Multiple Myeloma Associated Bone Disease
Abstract
:1. Introduction
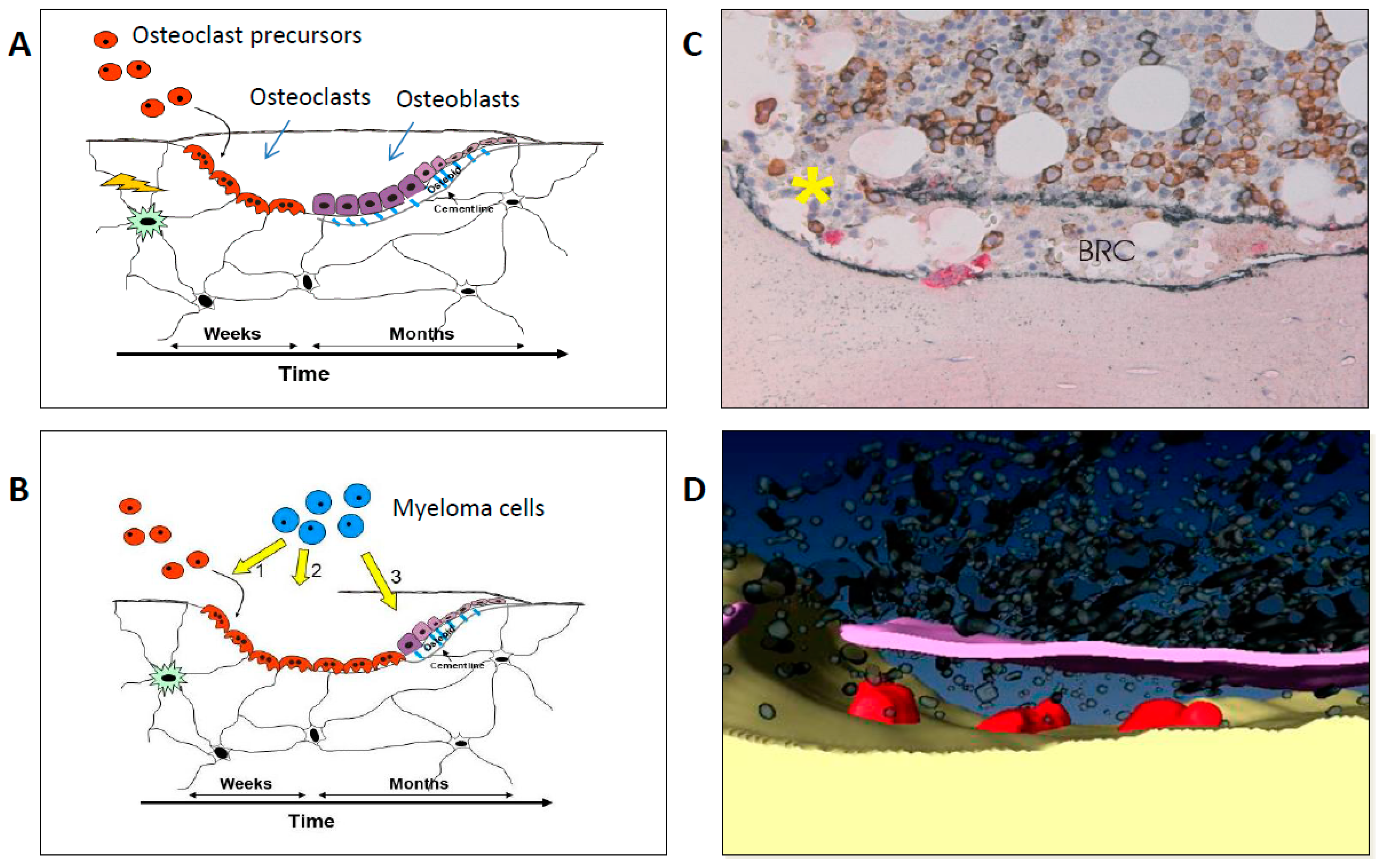
2. Pathophysiology
3. Imaging
3.1. Definition of Myeloma Associated Bone Disease
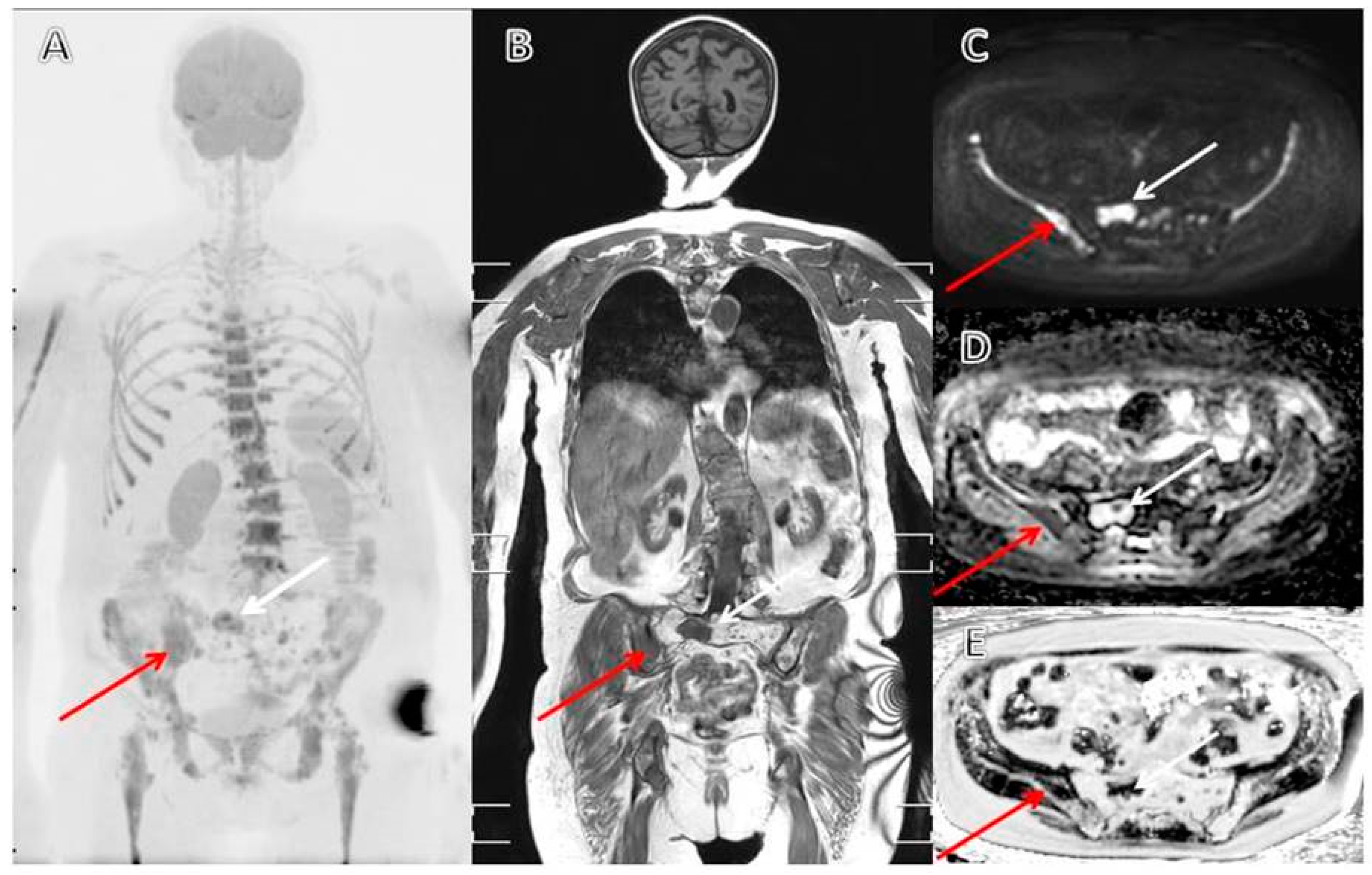
3.2. From Conventional Skeletal Survey to Whole-Body CT
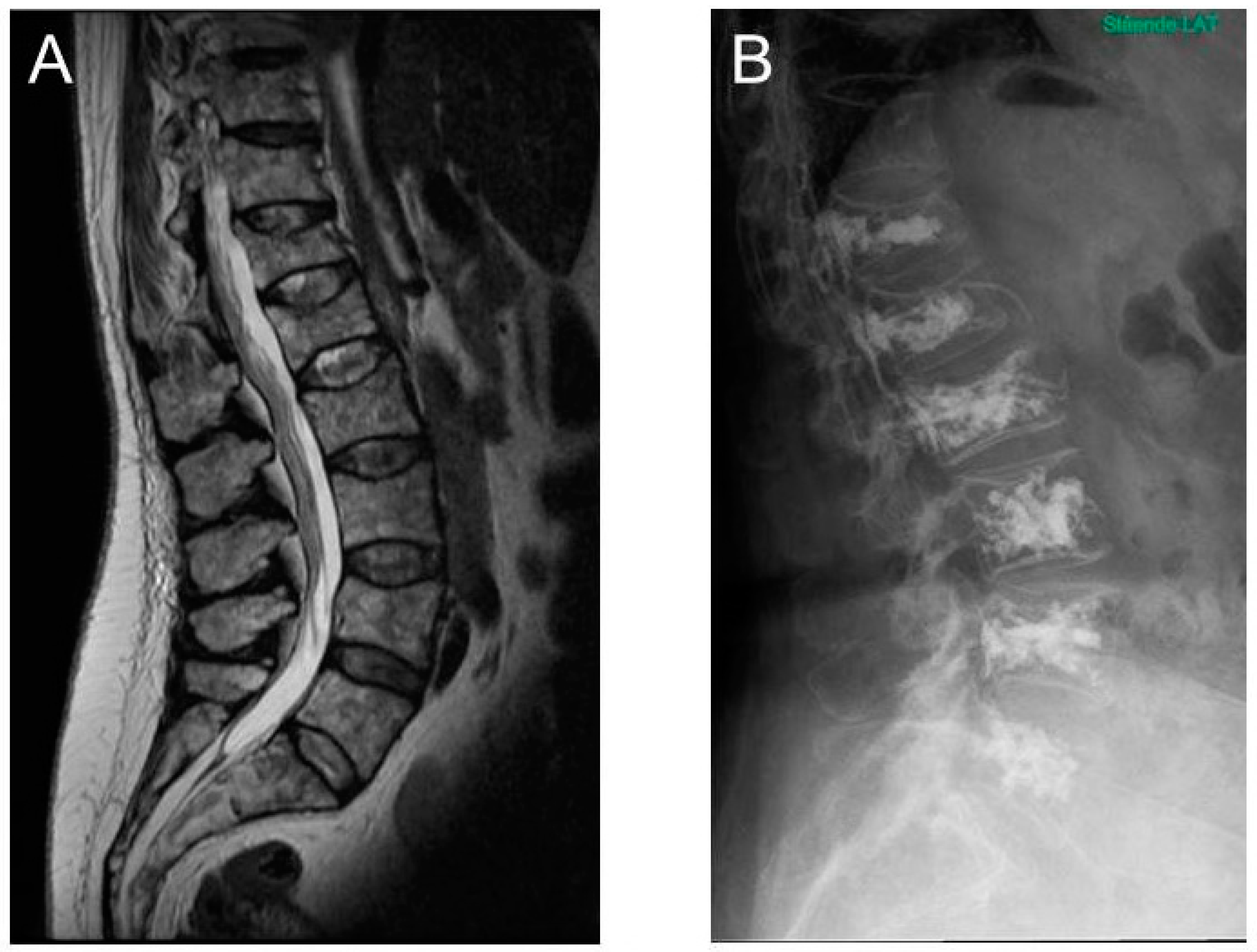
3.3. MRI as a Diagnostic and Prognostic Tool in Patients with Multiple Myeloma
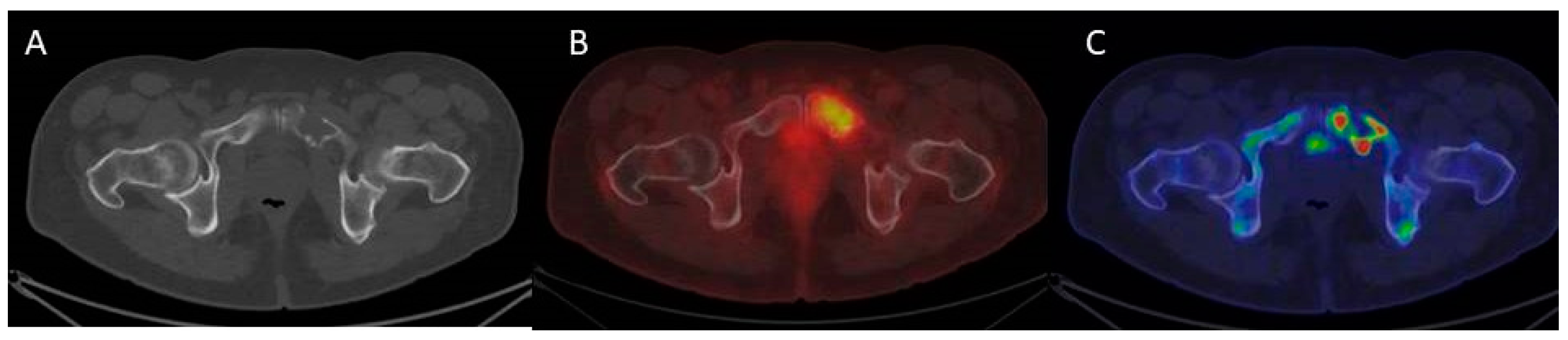
3.4. The Evolving Role of FDG-PET/CT in Multiple Myeloma
3.5. Follow-Up, Response Assessment, and Relapse
4. Medical Treatment
4.1. Bisphosphonates
4.2. Denosumab
5. Non-Pharmaceutical Treatment
5.1. Radiotherapy
5.2. Vertebral Augmentation
5.3. Rehabilitation and Exercise
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raab, M.S.; Podar, K.; Breitkreutz, I.; Richardson, P.G.; Anderson, K.C. Multiple myeloma. Lancet 2009, 374, 324–339. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Shortt, C.P.; Carty, F.; Murray, J.G. The Role of Whole-Body Imaging in the Diagnosis, Staging, and Follow-Up of Multiple Myeloma. Semin. Musculoskelet. Radiol. 2010, 14, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cocks, K.; Cohen, D.; Wisløff, F.; Sezer, O.; Lee, S.; Hippe, E.; Gimsing, P.; Turesson, I.; Hajek, R.; Smith, A.; et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur. J. Cancer 2007, 43, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Proskorovsky, I.; Lewis, P.; Ishak, J.; Payne, K.; Lordan, N.; Kyriakou, C.; Williams, C.D.; Peters, S.; Davies, F.E. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: Results of a European, multicenter cohort study. Support. Care Cancer 2014, 22, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Berenson, J.; Cook, R.J.; Lipton, A.; Coleman, R.E. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia 2010, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Augustson, B.M.; Begum, G.; Dunn, J.A.; Barth, N.J.; Davies, F.; Morgan, G.; Behrens, J.; Smith, A.; Child, J.A.; Drayson, M.T. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J. Clin. Oncol. 2005, 23, 9219–9226. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, L.; Abdul-Hay, M. Novel agents in the treatment of multiple myeloma: A review about the future. J. Hematol. Oncol. 2016, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Landgren, O.; Iskander, K. Modern multiple myeloma therapy: Deep, sustained treatment response and good clinical outcomes. J. Intern. Med. 2017, 281, 365–382. [Google Scholar] [CrossRef]
