Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
2.2. Isolation and Identification of Circulating Tumour Cells
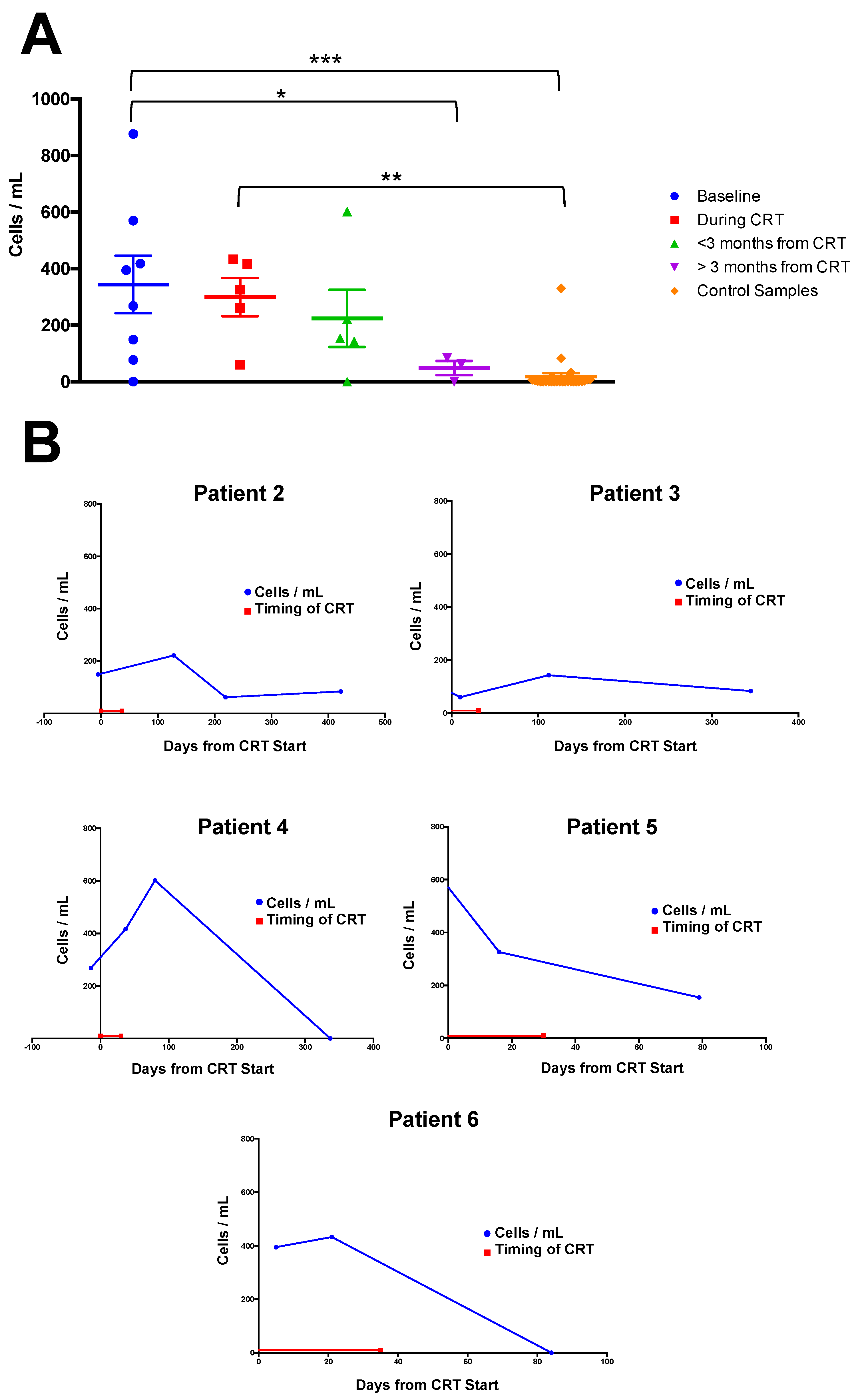
2.3. Correlation of CC Numbers with Clinical Timepoints
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer Statistics, 2011: The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Smith, M.; Canfell, K. Anal Cancer in High-Income Countries: Increasing Burden of Disease. PLoS ONE 2018, 13, e0205105. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Haque, W.; Butler, E.B.; Teh, B.S. Survival Outcomes and Patterns of Management for Anal Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Morris, E.; Downing, A.; Finan, P.; Aravani, A.; Thomas, J.; Sebag-Montefiore, D. The Rising Incidence of Anal Cancer in England 1990–2010: A Population-Based Study. Colorectal Dis. 2014, 16, O234–O239. [Google Scholar] [CrossRef]
- Patel, J.; Salit, I.E.; Berry, M.J.; de Pokomandy, A.; Nathan, M.; Fishman, F.; Palefsky, J.; Tinmouth, J. Environmental Scan of Anal Cancer Screening Practices: Worldwide Survey Results. Cancer Med. 2014, 3, 1052–1061. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Scholefield, J.; Smith, J.; Polacarz, S.; Rogers, K.; Sharp, F. Immunohistochemical Analysis of p53 Expression in Anal Squamous Neoplasia. J. Clin. Pathol. 1993, 46, 507–512. [Google Scholar] [CrossRef][Green Version]
- Glynne-Jones, R.; Saleem, W.; Harrison, M.; Mawdsley, S.; Hall, M. Background and Current Treatment of Squamous Cell Carcinoma of the Anus. Oncol. Ther. 2016, 4, 135–172. [Google Scholar] [CrossRef]
- Northover, J.; Glynne-Jones, R.; Sebag-Montefiore, D.; James, R.; Meadows, H.; Wan, S.; Jitlal, M.; Ledermann, J. Chemoradiation for the Treatment of Epidermoid Anal Cancer: 13-year Follow-Up of the First Randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 2010, 102, 1123–1128. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Nilsson, P.J.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Arnold, D. Anal Cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, iii10–iii20. [Google Scholar] [CrossRef]
