Laryngeal Mid-Cord Erythroleukoplakias: How to Modulate the Transoral CO2 Laser Excisional Biopsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
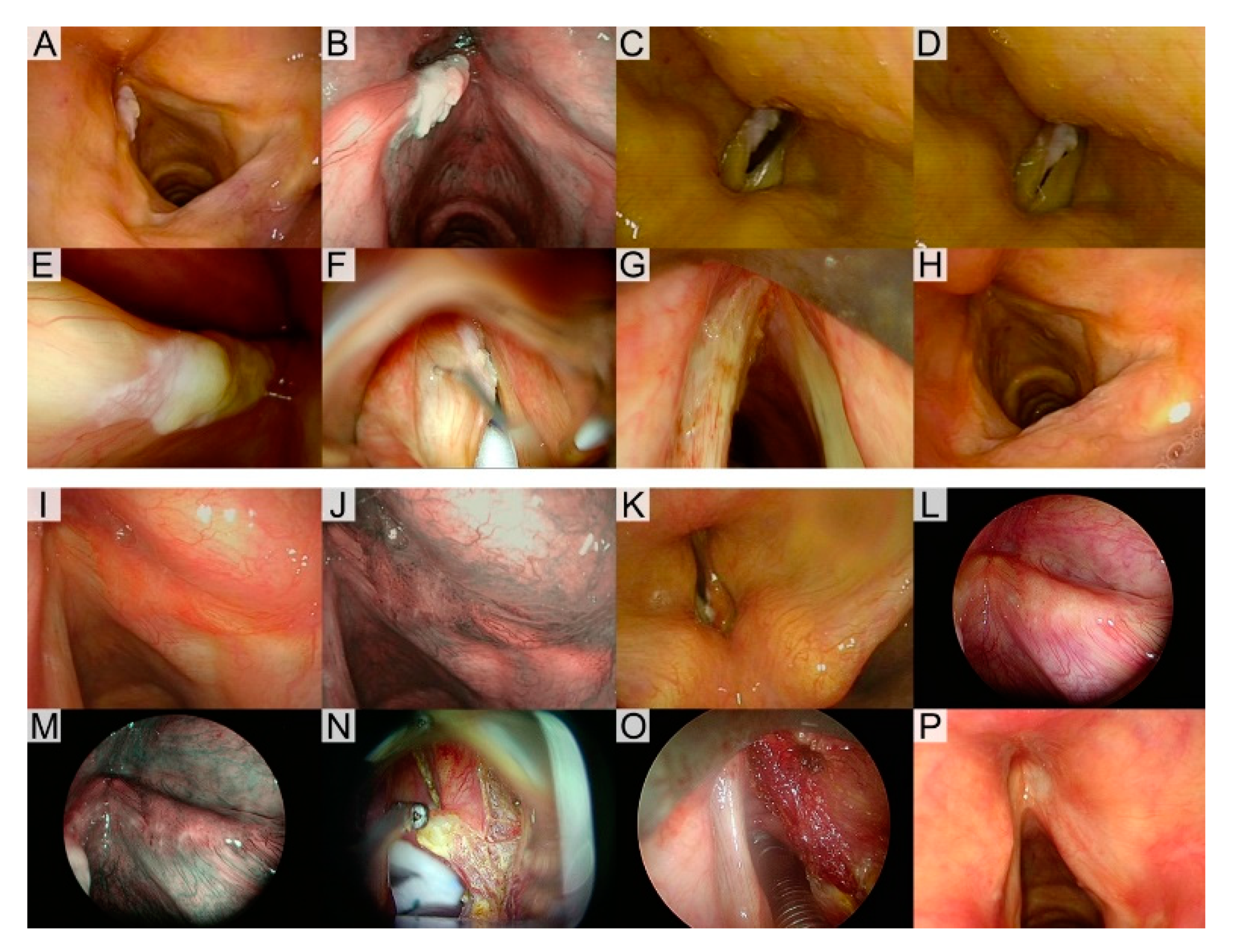
2.2. Diagnostic Workup
2.3. Treatments
2.4. Outcome Evaluation and Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Univariable Adequacy of Treatment Analysis
3.3. Multivariable Adequacy of Treatment Analysis
3.4. Margin Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
Informed Consent
References
- EI-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. (Eds.) WHO Classification of Head and Neck Tumors, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-4489-9. [Google Scholar]
- Marioni, G.; Agostini, M.; Cappellesso, R.; Bedin, C.; Ottaviano, G.; Marchese-Ragona, R.; Lovato, A.; Cacco, T.; Giacomelli, L.; Nitti, D.; et al. miR-19a and SOCS-1 expression in the differential diagnosis of laryngeal (glottic) verrucous squamous cell carcinoma. J. Clin. Pathol. 2016, 69, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Cappiello, J.; Nicolai, P.; Smussi, C.; Antonelli, A.R. Endoscopic laser excisional biopsy for selected glottic carcinomas. Laryngoscope 1994, 104, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Eckel, H.E.; Antonelli, A.; Brasnu, D.; Chevalier, D.; Friedrich, G.; Olofsson, J.; Rudert, H.H.; Thumfart, W.; De Vincentiis, M.; et al. Endoscopic cordectomy. A proposal for a classification by the working committee, European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2000, 257, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Van Haverbeke, C.; Eckel, H.; Bradley, P.; Chevalier, D.; Djukic, V.; De Vicentiis, M.; Friedrich, G.; Olofsson, J.; Peretti, G.; et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur. Arch. Otorhinolaryngol. 2007, 264, 613–615. [Google Scholar] [CrossRef]
- Piazza, C.; Del Bon, F.; Peretti, G.; Nicolai, P. Narrow band imaging in endoscopic evaluation of the larynx. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1. 2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; Colevas, A.D.; Dunphy, F.; Eisele, D.W.; et al. Head and neck cancers, version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 1454–1487. [Google Scholar] [CrossRef]
- Peretti, G.; Piazza, C.; Berlucchi, M.; Cappiello, J.; Giudice, M.; Nicolai, P. Pre- and intraoperative assessment of mid-cord erythroleukoplakias: A prospective study on 52 patients. Eur. Arch. Otorhinolaryngol. 2003, 260, 525–528. [Google Scholar] [CrossRef]
- Piazza, C.; Bon, F.D.; Peretti, G.; Nicolai, P. Biologic endoscopy: Optimization of upper aerodigestive tract cancer evaluation. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 67–76. [Google Scholar] [CrossRef]
- Staníková, L.; Walderová, R.; Jančatová, D.; Formánek, M.; Zeleník, K.; Komínek, P. Comparison of narrow band imaging and the Storz Professional Image Enhancement System for detection of laryngeal and hypopharyngeal pathologies. Eur. Arch. Otorhinolaryngol. 2018, 275, 1819–1825. [Google Scholar] [CrossRef]
