Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach
Abstract
:1. Introduction
2. Role of Inflammation, Oxidative Stress, Apoptosis, and Autophagy in Cancer Progression
3. Rutin: Sources and Pharmacological Effects
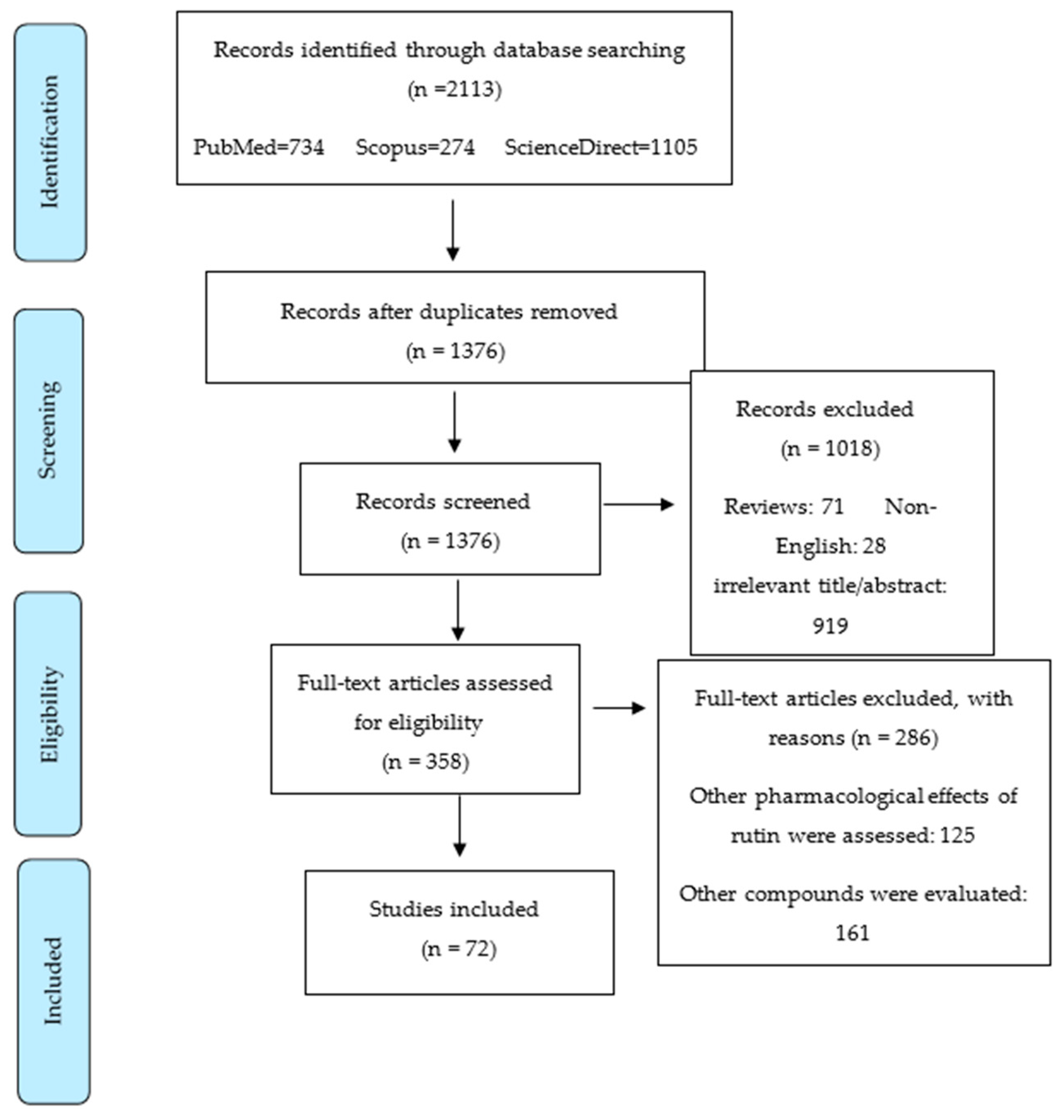
4. Methodology for Literature Search on Rutin and Cancer
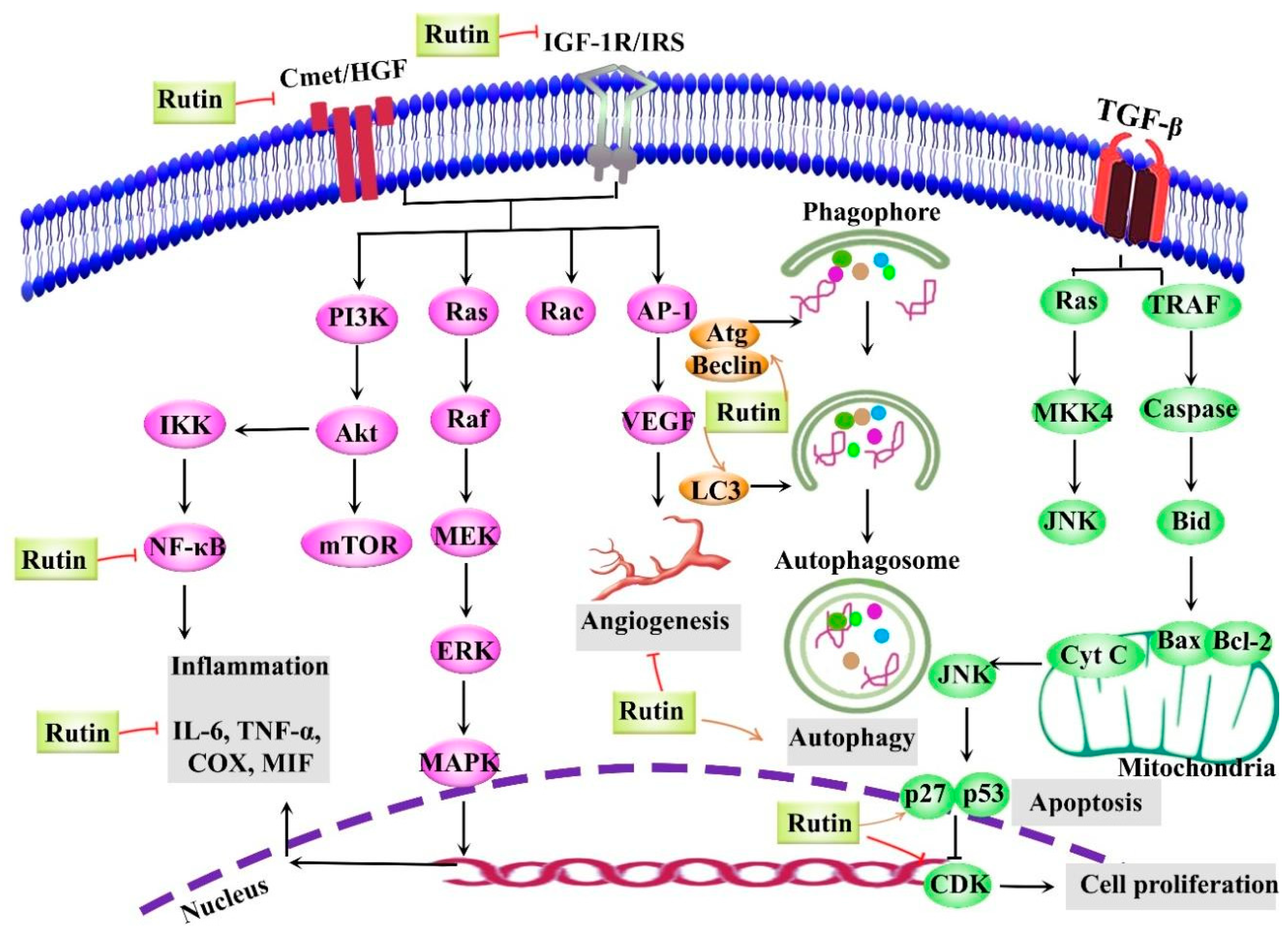
5. Anticancer Activities of Rutin
5.1. Rutin and Breast Cancer
5.2. Rutin and Lung Cancer
5.3. Rutin and Colon Cancer
5.4. Rutin and Brain Cancer
5.5. Rutin and Leukemia/Multiple Myeloma/Lymphoma
5.6. Rutin and Liver Cancer
5.7. Rutin and Gastric Cancer
5.8. Rutin and Prostate Cancer
5.9. Rutin and Other Cancers
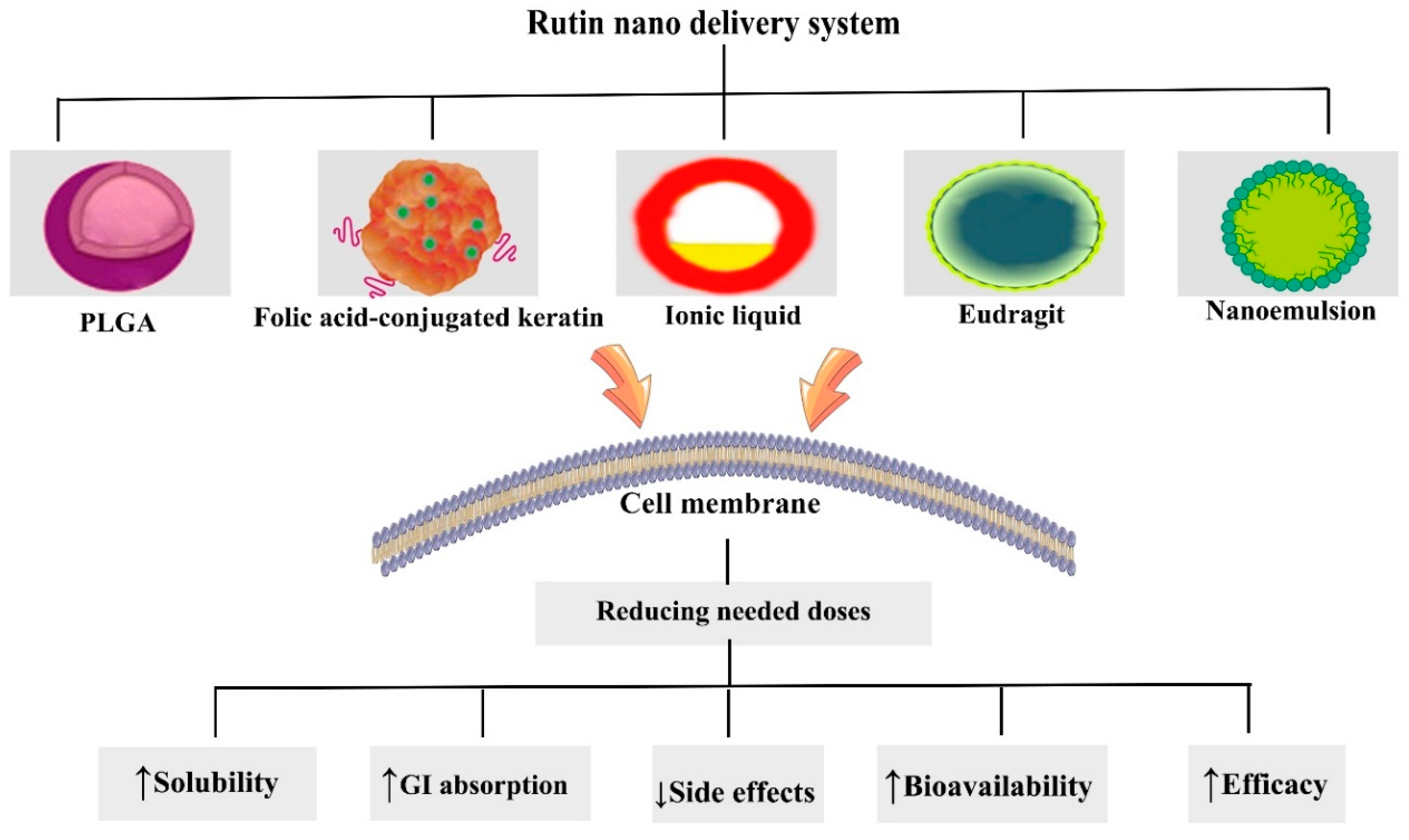
6. Nanostructured Formulations of Rutin in Combating Cancer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Henson, E.S.; Xiao, W.; Huang, D.; McMillan-Ward, E.M.; Israels, S.J.; Gibson, S.B. Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 2016, 12, 1029–1046. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M.; Pourgholami, M.H. The effects of anticancer medicinal herbs on vascular endothelial growth factor based on pharmacological aspects: A review study. Nutr. Cancer 2019, 1–15. [Google Scholar] [CrossRef]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef]
- Slattery, M.L.; Herrick, J.S.; Mullany, L.E.; Samowitz, W.S.; Sevens, J.R.; Sakoda, L.; Wolff, R.K. The co-regulatory networks of tumor suppressor genes, oncogenes, and miRNAs in colorectal cancer. Genes Chromosomes Cancer 2017, 56, 769–787. [Google Scholar] [CrossRef] [Green Version]
- Croce, C.M.; Reed, J.C. Finally, an apoptosis-targeting therapeutic for cancer. Cancer Res. 2016, 76, 5914–5920. [Google Scholar] [CrossRef] [Green Version]
- Monkkonen, T.; Debnath, J. Inflammatory signaling cascades and autophagy in cancer. Autophagy 2018, 14, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postovit, L.; Widmann, C.; Huang, P.; Gibson, S.B. Harnessing oxidative stress as an innovative target for cancer therapy. Hindawi 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mileo, A.M.; Miccadei, S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, L.; Li, B.; Liu, C.; Hong, S.; Min, J.; Hong, L. Knockdown of hypoxia-inducible factor 1α (HIF-1α) promotes autophagy and inhibits Phosphatidylinositol 3-Kinase (PI3K)/AKT/Mammalian target of rapamycin (mTOR) signaling pathway in ovarian cancer cells. Med. Sci. Monit. 2019, 25, 4250–4263. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Ajami, M.; Maleki, S.N.; Pazoki-Toroudi, H.; Daglia, M.; Sokeng, A.J.T.; Di Lorenzo, A.; Nabavi, S.F.; Devi, K.P.; Nabavi, S.M. Targeting signal transducers and activators of transcription (STAT) in human cancer by dietary polyphenolic antioxidants. Biochimie 2017, 142, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Yarla, N.S.; Ambra, R.; Pastore, G.; Perry, G. MAPK signalling pathway in cancers: Olive products as cancer preventive and therapeutic agents. Semin. Cancer Biol. 2019, 56, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 transcription factors as regulators of immune responses in cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef] [Green Version]
- Marelli, G.; Sica, A.; Vannucci, L.; Allavena, P. Inflammation as target in cancer therapy. Curr. Opin. Pharmacol. 2017, 35, 57–65. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jaafari, M.; Mirzaei, H.; Mirzaei, H. Mesenchymal stem cell: A new horizon in cancer gene therapy. Cancer Gene Ther. 2016, 23, 285–286. [Google Scholar] [CrossRef]
- Fujishiro, T.; Nonoguchi, N.; Pavliukov, M.; Ohmura, N.; Kawabata, S.; Park, Y.; Kajimoto, Y.; Ishikawa, T.; Nakano, I.; Kuroiwa, T. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn. Ther. 2018, 24, 58–68. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Castillo, R.R.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef]
- Khurana, R.K.; Jain, A.; Jain, A.; Sharma, T.; Singh, B.; Kesharwani, P. Administration of antioxidants in cancer: Debate of the decade. Drug Discov. Today 2018, 23, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anticancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; De Luca, C.; Kostyuk, V.; Pastore, S. Plant polyphenols and tumors: From mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009, 16, 3943–3965. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Khodamorady, M.; Naseri, M.; Farzaei, M.H.; Khan, H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020, 159, 104895. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Zanoaga, O.; Zimta, A.-A.; Tigu, A.B.; Kilpatrick, K.L.; Bishayee, A.; Nabavi, S.M.; Berindan-Neagoe, I. Natural compounds modulate the crosstalk between apoptosis-and autophagy-regulated signaling pathways: Controlling the uncontrolled expansion of tumor cells. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Satari, A.; Amini, S.A.; Raeisi, E.; Lemoigne, Y.; Heidarian, E. Synergetic impact of combined 5-fluorouracil and rutin on apoptosis in PC3 cancer cells through the modulation of P53 gene expression. Adv. Pharm. Bull. 2019, 9, 462. [Google Scholar] [CrossRef] [Green Version]
- ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Song, H.-l.; Zhang, X.; Wang, W.-z.; Liu, R.-h.; Zhao, K.; Liu, M.-y.; Gong, W.-m.; Ning, B. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway. Neural Regen Res. 2018, 13, 128. [Google Scholar]
- Gautam, R.; Singh, M.; Gautam, S.; Rawat, J.K.; Saraf, S.A.; Kaithwas, G. Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement. Altern. Med. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-Y.; Hsiu, S.-L.; Wen, K.-C.; Lin, S.-P.; Tsai, S.-Y. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J. Food Drug Anal. 2005, 13. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Z.; Fan, L.; Meng, S.; Song, C.; Qiu, L.; Xu, P.; Chen, J. Dietary supplementation with rutin has pro-/anti-inflammatory effects in the liver of juvenile GIFT tilapia, Oreochromis niloticus. Fish. Shellfish Immunol. 2017, 64, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.-R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef]
- Prasad, R.; Prasad, S.B. A review on the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J. Pharm. Pharmacol. 2019, 5, 1–20. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi. Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Perk, A.A.; Shatynska-Mytsyk, I.; Gerçek, Y.C.; Boztaş, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta Rev. Cancer 2014, 1845, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Tsao, S.-m.; Hsia, T.-c.; Yin, M.-c. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-κB pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- de Pasquali, M.A.B.; Gelain, D.P.; Zeidán-Chuliá, F.; Pires, A.S.; Gasparotto, J.; Terra, S.R.; Moreira, J.C.F. Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell Signal. 2013, 25, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, L.; Zhang, M.; Li, R.; Li, Y.; Hu, X.; Wang, S.; Bao, Z. Characterization of three mitogen-activated protein kinases (MAPK) genes reveals involvement of ERK and JNK, not p38 in defense against bacterial infection in Yesso scallop Patinopecten yessoensis. Fish Shellfish Immunol. 2016, 54, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Piao, H.; Jiang, J.; Jin, G.; Zheng, M.; Yang, J.; Jin, X.; Sun, T.; Choi, Y.H.; Li, L. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Teplinsky, E.; Silvera, D.; Valeta-Magara, A.; Arju, R.; Giashuddin, S.; Sarfraz, Y.; Alexander, M.; Darvishian, F.; Levine, P.H. Hyperactivated mTOR and JAK2/STAT3 pathways: Molecular drivers and potential therapeutic targets of inflammatory and invasive ductal breast cancers after neoadjuvant chemotherapy. Clin. Breast Cancer 2016, 16, 113–122.e1. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, H.Y.; Elsayed, H.E.; Mohyeldin, M.M.; Akl, M.R.; Bhattacharjee, J.; Egbert, S.; Sayed, K.A.E. Norstictic acid inhibits breast cancer cell proliferation, migration, invasion, and in vivo invasive growth through targeting C-Met. Phytother. Res. 2016, 30, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Merrett, N.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Targeting the HGF/c-MET pathway: Stromal remodelling in pancreatic cancer. Oncotarget 2017, 8, 76722. [Google Scholar] [CrossRef] [Green Version]
- Vahidnezhad, H.; Youssefian, L.; Uitto, J. Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses: Diagnostic implications and treatment opportunities. J. Invest. Dermatol. 2016, 136, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-Y.; Yu, S.-N.; Lee, S.-Y.; Chun, S.-S.; Choi, Y.-L.; Park, Y.-M.; Song, C.S.; Chatterjee, B.; Ahn, S.-C. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem. Biophys. Res. Commun. 2011, 413, 80–86. [Google Scholar] [CrossRef]
- Kongara, S.; Karantza, V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012, 2, 171. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.M. The dual role of oxidative stress in lung cancer. In Oxidative Stress in Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–113. [Google Scholar]
- Menegon, S.; Columbano, A.; Giordano, S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic regulation of apoptosis in cancer. Int. Rev. Cell Mol. Biol. 2016, 327, 43–87. [Google Scholar] [PubMed] [Green Version]
- Safa, A.R. Cancer stem cells, apoptosis pathways and mechanisms of death resistance. In Oncogenomics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–101. [Google Scholar]
- Hongmei, Z. Extrinsic and intrinsic apoptosis signal pathway review. In Apoptosis and Medicine; InTechOpen: London, UK, 2012. [Google Scholar]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akl, M.R.; Elsayed, H.E.; Ebrahim, H.Y.; Haggag, E.G.; Kamal, A.M.; Sayed, K.A.E. 3-O-[N-(p-fluorobenzenesulfonyl)-carbamoyl]-oleanolic acid, a semisynthetic analog of oleanolic acid, induces apoptosis in breast cancer cells. Eur. J. Pharmacol. 2014, 740, 209–217. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [Green Version]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [Green Version]