- Moscicki, A.-B.; Darragh, T.M.; Berry-Lawhorn, J.M.; Roberts, J.M.; Khan, M.J.; Boardman, L.A.; Chiao, E.; Einstein, M.H.; Goldstone, S.E.; Jay, N. Screening for Anal Cancer in Women. J. Low. Genit. Tract Dis. 2015, 19, S26. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Sebag-Montefiore, D.; Meadows, H.M.; Cunningham, D.; Begum, R.; Adab, F.; Benstead, K.; Harte, R.J.; Stewart, J.; Beare, S. Best Time to Assess Complete Clinical Response after Chemoradiotherapy in Squamous Cell Carcinoma of the Anus (Act Ii): A Post-Hoc Analysis of Randomised Controlled Phase 3 Trial. Lancet Oncol. 2017, 18, 347–356. [Google Scholar] [CrossRef]
- Kochhar, R.; Renehan, A.G.; Mullan, D.; Chakrabarty, B.; Saunders, M.P.; Carrington, B.M. The Assessment of Local Response Using Magnetic Resonance Imaging at 3- and 6-month Post Chemoradiotherapy in Patients with Anal Cancer. Eur. Radiol. 2017, 27, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Dewdney, A.; Rao, S. Metastatic Squamous Cell Carcinoma of the anus: Time for a Shift in the Treatment Paradigm? ISRN Oncol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Khoo, B.L.; Grenci, G.; Jing, T.; Lim, Y.B.; Lee, S.C.; Thiery, J.P.; Han, J.; Lim, C.T. Liquid Biopsy and Therapeutic Response: Circulating Tumor Cell Cultures for Evaluation of Anticancer Treatment. Sci. Adv. 2016, 2, e1600274. [Google Scholar] [CrossRef]
- Huang, W.-L.; Chen, Y.-L.; Yang, S.-C.; Ho, C.-L.; Wei, F.; Wong, D.T.; Su, W.-C.; Lin, C.-C. Liquid Biopsy Genotyping in Lung Cancer: Ready for Clinical Utility? Oncotarget 2017, 8, 18590. [Google Scholar] [CrossRef]
- Nawroz, H.; Koch, W.; Anker, P.; Stroun, M.; Sidransky, D. Microsatellite Alterations in Serum Dna of Head and Neck Cancer Patients. Nat. Med. 1996, 2, 1035–1037. [Google Scholar] [CrossRef]
- Chen, X.Q.; Stroun, M.; Magnenat, J.-L.; Nicod, L.P.; Kurt, A.-M.; Lyautey, J.; Lederrey, C.; Anker, P. Microsatellite Alterations in Plasma DNA of Small Cell Lung Cancer Patients. Nat. Med. 1996, 2, 1033–1035. [Google Scholar] [CrossRef]
- Wan, J.C.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid Biopsies Come of Age: Towards Implementation of Circulating Tumour DNA. Nat. Rev. Cancer 2017, 17, 223. [Google Scholar] [CrossRef]