- Vallet, S.; Filzmoser, J.-M.; Pecherstorfer, M.; Podar, K. Myeloma Bone Disease: Update on Pathogenesis and Novel Treatment Strategies. Pharmaceutics 2018, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral. Biol. 2016, 18, 9–16. [Google Scholar] [PubMed]
- Giuliani, N.; Rizzoli, V.; Roodman, G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006, 108, 3992–3996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Giuliani, N.; Bataille, R.; Mancini, C.; Lazzaretti, M.; Barillé, S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 2001, 98, 3527–3533. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: Proposal for a novel prognostic index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef]
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013, 5, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Y.-W.; Chen, Y.; Stephens, O.; Brown, N.; Chen, B.; Epstein, J.; Barlogie, B.; Shaughnessy, J.D. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: A potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 2008, 112, 196–207. [Google Scholar] [CrossRef]
- Kristensen, I.B.; Christensen, J.H.; Lyng, M.B.; Møller, M.B.; Pedersen, L.M.; Rasmussen, L.M.; Ditzel, H.J.; Abildgaard, N. Expression of osteoblast and osteoclast regulatory genes in the bone marrow microenvironment in multiple myeloma: Only up-regulation of Wnt inhibitors SFRP3 and DKK1 is associated with lytic bone disease. Leuk. Lymphoma 2014, 55, 911–919. [Google Scholar] [CrossRef]
- Palma, B.D.; Guasco, D.; Pedrazzoni, M.; Bolzoni, M.; Accardi, F.; Costa, F.; Sammarelli, G.; Craviotto, L.; Filippo, M.D.; Ruffini, L.; et al. Osteolytic lesions, cytogenetic features and bone marrow levels of cytokines and chemokines in multiple myeloma patients: Role of chemokine (C-C motif) ligand 20. Leukemia 2016, 30, 409–416. [Google Scholar] [CrossRef]