- Garofolo, S.; Piazza, C.; Del Bon, F.; Mangili, S.; Guastini, L.; Mora, F.; Nicolai, P.; Peretti, G. Intraoperative narrow band imaging better delineates superficial resection margins during transoral laser microsurgery for early glottic cancer. Ann. Otol. Rhinol. Laryngol. 2015, 124, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.A.; Friedman, A.D.; Lutch, M.J.; Zeitels, S.M. Subepithelial vocal fold infusion: A useful diagnostic and therapeutic technique. Ann. Otol. Rhinol. Laryngol. 2012, 121, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Arens, C.; Eldin, M.B.; Campos, G.; Estomba, C.C.; Dulguerov, P.; Fiz, I.; Hantzakos, A.; Keghian, J.; Mora, F.; et al. Laser-assisted surgery of the upper aero-digestive tract: A clarification of nomenclature. A consensus statement of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2017, 274, 3723–3727. [Google Scholar] [CrossRef]
- Arens, C.; Piazza, C.; Andrea, M.; Dikkers, F.G.; Tjon Pian Gi, R.E.A.; Voigt-Zimmermann, S.; Peretti, G. Proposal for a descriptive guideline of vascular changes in lesions of the vocal folds by the committee on endoscopic laryngeal imaging of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2016, 273, 1207–1214. [Google Scholar] [CrossRef]
- Damm, M.; Sittel, C.; Streppel, M.; Eckel, H.E. Transoral CO2 laser for surgical management of glottic carcinoma in situ. Laryngoscope 2000, 110, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Scheweinfurth, J.M.; Powitzky, E.; Ossoff, R.H. Regression of laryngeal dysplasia after serial microflap excision. Ann. Otol. Rhinol. Laryngol. 2001, 110, 811–814. [Google Scholar] [CrossRef]
- Ahn, A.; Wang, L.; Slaughter, J.C.; Nguyen, A.M.; Ossoff, R.H.; Francis, D.O. Serial full-thickness excision of dysplastic vocal fold leukoplakia: Diagnostic or therapeutic? Laryngoscope 2016, 126, 923–927. [Google Scholar] [CrossRef]
- Hamzany, Y.; Shoffel-Havakuk, H.; Devons-Sberro, S.; Shteinberg, S.; Yaniv, D.; Mizrachi, A. Single stage transoral laser microsurgery for early glottic cancer. Front. Oncol. 2018, 8, 298. [Google Scholar] [CrossRef]
- Davaris, N.; Lux, A.; Esmaeili, N.; Illanes, A.; Boese, A.; Friebe, M.; Arens, C. Evaluation of vascular patterns using contact endoscopy and narrow-band imaging (CE-NBI) for the diagnosis of vocal fold malignancy. Cancers 2020, 12, 248. [Google Scholar] [CrossRef]
- Puxeddu, R.; Sionis, S.; Gerosa, C.; Carta, F. Enhanced contact endoscopy for the detection of neoangiogenesis in tumors of the larynx and hypopharynx. Laryngoscope 2015, 125, 1600–1606. [Google Scholar] [CrossRef]
- Djukic, V.; Milovanovic, J.; Jotic, A.D.; Vukasinovic, M. Stroboscopy in detection of laryngeal dysplasia effectiveness and limitations. J. Voice 2014, 28, 262. [Google Scholar] [CrossRef]
- Colden, D.; Zeitels, S.M.; Hillman, R.E.; Jarboe, J.; Bunting, G.; Spanou, K. Stroboscopic assessment of vocal fold keratosis and glottic cancer. Ann. Otol. Rhinol. Laryngol. 2001, 110, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Redaelli de Zinis, L.O.; Nicolai, P.; Valentini, S.; Piazza, C.; Antonelli, A.R. Oncological results of endoscopic resections of Tis and T1 glottic carcinomas by carbon dioxide laser. Ann. Otol. Rhinol. Laryngol. 2001, 110, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Cantarella, G.; Balzanelli, C.; Nicolai, P. Vocal outcome after endoscopic cordectomies for Tis and T1 glottic carcinomas. Ann. Otol. Rhinol. Laryngol. 2003, 112, 174–179. [Google Scholar] [CrossRef] [PubMed]