- Rothwell, D.G.; Ayub, M.; Cook, N.; Thistlethwaite, F.; Carter, L.; Dean, E.; Smith, N.; Villa, S.; Dransfield, J.; Clipson, A. Utility of Ctdna to Support Patient Selection for Early Phase Clinical Trials: The Target Study. Nat. Med. 2019, 25, 738–743. [Google Scholar] [CrossRef]
- Veyer, D.; Pavie, J.; Pernot, S.; Mandavit, M.; Garrigou, S.; Lucas, M.-L.; Gibault, L.; Taly, V.; Weiss, L.; Péré, H. HPV-Circulating Tumoural DNA by Droplet-Based Digital Polymerase Chain Reaction, A New Molecular Tool for Early Detection of HPV Metastatic Anal Cancer? A Case Report. Eur. J. Cancer 2019, 112, 34–37. [Google Scholar] [CrossRef]
- Morris, V.K. Circulating Tumor DNA in Advanced Anal Cancer: A Blood Biomarker Goes Viral. Clin. Cancer Res. 2019, 25, 2030–2032. [Google Scholar] [CrossRef]
- Chudasama, D.; Katopodis, P.; Stone, N.; Haskell, J.; Sheridan, H.; Gardner, B.; Urnovitz, H.; Schuetz, E.; Beck, J.; Hall, M. Liquid Biopsies in Lung Cancer: Four Emerging Technologies and Potential Clinical Applications. Cancers 2019, 11, 331. [Google Scholar] [CrossRef]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.-W.; Lim, W.-T.; Han, J.; Bhagat, A.A.S.; Lim, C.T. Isolation and Retrieval of Circulating Tumor Cells Using Centrifugal Forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Hou, J.-M.; Ward, T.H.; Blackhall, F.H.; Dive, C. Circulating Tumour Cells: Their Utility in Cancer Management and Predicting Outcomes. Ther. Adv. Med. Oncol. 2010, 2, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Dent, B.M.; Ogle, L.F.; O′Donnell, R.L.; Hayes, N.; Malik, U.; Curtin, N.J.; Boddy, A.V.; Plummer, E.R.; Edmondson, R.J.; Reeves, H.L. High-Resolution Imaging for the Detection and Characterisation of Circulating Tumour Cells from Patients with Oesophageal, Hepatocellular, Thyroid and Ovarian Cancers. Int. J. Cancer 2016, 138, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.; Morris, K.; Pritchard, S.; Kirwan, C.C. Challenges in Enumeration of CTCs in Breast Cancer Using Techniques Independent of Cytokeratin Expression. PLoS ONE 2017, 12, e0175647. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.-P.; Ngan, S.Y.; Michael, M.; Lynch, A.C.; Heriot, A.G.; Ramsay, R.G.; Phillips, W.A. Molecular Biology of Anal Squamous Cell Carcinoma: Implications for Future Research and Clinical Intervention. Lancet Oncol. 2015, 16, e611–e621. [Google Scholar] [CrossRef]