- Andersen, T.L.; Søe, K.; Sondergaard, T.E.; Plesner, T.; Delaisse, J.-M. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br. J. Haematol. 2010, 148, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Børset, M.; Sundan, A.; Waage, A.; Standal, T. Why do myeloma patients have bone disease? A historical perspective. Blood Rev. 2020, 41, 100646. [Google Scholar] [CrossRef]
- Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [CrossRef] [Green Version]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzeau, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, S.; Minarik, J.; Stork, M.; Jelinek, T.; Pour, L.; Hajek, R. Extramedullary disease in multiple myeloma-controversies and future directions. Blood Rev. 2019, 36, 32–39. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.-V.; Lonial, S.; Joao, C.; Anderson, K.C.; García-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Terpos, E.; Comenzo, R.L.; Tosi, P.; Beksac, M.; Sezer, O.; Siegel, D.; Lokhorst, H.; Kumar, S.; Rajkumar, S.V.; et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia 2009, 23, 1545–1556. [Google Scholar] [CrossRef] [Green Version]
- Edelstyn, G.A.; Gillespie, P.J.; Grebbell, F.S. The radiological demonstration of osseous metastases. Experimental observations. Clin. Radiol. 1967, 18, 158–162. [Google Scholar] [CrossRef]
- Hinge, M.; Andersen, K.T.; Lund, T.; Jørgensen, H.B.; Holdgaard, P.C.; Ormstrup, T.E.; Østergaard, L.L.; Plesner, T. Baseline bone involvement in multiple myeloma—A prospectiv prospective comparison of conventional X-ray, low-dose computed tomography, and 18flourodeoxyglucose positron emission tomography in previously untreated patients. Haematologica 2016, 101, e415–e418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillengass, J.; Moulopoulos, L.A.; Delorme, S.; Koutoulidis, V.; Mosebach, J.; Hielscher, T.; Drake, M.; Rajkumar, S.V.; Oestergaard, B.; Abildgaard, N.; et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: A study of the International Myeloma Working Group. Blood Cancer J. 2017, 7, e599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsue, K.; Kobayashi, H.; Matsue, Y.; Abe, Y.; Narita, K.; Kitadate, A.; Takeuchi, M. Prognostic significance of bone marrow abnormalities in the appendicular skeleton of patients with multiple myeloma. Blood Adv. 2018, 2, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Moulopoulos, L.A.; Dimopoulos, M.A. Magnetic Resonance Imaging of the Bone Marrow in Hematologic Malignancies. Blood 1997, 90, 2127–2147. [Google Scholar] [CrossRef]