- Elena-Real, C.A.; Díaz-Quintana, A.; González-Arzola, K.; Velázquez-Campoy, A.; Orzáez, M.; López-Rivas, A.; Gil-Caballero, S.; Miguel, Á.; Díaz-Moreno, I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.S. The functional interactions between the MAPK and p53 signaling pathways. Cancer Biol. Ther. 2004, 3, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.-Z.; Bacon, P.; Chaturvedi, V.; Nickoloff, B.J. Role of NF-κB activity in apoptotic response of keratinocytes mediated by interferon-γ, tumor necrosis factor-α, and tumor-necrosis-factor-related apoptosis-inducing ligand. J. Invest. Dermatol. 2001, 117, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.-Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, B.; Wang, Z.; Zhang, W.; Hao, K.; Chen, Y.; Li, K.; Wang, T.; Xie, Y.; Huang, Z. Marsdenia tenacissimae extraction (MTE) inhibits the proliferation and induces the apoptosis of human acute T cell leukemia cells through inactivating PI3K/AKT/mTOR signaling pathway via PTEN enhancement. Oncotarget 2016, 7, 82851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Tortola, L.; Perlot, T.; Wirnsberger, G.; Novatchkova, M.; Nitsch, R.; Sykacek, P.; Frank, L.; Schramek, D.; Komnenovic, V. A dual role for autophagy in a murine model of lung cancer. Nat. Commun. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.S.; Vats, S.; Chia, A.Y.-Q.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Dower, C.M.; Wills, C.A.; Frisch, S.M.; Wang, H.-G. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy 2018, 14, 1110–1128. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, X.; Li, Y.; Lu, S.; Lu, S.; Li, J.; Wang, Y.; Tian, X.; Wei, J.-j.; Shao, C. Neferine induces autophagy of human ovarian cancer cells via p38 MAPK/JNK activation. Tumor. Biol. 2016, 37, 8721–8729. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Park, K.-I.; Kim, S.-H.; Yu, S.-N.; Park, S.-G.; Kim, Y.W.; Seo, Y.-K.; Ma, J.-Y.; Ahn, S.-C. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Ro, S.-H.; Cao, J.; Otto, N.M.; Kim, D.-H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 457–479. [Google Scholar]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Abdullah, Y.; Schneider, B.; Petersen, M. Occurrence of rosmarinic acid, chlorogenic acid and rutin in Marantaceae species. Phytochem. Lett. 2008, 1, 199–203. [Google Scholar] [CrossRef]
- Kreft, S.; Štrukelj, B.; Gaberščik, A.; Kreft, I. Rutin in buckwheat herbs grown at different UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002, 53, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Abdel-Ghaffar, O.; Mahmoud, S.T.; Said, A.A.; Sanad, F.A.-A.Y. Hepatoprotective effect of rutin against oxidative stress of Isoniazid in albino rats. Int. J. Pharmacol. 2017, 13, 516–528. [Google Scholar]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Vladimir-Knežević, S.; Cvijanović, O.; Tadić, Ž.; Romić, Ž.; Rahelić, D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl 4-intoxicated BALB/cN mice. Acta Pharmacol. Sin. 2012, 33, 1260–1270. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Chen, X.-Y.; Zhang, J.; Yuan, Y.-L.; Zhao, W.; Wei, B. Upregulation of SIRT1 contributes to the cardioprotective effect of Rutin against myocardial ischemia-reperfusion injury in rats. J. Funct. Foods 2018, 46, 227–236. [Google Scholar] [CrossRef]
- Sheu, J.-R.; Hsiao, G.; Chou, P.-H.; Shen, M.-Y.; Chou, D.-S. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J. Agric. Food Chem. 2004, 52, 4414–4418. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.-h.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ugusman, A.; Zakaria, Z.; Chua, K.H.; Nordin, N.A.M.M.; Mahdy, Z.A. Role of rutin on nitric oxide synthesis in human umbilical vein endothelial cells. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Maoqiang, L.; Fan, H.; Zhenyu, B.; Qifang, H.; Xuepeng, W.; Liulong, Z. Rutin attenuates neuroinflammation in spinal cord injury rats. J. Surg. Res. 2016, 203, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Radwan, R.R.; Fattah, S.M.A. Mechanisms involved in the possible nephroprotective effect of rutin and low dose γ irradiation against cisplatin-induced nephropathy in rats. J. Photochem. Photobiol. B 2017, 169, 56–62. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Lang, F.; Hu, L.; Tang, Q.; Wang, H.; Zhang, Y.; Hao, Z. Rutin attenuates vancomycin-induced nephrotoxicity by ameliorating oxidative stress, apoptosis, and inflammation in rats. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Caglayan, C.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Eser, G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019, 54, 69–78. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Kravchenko, J.; Akushevich, I.; Seewaldt, V.L.; Abernethy, A.P.; Lyerly, H.K. Breast cancer as heterogeneous disease: Contributing factors and carcinogenesis mechanisms. Breast Cancer Res. Treat. 2011, 128, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Biswas, J.; Nabavi, S.M.; Bishayee, A. Tea phytochemicals for breast cancer prevention and intervention: From bench to bedside and beyond. Semin. Cancer Biol. 2017, 46, 33–54. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Katdare, M.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. The nutritional herb Epimedium grandiflorum inhibits the growth in a model for the Luminal A molecular subtype of breast cancer. Oncol. Lett. 2017, 13, 2477–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agelaki, S.; Dragolia, M.; Markonanolaki, H.; Alkahtani, S.; Stournaras, C.; Georgoulias, V.; Kallergi, G. Phenotypic characterization of circulating tumor cells in triple negative breast cancer patients. Oncotarget 2017, 8, 5309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, M.M.; Akl, M.R.; Ebrahim, H.Y.; Dragoi, A.M.; Dykes, S.; Cardelli, J.A.; Sayed, K.A.E. The oleocanthal-based homovanillyl sinapate as a novel c-Met inhibitor. Oncotarget 2016, 7, 32247. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Kamal, A.M.; Haggag, E.G.; El Sayed, K.A. Rutin as a novel c-Met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer 2017, 69, 1256–1271. [Google Scholar] [CrossRef]