- Kumar, J.; Chudasama, D.; Roberts, C.; Kubista, M.; Sjöback, R.; Chatterjee, J.; Anikin, V.; Karteris, E.; Hall, M. Detection of Abundant Non-Haematopoietic Circulating Cancer-Related Cells in Patients with Advanced Epithelial Ovarian Cancer. Cells 2019, 8, 732. [Google Scholar] [CrossRef]
- Rogers-Broadway, K.R.; Kumar, J.; Sisu, C.; Wander, G.; Mazey, E.; Jeyaneethi, J.; Pados, G.; Tsolakidis, D.; Klonos, E.; Grunt, T. Differential Expression of mTOR Components in Endometriosis and Ovarian Cancer: Effects of Rapalogues and Dual Kinase Inhibitors on mTORC1 and mTORC2 Stoichiometry. Int. J. Mol. Med. 2019, 43, 47–56. [Google Scholar] [CrossRef]
- Barr, J.; Chudasama, D.; Rice, A.; Karteris, E.; Anikin, V. Lack of Association between Screencell-Detected Circulating Tumour Cells and Long-Term Survival of Patients Undergoing Surgery for Non-Small Cell Lung Cancer: A Pilot Clinical Study. Mol. Clin. Oncol. 2020, 12, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, D.; Burnside, N.; Beeson, J.; Karteris, E.; Rice, A.; Anikin, V. Perioperative Detection of Circulating Tumour Cells in Patients with Lung Cancer. Oncol. Lett. 2017, 14, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.A.; Anderson, R.L.; Russell, P.A.; Cox, R.A.; Ivashkevich, A.; Swierczak, A.; Doherty, J.P.; Jacobs, D.H.; Smith, J.; Siva, S. Mobilization of Viable Tumor Cells into the Circulation during Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 395–403. [Google Scholar] [CrossRef]
- Shishido, S.N.; Carlsson, A.; Nieva, J.; Bethel, K.; Hicks, J.B.; Bazhenova, L.; Kuhn, P. Circulating Tumor Cells as a Response Monitor in sTage IV Non-Small Cell Lung Cancer. J. Transl. Med. 2019, 17, 294. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Valero, V., III; Saunders, T.; Blackford, A.L.; Griffin, J.F.; Poling, J.; Hruban, R.H.; Anders, R.A.; Herman, J.; Zheng, L. Circulating Tumor Cell Phenotype Predicts Recurrence and Survival in Pancreatic Adenocarcinoma. Ann. Surg. 2016, 264, 1073. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.A.; Asad, D.; Amu, E.; Hensley, M.T.; Cores, J.; Vandergriff, A.; Tang, J.; Dinh, P.