- Wolf, M.B.; Murray, F.; Kilk, K.; Hillengass, J.; Delorme, S.; Heiss, C.; Neben, K.; Goldschmidt, H.; Kauczor, H.-U.; Weber, M.-A. Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur. J. Radiol. 2014, 83, 1222–1230. [Google Scholar] [CrossRef]
- Walker, R.; Barlogie, B.; Haessler, J.; Tricot, G.; Anaissie, E.; Shaughnessy, J.D.; Epstein, J.; van Hemert, R.; Erdem, E.; Hoering, A.; et al. Magnetic Resonance Imaging in Multiple Myeloma: Diagnostic and Clinical Implications. J. Clin. Oncol. 2007, 25, 1121–1128. [Google Scholar] [CrossRef] [Green Version]
- Baur-Melnyk, A.; Buhmann, S.; Becker, C.; Schoenberg, S.O.; Lang, N.; Bartl, R.; Reiser, M.F. Whole-Body MRI Versus Whole-Body MDCT for Staging of Multiple Myeloma. Am. J. Roentgenol. 2008, 190, 1097–1104. [Google Scholar] [CrossRef]
- Lecouvet, F.E.; Vande Berg, B.C.; Malghem, J.; Maldague, B.E. Magnetic resonance and computed tomography imaging in multiple myeloma. Semin. Musculoskelet. Radiol. 2001, 5, 43–55. [Google Scholar] [CrossRef]
- Kastritis, E.; Moulopoulos, L.A.; Terpos, E.; Koutoulidis, V.; Dimopoulos, M.A. The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia 2014, 28, 2402–2403. [Google Scholar] [CrossRef]
- Hillengass, J.; Fechtner, K.; Weber, M.-A.; Bäuerle, T.; Ayyaz, S.; Heiss, C.; Hielscher, T.; Moehler, T.M.; Egerer, G.; Neben, K.; et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J. Clin. Oncol. 2010, 28, 1606–1610. [Google Scholar] [CrossRef]
- Merz, M.; Hielscher, T.; Wagner, B.; Sauer, S.; Shah, S.; Raab, M.S.; Jauch, A.; Neben, K.; Hose, D.; Egerer, G.; et al. Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia 2014, 28, 1902–1908. [Google Scholar] [CrossRef]
- Pratt, G.; Morris, T.C. Review of the NICE guidelines for multiple myeloma. Int. J. Lab. Hematol. 2017, 39, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 1 Guidance|Metastatic Spinal Cord Compression in Adults: Risk Assessment, Diagnosis and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/CG75/chapter/1-Guidance#imaging (accessed on 4 March 2020).