- Hasani, N.A.H.; Amin, I.M.; Kamaludin, R.; Rosdyd, N.M.M.N.M.; Ibahim, M.J.; Kadir, S.H.S.A. P53 and cyclin B1 mediate apoptotic effects of apigenin and rutin in ERα+-breast cancer MCF-7 cells. J. Teknol. 2018, 80. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Mohana, S.; Ganesan, M.; Rajendra Prasad, N.; Ananthakrishnan, D.; Velmurugan, D. Flavonoids modulate multidrug resistance through wnt signaling in P-glycoprotein overexpressing cell lines. BMC Cancer 2018, 18, 1168. [Google Scholar] [CrossRef] [Green Version]
- Schindler, R.; Mentlein, R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006, 136, 1477–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, H.; Yang, X.; Zhao, H.; Zhu, Y. Evaluation of antiproliferative activities of rutin on human colon cancer lovo cells and breast cancer MCF-7 cells. Anal. Quant. Cytopathol. Histopathol. 2017, 39, 99–107. [Google Scholar]
- Zhu, H.; Wang, Y.; Liu, D.; Sun, X.; Wang, F. Vanadium-rutin complex sensitizes breast cancer cells via modulation of p53/BAx/BCl2/VEGF correlated with apoptotic events. Acta Pol. Pharm. Drug Res. 2020, 77, 89–98. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Theivendran, P.; Baskararaj, S.; Sankaranarayanan, B.; Palanisamy, P.; Saravanan, G.; Arunachalam, S.; Sankaranarayanan, M.; Natarajan, J.; Somasundaram, B. Modeling a pH-sensitive Zein-co-acrylic acid hybrid hydrogels loaded 5-fluorouracil and rutin for enhanced anticancer efficacy by oral delivery. Biotechnology 2019, 9, 185. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Fan, L.M.; Liu, K.; Zhang, N.; Li, S.W.; Zhu, H.; Gao, H.C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp. Ther. Med. 2017, 14, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Yeh, S.L.; Wang, W.Y.; Huang, C.S.; Hu, M.L. Flavonoids suppresses the enhancing effect of beta-carotene on DNA damage induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A549 cells. Chem. Biol. Interact. 2006, 160, 175–182. [Google Scholar] [CrossRef]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer lett 1995, 95, 221–225. [Google Scholar] [CrossRef]
- Conesa, C.M.; Ortega, V.V.; Yáñez Gascón, M.J.; Baños, M.A.; Jordana, M.C.; Benavente-García, O.; Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, S.; Song, Y.-R.; Kim, S.; Kim, H.-J.; Na, H.S.; Chung, J. Rutin induces autophagy in cancer cells. Int. J. Oral Biol. 2016, 41, 45–51. [Google Scholar] [CrossRef]
- Li, X.-H.; Liu, Z.-Y.; Gu, Y.; Lv, Z.; Chen, Y.; Gao, H.-C. Expression of NF-kappaB and p38 under intervention of rutin in lung cancer therapy. Biomed Res 2017, 14, 2344–2347. [Google Scholar]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Castro, A.J.; Domínguez, F.; García-Carrancá, A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch. Med. Res. 2013, 44, 346–351. [Google Scholar] [CrossRef]
- Vijay, M.; Sivagami, G.; Thayalan, K.; Nalini, N. Radiosensitizing potential of rutin against human colon adenocarcinoma HT-29 cells. Bratisl. Lek. Listy 2016, 117, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic interaction of rutin and silibinin on human colon cancer cell line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, P.N.; Zareian, S.; Nayeri, Z.; Salmanipour, R.; Parsafar, S.; Gharib, E.; Aghdaei, H.A.; Zali, M.R. A detailed image of rutin underlying intracellular signaling pathways in human SW480 colorectal cancer cells based on miRNAs-lncRNAs-mRNAs-TFs interactions. J. Cell Physiol. 2019, 234, 15570–15580. [Google Scholar] [CrossRef]
- Jantrawut, P.; Akazawa, H.; Ruksiriwanich, W. Anti-cancer activity of rutin encapsulated in low methoxyl pectin beads. Int. J. Pharmcy Pharm. Sci. 2014, 6, 199–202. [Google Scholar]
- Aherne, S.A.; O’Brien, N.M. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr. Cancer 1999, 34, 160–166. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Lack of effect of the flavonoids, myricetin, quercetin, and rutin, on repair of H2O2-induced DNA single-strand breaks in Caco-2, Hep G2, and V79 cells. Nutr. Cancer 2000, 38, 106–115. [Google Scholar] [CrossRef]
- Deschner, E.E.; Ruperto, J.; Wong, G.; Newmark, H.L. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis 1991, 12, 1193–1196. [Google Scholar] [CrossRef]