-U.; Shen, D.; Qiao, L. Circulating Tumor Cells Exit Circulation While Maintaining Multicellularity, Augmenting Metastatic Potential. J. Cell Sci. 2019, 132, jcs231563. [Google Scholar] [CrossRef]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M. Circulating Giant Macrophages as a Potential Biomarker of Solid Tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef]
- Broncy, L.; Paterlini-Bréchot, P. Cancer-Associated Circulating Atypical Cells with both Epithelial and Macrophage-Specific Markers. J. Lab. Precis. Med. 2018, 3, 91. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B. Cell Fusion Potentiates Tumor Heterogeneity and Reveals Circulating Hybrid Cells That Correlate With Stage and Survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Lin, P.P. Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis. Cells 2020, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.V.; Ko, G.; Raphael, M.J.; Booth, C.M.; Brogly, S.B.; Kalyvas, M.; Li, W.; Hanna, T. Salvage APR for Anal Squamous Cell Carcinoma: Utilization, Risk Factors and Outcomes in a Canadian Population. Dis. Colon Rectum 2020. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Saunders, M.P.; Schofield, P.F.; O′Dwyer, S.T. Patterns of Local Disease Failure and Outcome after Salvage Surgery in Patients with Anal Cancer. Br. J. Surg. 2005, 92, 605–614. [Google Scholar] [CrossRef]
- Su, P.-J.; Wu, M.-H.; Wang, H.-M.; Lee, C.-L.; Huang, W.-K.; Wu, C.-E.; Chang, H.-K.; Chao, Y.-K.; Tseng, C.-K.; Chiu, T.-K. Circulating Tumour Cells as an Independent Prognostic Factor in Patients with Advanced Oesophageal Squamous Cell Carcinoma Undergoing Chemoradiotherapy. Sci. Rep. 2016, 6, 31423. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Tessier, A.; Jeannot, E.; Guenat, D.; Debernardi, A.; Michel, M.; Proudhon, C.; Vincent-Salomon, A.; Bièche, I.; Pierga, J.-Y.; Buecher, B. Clinical Validity of HPV Circulating Tumor Dna in Advanced Anal Carcinoma: An Ancillary Study to the Epitopes-HPV02 Trial. Clin. Cancer Res. 2019, 25, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Damerla, R.R.; Lee, N.Y.; You, D.; Soni, R.; Shah, R.; Reyngold, M.; Katabi, N.; Wu, V.; McBride, S.M.; Tsai, C.J. Detection of Early Human Papillomavirus–Associated Cancers by Liquid Biopsy. JCO Precis. Oncol. 