- Mauch, J.T.; Carr, C.M.; Cloft, H.; Diehn, F.E. Review of the Imaging Features of Benign Osteoporotic and Malignant Vertebral Compression Fractures. Am. J. Neuroradiol. 2018, 39, 1584–1592. [Google Scholar] [CrossRef]
- Role of 18 F-FDG PET/CT in the Diagnosis and Management of Multiple Myeloma and Other Plasma Cell Disorders: A Consensus Statement by the International Myeloma Working Group-Clinical Key. Available online: https://www-clinicalkey-com.proxy2-bib.sdu.dk/#!/content/journal/1-s2.0-S1470204517301894 (accessed on 5 March 2020).
- Zamagni, E.; Nanni, C.; Patriarca, F.; Englaro, E.; Castellucci, P.; Geatti, O.; Tosi, P.; Tacchetti, P.; Cangini, D.; Perrone, G.; et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica 2007, 92, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Gariani, J.; Westerland, O.; Natas, S.; Verma, H.; Cook, G.; Goh, V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) in patients with myeloma: Systematic review of diagnostic performance. Crit. Rev. Oncol./Hematol. 2018, 124, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, T.B.; Haessler, J.; Brown, T.L.Y.; Shaughnessy, J.D.; van Rhee, F.; Anaissie, E.; Alpe, T.; Angtuaco, E.; Walker, R.; Epstein, J.; et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 2009, 114, 2068–2076. [Google Scholar] [CrossRef] [Green Version]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef] [Green Version]
- Usmani, S.Z.; Mitchell, A.; Waheed, S.; Crowley, J.; Hoering, A.; Petty, N.; Brown, T.; Bartel, T.; Anaissie, E.; van Rhee, F.; et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood 2013, 121, 1819–1823. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.H.; Choi, W.H.; Yoo, I.R.; Lee, S.J.; Paeng, J.C.; Jeong, S.Y.; Lee, S.-W.; Kim, K.; Choi, J.Y. Prognostic Value of Baseline 18F-Fluorodeoxyglucose PET/CT in Patients with Multiple Myeloma: A Multicenter Cohort Study. Korean J. Radiol. 2018, 19, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Blau, M.; Nagler, W.; Bender, M.A. Fluorine-18: A new isotope for bone scanning. J. Nucl. Med. 1962, 3, 332–334. [Google Scholar] [PubMed]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-Fluoride: Applying New Technology to an Old Tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Even-Sapir, E.; Mishani, E.; Flusser, G.; Metser, U. 18F-Fluoride Positron Emission Tomography and Positron Emission Tomography/Computed Tomography. Semin. Nucl. Med. 2007, 37, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Tateishi, U.; Shizukuishi, K.; Shishikura, A.; Yamazaki, E.; Shibata, H.; Yoneyama, T.; Ishigatsubo, Y.; Inoue, T. Role of 18F-fluoride PET/CT in the assessment of multiple myeloma: Initial experience. Ann. Nucl. Med. 2013, 27, 78–83. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Goldschmidt, H.; Hose, D.; Pan, L.; Cheng, C.; Kopka, K.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. PET/CT studies of multiple myeloma using (18) F-FDG and (18) F-NaF: Comparison of distribution patterns and tracers’ pharmacokinetics. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Ak, İ.; Onner, H.; Akay, O.M. Is there any complimentary role of F-18 NaF PET/CT in detecting of osseous involvement of multiple myeloma? A comparative study for F-18 FDG PET/CT and F-18 FDG NaF PET/CT. Ann. Hematol. 2015, 94, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Hillengass, J.; Goldschmidt, H.; Wagner, B.; Haberkorn, U.; Kopka, K.; Dimitrakopoulou-Strauss, A. Treatment response evaluation with 18F-FDG PET/CT and 18F-NaF PET/CT in multiple myeloma patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 50–62. [Google Scholar] [CrossRef] [PubMed]
- DeGrado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar]
- Yoshimoto, M.; Waki, A.; Yonekura, Y.; Sadato, N.; Murata, T.; Omata, N.; Takahashi, N.; Welch, M.J.; Fujibayashi, Y. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: Acetate metabolism in tumor cells. Nucl. Med. Biol. 2001, 28, 117–122. [Google Scholar] [CrossRef]
- Nanni, C.; Zamagni, E.; Cavo, M.; Rubello, D.; Tacchetti, P.; Pettinato, C.; Farsad, M.; Castellucci, P.; Ambrosini, V.; Montini, G.C.; et al. 11C-choline vs. 18F-FDG PET/CT in assessing bone involvement in patients with multiple myeloma. World J. Surg. Oncol. 2007, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Cassou-Mounat, T.; Balogova, S.; Nataf, V.; Calzada, M.; Huchet, V.; Kerrou, K.; Devaux, J.-Y.; Mohty, M.; Talbot, J.-N.; Garderet, L. 18F-fluorocholine versus 18F-fluorodeoxyglucose for PET/CT imaging in patients with suspected relapsing or progressive multiple myeloma: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1995–2004. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Caillot, D.; Macro, M.; Karlin, L.; Garderet, L.; Facon, T.; Benboubker, L.; Escoffre-Barbe, M.; Stoppa, A.-M.; et al. Prospective Evaluation of Magnetic Resonance Imaging and [18F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J. Clin. Oncol. 2017, 35, 2911–2918. [Google Scholar] [PubMed]
