J. Transl. Med. 2011, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochenek, J.; Puskulluoglu, M.; Krzemieniecki, K. The antineoplastic effect of low-molecular-weight heparins-a literature review. Contemp. Oncol. 2013, 17, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Ilich, A.; Simioni, P.; Key, N.S. The relationship between pancreatic cancer and hypercoagulability: A comprehensive review on epidemiological and biological issues. Br. J. Cancer 2019, 121, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Chapman, O.; Connor, C.; Poole, C.; Rose, P.; Kakkar, A.K. Thrombosis and cancer. Nat. Rev. Clin. Oncol. 2012, 9, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Grosskopf, I.; Shaish, A.; Ray, A.; Harats, D.; Kamari, Y. Low molecular weight heparin-induced increase in chylomicron-remnants clearance, is associated with decreased plasma TNF-alpha level and increased hepatic lipase activity. Thromb. Res. 2014, 133, 688–692. [Google Scholar] [CrossRef]
- Etulain, J.; Mena, H.A.; Negrotto, S.; Schattner, M. Stimulation of PAR-1 or PAR-4 promotes similar pattern of VEGF and endostatin release and pro-angiogenic responses mediated by human platelets. Platelets 2015, 26, 799–804. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Connors, J.M. The Role of Direct Oral Anticoagulants in Treatment of Cancer-Associated Thrombosis. Cancers 2018, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Kraaijpoel, N.; Carrier, M. How I treat cancer-associated venous thromboembolism. Blood 2019, 133, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Mansueto, M.F.; Raso, S.; Siragusa, S. Quality of Life in Patients With Cancer Under Prolonged Anticoagulation for High-Risk Deep Vein Thrombosis: A Long-Term Follow-Up. Clin. Appl. Thromb./Hemost. Off. J. Int. Acad. Clin. Appl. Thromb./Hemost. 2020, 26, 1076029620918290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patsopoulos, N.A. A pragmatic view on pragmatic trials. Dialogues Clin. Neurosci. 2011, 13, 217–224. [Google Scholar] [PubMed]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S. Subcommittee on Control of, A. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C. Subcommittee on Control of Anticoagulation of the, S.; Standardization Committee of the International Society on, T.; Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]

| Patients Characteristics | Value |
|---|---|
| Number of patients | 426 |
| Male (%) | 54.6% |
| Age (mean ± SD) | 65.3 ± 11.2 |
| Lung | 107 (25.1%) |
| Pancreas | 59 (13.9%) |
| Breast | 37 (8.7%) |
| Stomach | 36 (8.5%) |
| Ovarian | 33 (7.8%) |
| Colorectal | 31 (7.3%) |
| Bladder | 24 (5.6%) |
| Prostate | 14 (3.3%) |
| Testis | 6 (1.4%) |
| Other* | 79 (18.5%) |
| Patients Characteristics | Value |
|---|---|
| BMI (mean ± SD) | 26.1 ± 5.1 |
| BMI ≥35 kg/m2 | 6.3% |
| Anemia (Hg < 10 g/L) | 20.2% |
| PLT count ≥ 350 × 109/L | 37.1% |
| Leucocytes count > 11 × 109/L | 23.7% |
| Erythropoietin use | 16.7% |
| Metastatic disease | 69.5% |
| HTRCAs* | 67.1% |
| Khorana score ≤ 1 | 43.9% |
| Khorana score = 2 | 27.5% |
| Khorana score ≥ 3 | 28.6% |
| Cancer | Treatment | Patient | Biomarker | |||||
|---|---|---|---|---|---|---|---|---|