- Volate, S.R.; Davenport, D.M.; Muga, S.J.; Wargovich, M.J. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis 2005, 26, 1450–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dihal, A.A.; de Boer, V.C.; van der Woude, H.; Tilburgs, C.; Bruijntjes, J.P.; Alink, G.M.; Rietjens, I.M.; Woutersen, R.A.; Stierum, R.H. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J. Nutr. 2006, 136, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Volková, M.; Forstová-Křížová, V.; Skálová, L.; Trejtnar, F. Modulatory effects of quercetin and rutin on the activity, expression and inducibility of CYP1A1 in intestinal HCT-8 cells. Phytother. Res. 2013, 27, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, M.V.W.; Van Erk, M.J.; Doornbos, R.P.; Krul, C.A.M.; Woutersen, R.A. Do aberrant crypt foci have predictive value for the occurrence of colorectal tumours? Potential of gene expression profiling in tumours. Food Chem. Toxicol. 2004, 42, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.; Silva, A.; Pitanga, B.; Sousa, C.; Grangeiro, M.; Fragomeni, B.; Coelho, P.; Oliveira, M.; Menezes-Filho, N.; Costa, M.F.D.; et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011, 127, 404–411. [Google Scholar] [CrossRef]
- Freitas, S.; Costa, S.; Azevedo, C.; Carvalho, G.; Freire, S.; Barbosa, P.; Velozo, E.; Schaer, R.; Tardy, M.; Meyer, R.; et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother. Res. 2011, 25, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.; Oliveira, M.N.; Coelho, P.L.C.; Pitanga, B.P.S.; da Silva, A.B.; Adelita, T.; Silva, V.D.A.; Costa, M.F.D.; El-Bachá, R.S.; Tardy, M.; et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem. Biol. Interact. 2015, 242, 123–138. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Hao, Y.; Chen, S.; Jia, G.; Guo, Y.; Zhang, G.; Wang, C.; Cheng, R.; Hu, T.; Zhang, X. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl. Cancer Res. 2019, 8, 2005–2013. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, S.; Li, N.; Ho, A.S.W.; Kiang, K.M.Y.; Zhang, X.; Cheng, Y.S.; Poon, M.W.; Lee, D.; Pu, J.K.S. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J. Neurooncol. 2017, 132, 393–400. [Google Scholar] [CrossRef]
- Bourogaa, E.; Bertrand, J.; Despeaux, M.; Jarraya, R.; Fabre, N.; Payrastre, L.; Demur, C.; Fournié, J.-J.; Damak, M.; Feki, A.E. Hammada scoparia flavonoids and rutin kill adherent and chemoresistant leukemic cells. Leuk. Res. 2011, 35, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Yang, J.S.; Lin, J.J.; Lai, K.C.; Lu, H.F.; Ma, C.Y.; Sai-Chuen Wu, R.; Wu, K.C.; Chueh, F.S.; Wood, W.G. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012, 27, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-P.; Yang, J.-S.; Lu, C.-C.; Chiang, J.-H.; Wu, C.-L.; Lin, J.-J.; Lin, H.-L.; Yang, M.-D.; Liu, K.-C.; Chiu, T.-H. Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo. Leuk. Res. 2009, 33, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Huang, C.-S.; Hu, M.-L. Vitamin E and rutin synergistically inhibit expression of vascular endothelial growth factor through down-regulation of binding activity of activator protein-1 in human promyelocytic leukemia (HL-60) cells. Chem. Biol. Interact. 2010, 183, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.E.A.; Novak, E.M.; Maria, D.A.; Velosa, A.S.; Pereira, R.M.S. Synthesis, characterization and biological evaluation of Rutin–zinc (II) flavonoid-metal complex. Chem. Biol. Interact. 2015, 239, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Horváthová, K.; Novotný, L.; Tóthová, D.; Vachálková, A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma 2004, 51, 395–399. [Google Scholar]
- Dedoussis, G.V.Z.; Kaliora, A.C.; Andrikopoulos, N.K. Effect of phenols on natural killer (NK) cell-mediated death in the K562 human leukemic cell line. Cell Biol. Int. 2005, 29, 884–889. [Google Scholar] [CrossRef]
- Shen, S.C.; Chen, Y.C.; Hsu, F.L.; Lee, W.R. Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J. Cell Biochem. 2003, 89, 1044–1055. [Google Scholar] [CrossRef]
- Nadova, S.; Miadokova, E.; Cipak, L. Flavonoids potentiate the efficacy of cytarabine through modulation of drug-induced apoptosis. Neoplasma 2007, 54, 202–206. [Google Scholar]
- Canturk, Z.; Dikmen, M.; Artagan, O.; Ozarda, M.G.; Ozturk, N. Cytotoxic effects of resveratrol, rutin and rosmarinic acid on ARH–77 human (multiple myeloma) cell line. Nat. Prod. Commun. 2016, 11, 1441–1444. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Banerjee, S.; Kharshiing, C.; Bhattacharjee, A.; Prasad, S. Rutin-mediated apoptosis and glutathione changes in ascites daltons lymphoma cells: In silico analysis of rutin interactions with some antiapoptotic and glutathione-related proteins. Indian J. Pharm. Sci. 2019, 81, 720–728. [Google Scholar] [CrossRef]
- Marcarini, J.C.; Tsuboy, M.S.F.; Luiz, R.C.; Ribeiro, L.R.; Hoffmann-Campo, C.B.; Mantovani, M.S. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, S. Modulatory effects of rutin on the expression of cytochrome P450s and antioxidant enzymes in human hepatoma cells. Acta Pharm. 2016, 66, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Alía, M.; Mateos, R.; Ramos, S.; Lecumberri, E.; Bravo, L.; Goya, L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur. J. Nutr. 2006, 45, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Jang, H.D. Protective mechanism of quercetin and rutin using glutathione metabolism on HO-induced oxidative stress in HepG2 cells. Ann. N. Y. Acad. Sci. 2009, 1171, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Labh, A.K.; Priya, V.V.; Gayathri, R. Cytotoxic action of rutin isolated from Morinda citrifolia against hepatic carcinoma cell lines. Drug Invent. Today 2019, 12, 1904–1907. [Google Scholar]