- Pawlyn, C.; Fowkes, L.; Otero, S.; Jones, J.R.; Boyd, K.D.; Davies, F.E.; Morgan, G.J.; Collins, D.J.; Sharma, B.; Riddell, A.; et al. Whole-body diffusion-weighted MRI: A new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia 2016, 30, 1446–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenson, J.R.; Lichtenstein, A.; Porter, L.; Dimopoulos, M.A.; Bordoni, R.; George, S.; Lipton, A.; Keller, A.; Ballester, O.; Kovacs, M.J.; et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N. Engl. J. Med. 1996, 334, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Clézardin, P. Mechanisms of action of bisphosphonates in oncology: A scientific concept evolving from antiresorptive to anticancer activities. Bonekey Rep. 2013, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.A.; Coleman, R.E.; Reitsma, D.J.; et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. Cancer 2003, 98, 1735–1744. [Google Scholar] [CrossRef]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Cocks, K.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.T.; Owen, R.G.; Feyler, S.; et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet 2010, 376, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Morgan, G.J.; Child, J.A.; Gregory, W.M.; Szubert, A.J.; Cocks, K.; Bell, S.E.; Navarro-Coy, N.; Drayson, M.T.; Owen, R.G.; Feyler, S.; et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): Secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011, 12, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst. Rev. 2017, 12, CD003188. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Gage, B.; Luo, S.; Weilbaecher, K.; Tomasson, M.; Vij, R.; Colditz, G.; Carson, K. Comparative effectiveness on survival of zoledronic acid versus pamidronate in multiple myeloma. Leuk. Lymphoma 2015, 56, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Morgan, G.; Dimopoulos, M.A.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; García-Sanz, R.; Shimizu, K.; Turesson, I.; et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J. Clin. Oncol. 2013, 31, 2347–2357. [Google Scholar] [CrossRef] [Green Version]
- D’Arena, G.; Gobbi, P.G.; Broglia, C.; Sacchi, S.; Quarta, G.; Baldini, L.; Iannitto, E.; Falcone, A.; Guariglia, R.; Pietrantuono, G.; et al. Pamidronate versus observation in asymptomatic myeloma: Final results with long-term follow-up of a randomized study. Leuk. Lymphoma 2011, 52, 771–775. [Google Scholar] [CrossRef]
- Musto, P.; Petrucci, M.T.; Bringhen, S.; Guglielmelli, T.; Caravita, T.; Bongarzoni, V.; Andriani, A.; D’Arena, G.; Balleari, E.; Pietrantuono, G.; et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 2008, 113, 1588–1595. [Google Scholar] [CrossRef]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Szubert, A.J.; Bell, S.E.; Drayson, M.T.; Owen, R.G.; Ashcroft, A.J.; Jackson, G.H.; Child, J.A. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: The Medical Research Council Myeloma IX Trial. Blood 2012, 119, 5374–5383. [Google Scholar] [CrossRef] [Green Version]
- Jackson, G.H.; Morgan, G.J.; Davies, F.E.; Wu, P.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Coy, N.N.; Drayson, M.T.; Owen, R.G.; et al. Osteonecrosis of the jaw and renal safety in patients with newly diagnosed multiple myeloma: Medical Research Council Myeloma IX Study results. Br. J. Haematol. 2014, 166, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Aviles, A.; Nambo, M.-J.; Huerta-Guzman, J.; Cleto, S.; Neri, N. Prolonged Use of Zoledronic Acid (4 Years) Did Not Improve Outcome in Multiple Myeloma Patients. Clin. Lymphoma Myeloma Leuk. 2017, 17, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M. Use of Bisphosphonates in Multiple Myeloma: IMWG Response to Mayo Clinic Consensus Statement. Mayo Clin. Proc. 2007, 82, 516–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larocca, A.; Child, J.A.; Cook, G.; Jackson, G.H.; Russell, N.; Szubert, A.; Gregory, W.M.; Brioli, A.; Owen, R.G.; Drayson, M.T.; et al. The impact of response on bone-directed therapy in patients with multiple myeloma. Blood 2013, 122, 2974–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himelstein, A.L.; Foster, J.C.; Khatcheressian, J.L.; Roberts, J.D.; Seisler, D.K.; Novotny, P.J.; Qin, R.; Go, R.S.; Grubbs, S.S.; O’Connor, T.; et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA 2017, 317, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Vescio, R.; Montgomery, C.W.; Badros, A.; Munshi, N.; Orlowski, R.; Hadala, J.T.; Warsi, G.; Argonza-Aviles, E.; Ericson, S.G.; et al. Bone Marker-Directed Dosing of Zoledronic Acid for the Prevention of Skeletal Complications in Patients with Multiple Myeloma: Results of the Z-MARK Study. Clin. Cancer Res. 2016, 22, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- García-Sanz, R.; Oriol, A.; Moreno, M.J.; de la Rubia, J.; Payer, A.R.; Hernández, M.T.; Palomera, L.; Teruel, A.I.; Blanchard, M.J.; Gironella, M.; et al. Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: Results of the randomized AZABACHE Spanish trial. Haematologica 2015, 100, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; Garcia-Sanz, R.; Durie, B.; Legiec, W.; Krejci, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Identification of Risk Factors for Bisphosphonate-Associated Atypical Femoral Fractures and Osteonecrosis of the Jaw in a Pharmacovigilance Database-Rebecca S. Bejhed, Mohammad Kharazmi, Pär Hallberg. 2016. Available online: https://journals-sagepub-com.proxy1-bib.sdu.dk/doi/10.1177/1060028016649368 (accessed on 23 March 2020).