2019, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bertoldo, A.; Garolla, A.; Pizzol, D.; Mason, S.; Lenzi, A.; De Toni, L. Human Papillomavirus Proteins Are Found in Peripheral Blood and Semen Cd20+ and Cd56+ Cells during Hpv-16 Semen Infection. BMC Infect. Dis. 2013, 13, 593. [Google Scholar] [CrossRef] [PubMed]
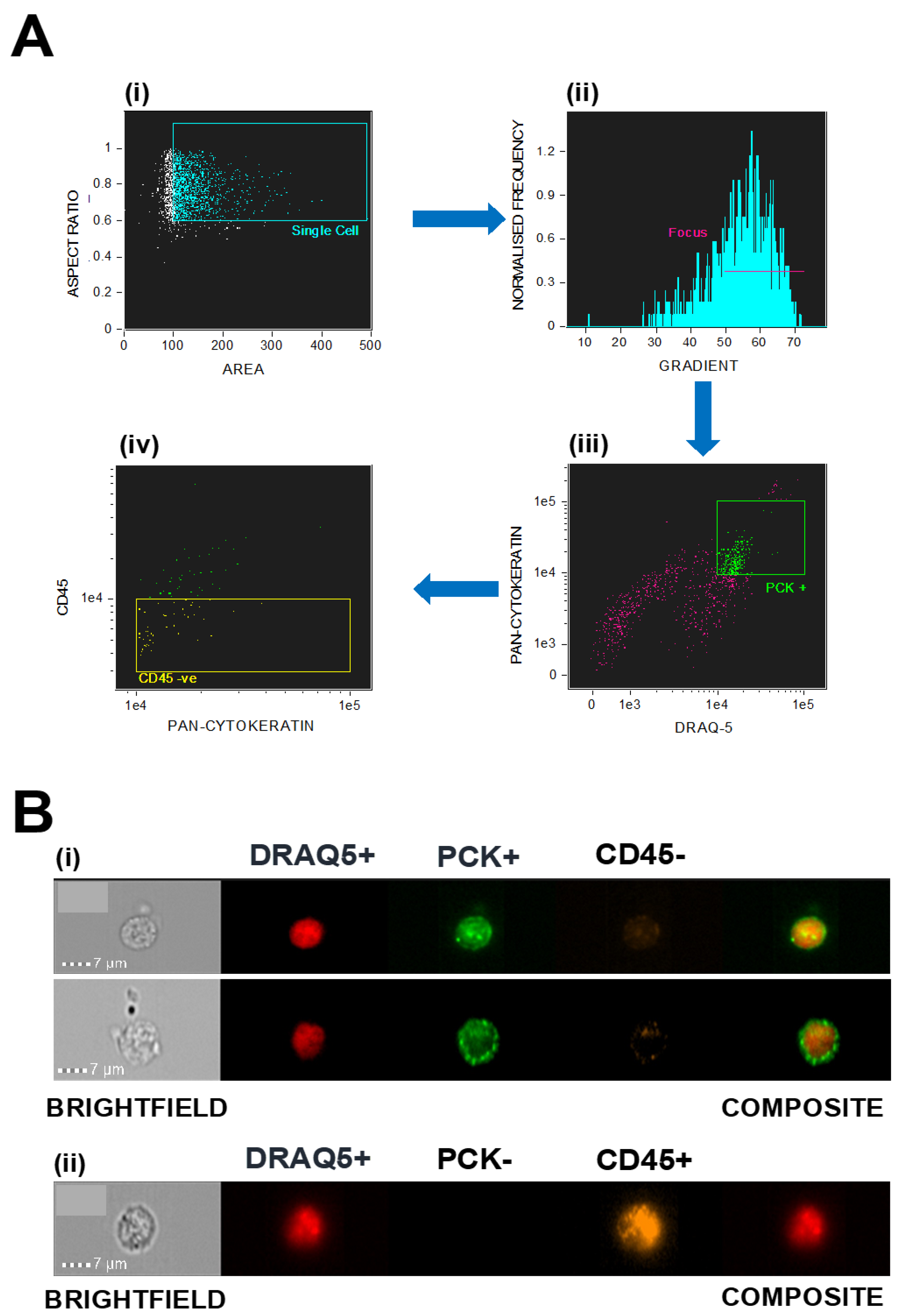
| Patient Number | Age at Diagnosis | Tumour Staging | Number of Samples | Imaging Follow Up |
|---|---|---|---|---|
| 1 | 51 | T4N0 | 1 | 12 m |
| 2 | 53 | T2N0 | 4 | 12 m |
| 3 | 64 | T1N0 | 4 | 6 m |
| 4 | 68 | T2N0 | 4 | 6 m |
| 5 | 74 | T1/2N0 | 3 | 6 m |
| 6 | 78 | T2N0 | 3 | 6 m |
| 7 | 53 | T2N1 | 1 | 6 m |
| 8 | 48 | T2N0 | 2 | EOT |
| Patient # | Baseline Sample | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| 1 | On Day 1 | NS | NS | NS |
| 2 | Before Day 1 | <3 m | >3 m | >3 m |
| 3 | On Day 1 | During CRT | <3 m | >3 m |
| 4 | Before Day 1 | During CRT | <3 m | >3 m |
| 5 | On Day 1 | During CRT | <3 m | NS |
| 6 | After Day 1 | During CRT | <3 m | NS |
| 7 | Before Day 1 | NS | NS | NS |
| 8 | Before Day 1 | During CRT | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, T.J.; Jeyaneethi, J.; Kumar, J.; Karteris, E.; Glynne-Jones, R.; Hall, M. Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients. Cancers 2020, 12, 2229. https://doi.org/10.3390/cancers12082229
Carter TJ, Jeyaneethi J, Kumar J, Karteris E, Glynne-Jones R, Hall M. Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients. Cancers. 2020; 12(8):2229. https://doi.org/10.3390/cancers12082229
Chicago/Turabian StyleCarter, Thomas J., Jeyarooban Jeyaneethi, Juhi Kumar, Emmanouil Karteris, Rob Glynne-Jones, and Marcia Hall. 2020. "Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients" Cancers 12, no. 8: 2229. https://doi.org/10.3390/cancers12082229
APA StyleCarter, T. J., Jeyaneethi, J., Kumar, J., Karteris, E., Glynne-Jones, R., & Hall, M. (2020). Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients. Cancers, 12(8), 2229. https://doi.org/10.3390/cancers12082229