- Chandra, Y.P.; Viswanathswamy, A. Chemopreventive effect of Rutin against N-nitrosodiethylamine-induced and phenobarbital-promoted hepatocellular carcinoma in Wistar rats. Indian J. Pharm. Educ. Res. 2018, 52, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.; Gawde, M.; Bhattacharya, R. Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett. 1996, 109, 185–191. [Google Scholar] [CrossRef]
- Li, Q.; Ren, L.; Zhang, Y.; Gu, Z.; Tan, Q.; Zhang, T.; Qin, M.; Chen, S. P38 signal transduction pathway has more cofactors on apoptosis of SGC-7901 gastric cancer cells induced by combination of rutin and oxaliplatin. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- George, K.; Malathi, R. Influence of the novel anticancer agents on the activity of outward rectifier potassium currents in human prostate cancer cell line-LNCaP. Asian J. Pharm. 2017, 11, S603–S608. [Google Scholar]
- Roy, A.S.; Tripathy, D.R.; Samanta, S.; Ghosh, S.K.; Dasgupta, S. DNA damaging, cell cytotoxicity and serum albumin binding efficacy of the rutin-Cu(ii) complex. Mol. Biosyst. 2016, 12, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Guruvayoorappan, C.; Kuttan, G. Antiangiogenic effect of rutin and its regulatory effect on the production of VEGF, IL-1β and TNF-α in tumor associated macrophages. J. Biol. Sci. 2007, 7, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, M.; Yu, Y.; He, J.; Hu, S.; Pan, M.; Lu, S.; Liao, K.; Pan, Z.; Zhou, Y. Effects of harmaline on cell growth of human liver cancer through the p53/p21 and Fas/FasL signaling pathways. Oncol. Lett. 2018, 15, 1931–1936. [Google Scholar] [CrossRef] [Green Version]
- Keay, S.; Nallar, S.C.; Gade, P.; Zhang, C.-O.; Kalvakolanu, D.V. Oncosuppressor protein p53 and cyclin-dependent kinase inhibitor p21 regulate interstitial cystitis associated gene expression. Cytokine 2018, 110, 110–115. [Google Scholar] [CrossRef]
- Munir, M.T.; Ponce, C.; Powell, C.A.; Tarafdar, K.; Yanagita, T.; Choudhury, M.; Gollahon, L.S.; Rahman, S.M. The contribution of cholesterol and epigenetic changes to the pathophysiology of breast cancer. J. Steroid Biochem. Mol. Biol. 2018, 183, 1–9. [Google Scholar] [CrossRef]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019, 21, 35. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 2016, 7, 63679. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559. [Google Scholar] [CrossRef] [Green Version]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, B.D.; Leslie, E.M. Efflux transporters in anti-cancer drug resistance: Molecular and functional identification and characterization of multidrug resistance proteins (MRPs/ABCCs). In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–65. [Google Scholar]
- Das, M.; Law, S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018, 103, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Frascini, F.; Iriti, M.; Maestri, P.; Rimondini, L.; Catalano, E.; Megna, S. Compositions Comprising Rutin Useful for the Treatment of Tumors Resistant to Chemotherapy. CN Patent Application No. CN105611945A, 25 May 2016. [Google Scholar]
- de Oliveira, C.T.; Colenci, R.; Pacheco, C.C.; Mariano, P.M.; do Prado, P.R.; Mamprin, G.P.R.; Santana, M.G.; Gambero, A.; de Carvalho, P.O.; Priolli, D.G. Hydrolyzed rutin decreases worsening of anaplasia in glioblastoma relapse. CNS Neurol. Disord. Drug Targets 2019, 18, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Moghanjoughi, A.A.; Khoshnevis, D.; Zarrabi, A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Silva Jr, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Judd, J.; Borghaei, H. Combining immunotherapy and chemotherapy for non–small cell lung cancer. Thorac. Surg. Clin. 2020, 30, 199–206. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shueng, P.W.; Chou, C.M.; Lin, B.X.; Lin, M.H.; Kuo, D.Y.; Tsai, I.L.; Wu, S.M.; Lin, C.W. Elevation of CD109 promotes metastasis and drug resistance in lung cancer via activation of EGFR-AKT-mTOR signaling. Cancer Sci. 2020, 111, 1652. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Zhang, X.; He, J.; Pan, Y.; Hao, F.; Xie, L.; Li, Q.; Qiu, X.; Wang, E. Inhibition of cytoplasmic GSK-3β increases cisplatin resistance through activation of Wnt/β-catenin signaling in A549/DDP cells. Cancer Lett. 2013, 336, 231–239. [Google Scholar] [CrossRef]
- Filla, M.S.; Dimeo, K.D.; Tong, T.; Peters, D.M. Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp. Eye Res. 2017, 165, 7–19. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, N.-J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stramucci, L.; Pranteda, A.; Bossi, G. Insights of crosstalk between p53 protein and the MKK3/MKK6/p38 MAPK signaling pathway in cancer. Cancers 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradham, C.; McClay, D.R. p38 MAPK in development and cancer. Cell Cycle 2006, 5, 824–828. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, S.; Cao, L.; Ren, X.; Li, Y.; Shao, J.; Xu, L. Lead acetate induces apoptosis in Leydig cells by activating PPARγ/caspase-3/PARP pathway. Int. J. Environ. Health Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Qi, X.; Du, L.; Chen, X.; Chen, L.; Yi, T.; Chen, X.; Wen, Y.; Wei, Y.; Zhao, X. VEGF-D-enhanced lymph node metastasis of ovarian cancer is reversed by vesicular stomatitis virus matrix protein. Int. J. Oncol. 2016, 49, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Jiangjiang, Q.; Wei, W.; Zhang, R. Novel natural product therapeutics targeting both inflammation and cancer. Chin. J. Nat. Med. 2017, 15, 401–416. [Google Scholar]