- Dimopoulos, M.A.; Kastritis, E.; Bamia, C.; Melakopoulos, I.; Gika, D.; Roussou, M.; Migkou, M.; Eleftherakis-Papaiakovou, E.; Christoulas, D.; Terpos, E.; et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann. Oncol. 2009, 20, 117–120. [Google Scholar] [CrossRef]
- Khan, S.A.; Kanis, J.A.; Vasikaran, S.; Kline, W.F.; Matuszewski, B.K.; McCloskey, E.V.; Beneton, M.N.; Gertz, B.J.; Sciberras, D.G.; Holland, S.D.; et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J. Bone Miner. Res. 1997, 12, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef]
- Montefusco, V.; Gay, F.; Spina, F.; Miceli, R.; Maniezzo, M.; Ambrosini, M.T.; Farina, L.; Piva, S.; Palumbo, A.; Boccadoro, M.; et al. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk. Lymphoma 2008, 49, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Fink, L.; Koneswaran, N.; Intorcia, M.; Giannopoulou, C.; Niepel, D.; Cavo, M. Bone complications in patients with multiple myeloma in five European countries: A retrospective patient chart review. BMC Cancer 2020, 20, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, R.; Ferrari, S.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; García-Sanz, R.; Shimizu, K.; Willenbacher, W.; Glennane, A.; Dai, T.; Pasteiner, W.; Raje, N.S. Progression-Free Survival Analysis of Denosumab Vs Zoledronic Acid in Intent to Transplant Multiple Myeloma Patients Based on Treatment Regimen and Baseline Characteristics. Blood 2019, 134, 606. [Google Scholar] [CrossRef]
- Lawson, M.A.; McDonald, M.M.; Kovacic, N.; Hua Khoo, W.; Terry, R.L.; Down, J.; Kaplan, W.; Paton-Hough, J.; Fellows, C.; Pettitt, J.A.; et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015, 6, 8983. [Google Scholar] [CrossRef] [Green Version]
- Watkins, K.R.; Rogers, J.E.; Atkinson, B. Tolerability of denosumab in metastatic solid tumor patients with renal insufficiency. Support. Care Cancer 2015, 23, 1657–1662. [Google Scholar] [CrossRef]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.-C.; Grazette, L.; San Martin, J.; Gallagher, J.C. Effects of Denosumab Treatment and Discontinuation on Bone Mineral Density and Bone Turnover Markers in Postmenopausal Women with Low Bone Mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Molon, R.S.; Shimamoto, H.; Bezouglaia, O.; Pirih, F.Q.; Dry, S.M.; Kostenuik, P.; Boyce, R.W.; Dwyer, D.; Aghaloo, T.L.; Tetradis, S. OPG-Fc but Not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice. J. Bone Miner. Res. 2015, 30, 1627–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, O.; Stoll, D.; Aubry-Rozier, B.; Rodriguez, E.G. Stopping Denosumab. Curr. Osteoporos. Rep. 2019, 17, 8–15. [Google Scholar] [CrossRef]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guañabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- eTalamo, G.; eDimaio, C.; Abbi, K.K.; Pandey, M.K.; eMalysz, J.; Creer, M.H.; Zhu, J.; Mir, M.A.; Varlotto, J.M. Current Role of Radiation Therapy for Multiple Myeloma. Front. Oncol. 2015, 5, 40. [Google Scholar]
- Terpos, E.; Kleber, M.; Engelhardt, M.; Zweegman, S.; Gay, F.; Kastritis, E.; van de Donk, N.W.C.J.; Bruno, B.; Sezer, O.; Broijl, A.; et al. European Myeloma Network Guidelines for the Management of Multiple Myeloma-related Complications. Haematologica 2015, 100, 1254–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rades, D.; Conde-Moreno, A.J.; Cacicedo, J.; Segedin, B.; Rudat, V.; Schild, S.E. Excellent outcomes after radiotherapy alone for malignant spinal cord compression from myeloma. Radiol. Oncol. 2016, 50, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.M.; Chouake, R.J.; Sanfilippo, N.J.; Rapp, T.B.; Cook, P.; Formenti, S.C.; Mazumder, A.; Silverman, J.S. Feasibility and Efficacy of Local Radiotherapy With Concurrent Novel Agents in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2014, 14, 480–484. [Google Scholar] [CrossRef]
- Berenson, J.; Pflugmacher, R.; Jarzem, P.; Zonder, J.; Schechtman, K.; Tillman, J.B.; Bastian, L.; Ashraf, T.; Vrionis, F. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: A multicentre, randomised controlled trial. Lancet Oncol. 2011, 12, 225–235. [Google Scholar] [CrossRef]
- Julka, A.; Tolhurst, S.; Srinivasan, R.; Graziano, G. Functional Outcomes and Height Restoration for Patients with Multiple Myeloma-Related Osteolytic Vertebral Compression Fractures Treated with Kyphoplasty. J. Spinal Disord. Tech. 2014, 27, 342–346. [Google Scholar] [CrossRef]