| Histology | Treatment | |
|---|---|---|
| Type I Cordectomy | Type II Cordectomy | |
| Keratosis without atypia | Adequate | Not adequate |
| SIN1-2 | Adequate | Adequate |
| SIN3-CIS | Adequate | Adequate |
| SCC | Not adequate | Adequate |
| Variables | All | Suspicious for Invasive SCC | Not Clearly Suspicious for Invasive SCC | ||
|---|---|---|---|---|---|
| Group A (BE + VLS) | Group B (BE + VLS) | Group C (BE + VLS + SI) | |||
| Type II Cordectomy | Type I Cordectomy | Type I Cordectomy | Type II Cordectomy | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Histology | |||||
| Keratosis without atypia | 26 (17) | 1 (1) | 4 (16) | 19 (37) | 2 (18) |
| SIN1-2 | 47 (30) | 6 (9) | 10 (40) | 26 (51) | 5 (45) |
| SIN3-CIS | 21 (14) | 18 (26) | 1 (4) | 2 (4) | 0 (0) |
| SCC | 61 (39) | 43 (63) | 10 (40) | 4 (8) | 4 (36) |
| Adequacy of treatment | |||||
| Adequate | 138 (89) | 67 (99) | 15 (60) | 47 (92) | 9 (82) |
| Not adequate | 17 (11) | 1 (1) | 10 (40) | 4 (8) | 2 (18) |
| Histopathologic Diagnosis | Endoscopic Findings | ||
|---|---|---|---|
| Suspicious for SCC | Not clearly Suspicious for SCC | p | |
| N (%) | N (%) | ||
| Keratosis without atypia | 1 (1) | 25 (29) | <0.001 |
| SIN 1-2 | 6 (9) | 41 (47) | |
| SIN3-CIS | 18 (26) | 3 (3) | |
| SCC | 43 (63) | 18 (21) | |
| Not Clearly Suspicious for Invasive SCC | All | Treatment | |||
|---|---|---|---|---|---|
| Adequate | Not Adequate | ||||
| N (%) | N (%) | N (%) | p | ||
| Workup | Group B (BE + VLS) | 25 (29) | 15 (60) | 10 (40) | 0.006 |
| Group C (BE + VLS + SI) | 62 (71) | 56 (90) | 6 (10) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, F.; Carta, F.; Missale, F.; Laborai, A.; Parrinello, G.; Piazza, C.; Puxeddu, R.; Peretti, G. Laryngeal Mid-Cord Erythroleukoplakias: How to Modulate the Transoral CO2 Laser Excisional Biopsy. Cancers 2020, 12, 2165. https://doi.org/10.3390/cancers12082165
Mora F, Carta F, Missale F, Laborai A, Parrinello G, Piazza C, Puxeddu R, Peretti G. Laryngeal Mid-Cord Erythroleukoplakias: How to Modulate the Transoral CO2 Laser Excisional Biopsy. Cancers. 2020; 12(8):2165. https://doi.org/10.3390/cancers12082165
Chicago/Turabian StyleMora, Francesco, Filippo Carta, Francesco Missale, Andrea Laborai, Giampiero Parrinello, Cesare Piazza, Roberto Puxeddu, and Giorgio Peretti. 2020. "Laryngeal Mid-Cord Erythroleukoplakias: How to Modulate the Transoral CO2 Laser Excisional Biopsy" Cancers 12, no. 8: 2165. https://doi.org/10.3390/cancers12082165
APA StyleMora, F., Carta, F., Missale, F., Laborai, A., Parrinello, G., Piazza, C., Puxeddu, R., & Peretti, G. (2020). Laryngeal Mid-Cord Erythroleukoplakias: How to Modulate the Transoral CO2 Laser Excisional Biopsy. Cancers, 12(8), 2165. https://doi.org/10.3390/cancers12082165