- Fakhri, S.; Moradi, S.Z.; Farzaei, M.H.; Bishayee, A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Su, S.; Wang, J.; Vargas, E.; Wei, J.; Martínez-Zaguilán, R.; Sennoune, S.R.; Pantoya, M.L.; Wang, S.; Chaudhuri, J.; Qiu, J. Porphyrin immobilized nanographene oxide for enhanced and targeted photothermal therapy of brain cancer. ACS Biomater. Sci. Eng. 2016, 2, 1357–1366. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Sivaprasad, U.; Basu, A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-α-induced cell death in MCF-7 cells. J. Cell Mol. Med. 2008, 12, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Zeid, R.; Lawlor, M.A.; Poon, E.; Reyes, J.M.; Fulciniti, M.; Lopez, M.A.; Scott, T.G.; Nabet, B.; Erb, M.A.; Winter, G.E. Enhancer invasion shapes MYCN-dependent transcriptional amplification in neuroblastoma. Nat. Genet. 2018, 50, 515–523. [Google Scholar] [CrossRef]
- Moosavi, M.A.; Haghi, A.; Rahmati, M.; Taniguchi, H.; Mocan, A.; Echeverría, J.; Gupta, V.K.; Tzvetkov, N.T.; Atanasov, A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018, 424, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Autophagy in cancer therapy. Front. Oncol. 2017, 7, 128. [Google Scholar] [CrossRef]
- Vasilevskaya, I.A.; Selvakumaran, M.; Roberts, D.; O’Dwyer, P.J. JNK1 inhibition attenuates hypoxia-induced autophagy and sensitizes to chemotherapy. Mol. Cancer Res. 2016, 14, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saultz, J.N.; Garzon, R. Acute myeloid leukemia: A concise review. J. Clin. Med. 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddiconto, G.; Toto, C.; Palamà, I.; De Leo, S.; De Luca, E.; De Matteis, S.; Dini, L.; Passerini, C.G.; Di Renzo, N.; Maffia, M. Targeting of GSK3β promotes imatinib-mediated apoptosis in quiescent CD34+ chronic myeloid leukemia progenitors, preserving normal stem cells. Blood 2012, 119, 2335–2345. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, C.; Li, X.; Ding, K. Sulfated polysaccharide JCS1S2 inhibits angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Carbohydr. Polym. 2019, 207, 502–509. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, H.L.; Gu, C.J.; Liu, Y.K.; Shao, J.; Zhu, R.; He, Y.Y.; Zhu, X.Y.; Li, M.Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.; Chen, F.; Chen, J.; Ruan, G.; He, M.; Chen, C.; Tang, J.; Wang, D.W. Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.H.; Nazir, M.; Muhammad, W.; Hashmi, S.S.; Abbasi, R.; Rahman, L.; Hano, C. A comparative evaluation of the antiproliferative activity against Hepg2 liver carcinoma cells of plant-derived silver nanoparticles from basil extracts with contrasting anthocyanin contents. Biomolecules 2019, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-L.; Wu, Q.; Tao, Y.-Y.; Liu, C.-H.; El-Nezami, H. Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 502–508. [Google Scholar] [CrossRef]
- Aloud, A.A.; Veeramani, C.; Govindasamy, C.; Alsaif, M.A.; Al-Numair, K.S. Galangin ameliorates changes of membrane–bound enzymes in rats with streptozotocin–induced hyperglycemia. Asian Pac. J. Trop. Biomed. 2019, 9, 284. [Google Scholar]
- Rajamanickam, E.; Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Chemopreventive effect of Acanthus ilicifolius extract on modulating antioxidants, lipid peroxidation and membrane bound enzymes in diethyl nitrosamine induced liver carcinogenesis. Int. J. Cancer Res. 2016, 12, 1–16. [Google Scholar]
- Nriagu, J.; Darroudi, F.; Shomar, B. Health effects of desalinated water: Role of electrolyte disturbance in cancer development. Environ. Res. 2016, 150, 191–204. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Sun, Q.; Zang, W.; Li, M.; Dong, Z.; Zhao, G. Retraction note: Overexpression of A613T and G462T variants of DNA polymerase β weakens chemotherapy sensitivity in esophageal cancer cell lines. Cancer Cell Int. 2019, 19, 19. [Google Scholar] [CrossRef] [Green Version]
- Sallmyr, A.; Matsumoto, Y.; Roginskaya, V.; Van Houten, B.; Tomkinson, A.E. Inhibiting mitochondrial DNA ligase IIIα activates caspase 1–Dependent apoptosis in cancer cells. Cancer Res. 2016, 76, 5431–5441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.Y.; Kraus, W.L. PARP inhibitors for cancer therapy. Cell 2017, 169, 183. [Google Scholar] [CrossRef] [PubMed]
- Haghi, A.; Azimi, H.; Rahimi, R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Berlth, F.; Bollschweiler, E.; Drebber, U.; Hoelscher, A.H.; Moenig, S. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. 2014, 20, 5679. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.-Q.; Li, Q.; Zhang, Y. Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed. Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Blank, M. Targeting p38 MAP kinase signaling in cancer through post-translational modifications. Cancer Lett. 2017, 384, 19–26. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 pathway: From biology to cancer therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef] [Green Version]