- Khan, O.A.; Brinjikji, W.; Kallmes, D.F. Vertebral Augmentation in Patients with Multiple Myeloma: A Pooled Analysis of Published Case Series. Am. J. Neuroradiol. 2014, 35, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, T.R.; Koyyalagunta, D.; Burton, A.W.; Thomas, S.K.; Phan, M.-H.V.; Giralt, S.A.; Shah, J.J.; Cleeland, C.S. Changes in pain and other symptoms in patients with painful multiple myeloma-related vertebral fracture treated with kyphoplasty or vertebroplasty. J. Pain 2012, 13, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, K.; Butler, J.S.; Yu, H.M.; Selvadurai, S.; D’Sa, S.; Rabin, N.; Kyriakou, C.; Yong, K.; Molloy, S. Spinal disease in myeloma: Cohort analysis at a specialist spinal surgery centre indicates benefit of early surgical augmentation or bracing. BMC Cancer 2016, 16, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simony, A.; Hansen, E.J.; Gaurilcikas, M.; Abildgaard, N.; Andersen, M.O. Pain reduction after percutaneous vertebroplasty for myeloma-associated vertebral fractures. Dan. Med. J. 2014, 61, A4945. [Google Scholar]
- Health Quality Ontario. Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: A Systematic Review. Ont. Health Technol. Assess. Ser. 2016, 16, 1–202. [Google Scholar]
- Tosi, P.; Sintini, M.; Molinari, A.L.; Imola, M.; Ciotta, G.; Tomassetti, S.; Mianulli, A.M.; Ratta, M.; Mangianti, S.; Merli, A.; et al. Early application of percutaneous vertebroplasty reduces pain without affecting peripheral blood stem cell (PBSC) collection and transplant in newly diagnosed multiple myeloma (MM) patients. Eur. J. Cancer Care 2014, 23, 773–778. [Google Scholar] [CrossRef]
- Kyriakou, C.; Molloy, S.; Vrionis, F.; Alberico, R.; Bastian, L.; Zonder, J.A.; Giralt, S.; Raje, N.; Kyle, R.A.; Roodman, D.G.D.; et al. The role of cement augmentation with percutaneous vertebroplasty and balloon kyphoplasty for the treatment of vertebral compression fractures in multiple myeloma: A consensus statement from the International Myeloma Working Group (IMWG). Blood Cancer J. 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Br. Med. J. 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Rousing, R.; Kirkegaard, A.O.; Nielsen, M.; Holtved, E.; Sørensen, L.H.; Lund, T.; Olesen, V.; Andersen, M.Ø. Percutaneous vertebroplasty as treatment of malignant vertebral lesions: A systematic review and GRADE evaluation resulting in a Danish national clinical guideline. Eur. Spine J. 2020, 29, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Sweegers, M.G.; Altenburg, T.M.; Chinapaw, M.J.; Kalter, J.; Verdonck-de Leeuw, I.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Brug, J.; et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2018, 52, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.; McCourt, O.; Henrich, M.; Paton, B.; Yong, K.; Wardle, J.; Fisher, A. Multiple myeloma and physical activity: A scoping review. BMJ Open 2015, 5, e009576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, J.H.; Sim, C.Y.L.; Santorelli, L.A. The effectiveness of exercise programmes in patients with multiple myeloma: A literature review. Crit. Rev. Oncol. Hematol. 2016, 98, 275–289. [Google Scholar] [CrossRef]
- Larsen, R.F.; Jarden, M.; Minet, L.R.; Frølund, U.C.; Möller, S.; Abildgaard, N. Physical function in patients newly diagnosed with multiple myeloma; a Danish cohort study. BMC Cancer 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, R.F.; Jarden, M.; Minet, L.R.; Frølund, U.C.; Abildgaard, N. Supervised and home-based physical exercise in patients newly diagnosed with multiple myeloma—A randomized controlled feasibility study. Pilot Feasibil. Stud. 2019, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.F.; Jaden, M.; Minet, L.R.; Frølund, U.C.; Möller, S.; Abildgaard, N. Exercise in Newly Diagnosed Patients with Multiple Myeloma—A Randomized, Controlled Trial of Effects on Physical Function, Physical Activity. European Haematology Association (EHA) Congress 2020. Available online: https://library.ehaweb.org/eha/2020/eha25th/294240/rikke.faebo.larsen.exercise.in.newly.diagnosed.patients.with.multiple.myeloma.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dep1760 (accessed on 12 June 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasch, S.; Lund, T.; Asmussen, J.T.; Lerberg Nielsen, A.; Faebo Larsen, R.; Østerheden Andersen, M.; Abildgaard, N. Multiple Myeloma Associated Bone Disease. Cancers 2020, 12, 2113. https://doi.org/10.3390/cancers12082113
Rasch S, Lund T, Asmussen JT, Lerberg Nielsen A, Faebo Larsen R, Østerheden Andersen M, Abildgaard N. Multiple Myeloma Associated Bone Disease. Cancers. 2020; 12(8):2113. https://doi.org/10.3390/cancers12082113
Chicago/Turabian StyleRasch, Stine, Thomas Lund, Jon Thor Asmussen, Anne Lerberg Nielsen, Rikke Faebo Larsen, Mikkel Østerheden Andersen, and Niels Abildgaard. 2020. "Multiple Myeloma Associated Bone Disease" Cancers 12, no. 8: 2113. https://doi.org/10.3390/cancers12082113
APA StyleRasch, S., Lund, T., Asmussen, J. T., Lerberg Nielsen, A., Faebo Larsen, R., Østerheden Andersen, M., & Abildgaard, N. (2020). Multiple Myeloma Associated Bone Disease. Cancers, 12(8), 2113. https://doi.org/10.3390/cancers12082113