| Primary Site | Patients (N, %) | Metastatic (N, %) | HTRCAs (N, %) | Age (≥65, N, %) | Comorbidities (N, %) | PLT Count (≥350 × 109/L, N, %) | Anemia (Hg < 10 g/L, N, %) | Leucocytes (>11 × 109/L, N, %) |
| Lung | 107 (25.1%) | 80 (74.8%) | 70 (65.4%) | 59 (55.1%) | 27 (25.2%) | 48 (44.9%) | 26 (24.3%) | 35 (32.7%) |
| Pancreas | 59 (13.9%) | 43 (72.9%) | 55 (93.2%) | 35 (59.3%) | 16 (27.1%) | 21 (35.6%) | 11 (18.6%) | 13 (22.0%) |
| Breast | 37 (8.7%) | 25 (67.6%) | 13 (35.1%) | 18 (48.7%) | 5 (13.9%) | 4 (10.8%) | 4 (10.8%) | 1 (2.7%) |
| Stomach | 36 (8.5%) | 29 (80.6%) | 31 (86.1%) | 24 (66.7%) | 9 (25%) | 14 (38.9%) | 9 (25%) | 6 (16.7%) |
| Ovarian | 33 (7.8%) | 19 (57.6%) | 25 (75.8%) | 19 (57.6%) | 9 (27.3%) | 12 (36.4%) | 6 (18.2%) | 5 (15.2%) |
| Colorectal | 31 (7.3%) | 23 (74.2%) | 25 (80.7%) | 18 (58.1%) | 8 (25.8%) | 7 (22.6%) | 7 (22.6%) | 4 (12.9%) |
| Bladder | 24 (5.6%) | 17 (70.8%) | 19 (79.2%) | 17 (70.8%) | 8 (33.3%) | 12 (50%) | 5 (20.8%) | 8 (33.3%) |
| Prostate | 14 (3.3%) | 12 (85.7% | 1 (7.1%) | 10 (71.4%) | 3 (21.4%) | 4 (28.6%) | 4 (28.6%) | |
| Testis | 6 (1.4%) | 2 (33.3%) | 6 (100%) | 1 (16.7%) | 1 (16.7%) | 4 (66.7%) | 0 (0%) | 2 (33.3%) |
| Other | 79 (18.5%) | 47 (59.5%) | 41 (51.9%) | 38 (48.1%) | 29 (36.7%) | 32 (40.5%) | 14 (17.7%) | 27 (34.2%) |
| Risk Group | Factor |
|---|---|
| Cancer Related | Cancer type has a very high risk for CAT according to the Khorana score |
| Cancer type has a high risk for CAT according to the Khorana score | |
| Cancer type is not included in the Khorana score | |
| Metastatic disease | |
| Treatment Related | Use of HTRCAs during treatment |
| Cancer surgery was performed | |
| Hospitalization of patient | |
| Use of erythropoietin | |
| Use of hormonotherapy (tamoxifen) | |
| Patient Related | BMI ≥35 kg/m2 |
| Age >65 years | |
| Smoking | |
| Co-morbidities | |
| History of trauma or heart failure or thrombosis | |
| Biomarkers | Pre-chemotherapy platelets count ≥350 × 10⁹/L |
| Hemoglobin level <10 g/dL or use of erythropoietin | |
| Pre-chemotherapy leukocytes count >11 × 10⁹/L |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoukalas, N.; Papakotoulas, P.; Christopoulou, A.; Ardavanis, A.; Koumakis, G.; Papandreou, C.; Papatsimpas, G.; Papakostas, P.; Samelis, G.; Andreadis, C.; et al. Real-World Data on Thromboprophylaxis in Active Cancer Patients: Where Are We? Are We Getting There? Cancers 2020, 12, 1907. https://doi.org/10.3390/cancers12071907
Tsoukalas N, Papakotoulas P, Christopoulou A, Ardavanis A, Koumakis G, Papandreou C, Papatsimpas G, Papakostas P, Samelis G, Andreadis C, et al. Real-World Data on Thromboprophylaxis in Active Cancer Patients: Where Are We? Are We Getting There? Cancers. 2020; 12(7):1907. https://doi.org/10.3390/cancers12071907
Chicago/Turabian StyleTsoukalas, Nikolaos, Pavlos Papakotoulas, Athina Christopoulou, Alexandros Ardavanis, Georgios Koumakis, Christos Papandreou, Georgios Papatsimpas, Pavlos Papakostas, Georgios Samelis, Charalambos Andreadis, and et al. 2020. "Real-World Data on Thromboprophylaxis in Active Cancer Patients: Where Are We? Are We Getting There?" Cancers 12, no. 7: 1907. https://doi.org/10.3390/cancers12071907