- Kawan, M.A.; Kyrou, I.; Ramanjaneya, M.; Williams, K.; Jeyaneethi, J.; Randeva, H.S.; Karteris, E. Involvement of the glutamine RF-amide peptide and its cognate receptor GPR103 in prostate cancer. Oncol. Rep. 2019, 41, 1140–1150. [Google Scholar] [CrossRef]
- Abidi, S.H.; Bilwani, F.; Ghias, K.; Abbas, F. Viral etiology of prostate cancer: Genetic alterations and immune response. A literature review. Int. J. Surg. 2018, 52, 136–140. [Google Scholar] [CrossRef]
- de Júnior, R.G.O.; Adrielly, A.F.C.; da Almeida, J.R.G.S.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Abdul, M.; Hoosein, N. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 2002, 186, 99–105. [Google Scholar] [CrossRef]
- Flahaut, M.; Meier, R.; Coulon, A.; Nardou, K.; Niggli, F.; Martinet, D.; Beckmann, J.; Joseph, J.; Mühlethaler-Mottet, A.; Gross, N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/β-catenin pathway. Oncogene 2009, 28, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [Green Version]
- Pashaei-Asl, F.; Pashaei-Asl, R.; Khodadadi, K.; Akbarzadeh, A.; Ebrahimie, E.; Pashaiasl, M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Arima, H.; Hirayama, F.; Yamamoto, M.; Horikawa, T.; Sumiyoshi, H.; Noda, S.; Uekama, K. Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-β-cyclodextrin. Pharm. Dev. Technol. 2000, 5, 399–407. [Google Scholar] [CrossRef]
- Arora, D.; Jaglan, S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci Technol. 2016, 54, 114–126. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019, in press. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Nel, A.; Ruoslahti, E.; Meng, H. New insights into “permeability” as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano 2017. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921. [Google Scholar] [CrossRef] [Green Version]
- Kunjiappan, S.; Panneerselvam, T.; Govindaraj, S.; Parasuraman, P.; Baskararaj, S.; Sankaranarayanan, M.; Arunachalam, S.; Babkiewicz, E.; Jeyakumar, A.; Lakshmanan, M. Design, in silico modelling, and functionality theory of novel folate receptor targeted rutin encapsulated folic acid conjugated keratin nanoparticles for effective cancer treatment. Anticancer Agents Med. Chem. 2019, 19, 1966–1982. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; He, X.; Zhang, Y.; Zhang, Y.; Chai, F.; Jiang, L.; Webster, T.J.; Zheng, C. A dabigatran etexilate phospholipid complex nanoemulsion system for further oral bioavailability by reducing drug-leakage in the gastrointestinal tract. Nanomedicine 2018, 14, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Ansari, V.A.; Hussain, Z.; Siddique, N.F. Nanoemulsion: A Droplet Nanocarrier System for Enhancing Bioavailability of Poorly Water Soluble Drugs. Res. J. Pharm. Technol. 2018, 11, 5191–5196. [Google Scholar] [CrossRef]
- Ahmad, M.; Sahabjada, J.A.; Hussain, A.; Badaruddeen, M.A.; Mishra, A. Development of a new rutin nanoemulsion and its application on prostate carcinoma PC3 cell line. Excli. J. 2017, 16, 810. [Google Scholar]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Asfour, M.H.; Mohsen, A.M. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J. Adv. Res. 2018, 9, 17–26. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Sankaranarayanan, M.; Chowdhury, R.; Chowdhury, A.; Bhattacharjee, C. Design, in silico modeling, biodistribution study of rutin and quercetin loaded stable human hair keratin nanoparticles intended for anticancer drug delivery. Biomed. Phys. Eng. Express 2018, 4, 025019. [Google Scholar] [CrossRef]
- Pandey, P.; Rahman, M.; Bhatt, P.C.; Beg, S.; Paul, B.; Hafeez, A.; Al-Abbasi, F.A.; Nadeem, M.S.; Baothman, O.; Anwar, F. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine 2018, 13, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Vimala, R.; Sivasubramanian, S.; Jeganathan, K.; Thirumurugan, R. Dual drug loaded PLGA nanospheres for synergistic efficacy in breast cancer therapy. Mater. Sci. Eng. C 2019, 103, 109716. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L.; Miao, Y.; Fang, B.; Yang, Z. Anticancer and apoptotic-inducing effects of rutin-chitosan nanoconjugates in triple negative breast cancer cells. J. Clust. Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Bharathi, D.; Bhuvaneshwari, V. Synthesis of zinc oxide nanoparticles (ZnO NPs) using pure bioflavonoid rutin and their biomedical applications: Antibacterial, antioxidant and cytotoxic activities. Res. Chem. Intermediat. 2019, 45, 2065–2078. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019, 109, 1181–1195. [Google Scholar] [CrossRef]
- Radwan, R.R.; Ali, H.E. Radiation-synthesis of chitosan/poly (acrylic acid) nanogel for improving the antitumor potential of rutin in hepatocellular carcinoma. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Khonkarn, R.; Daowtak, K.; Okonogi, S. Chemotherapeutic efficacy enhancement in P-gp-Overexpressing cancer cells by flavonoid-loaded polymeric micelles. AAPS Pharm. Sci. Tech. 2020, 21, 121. [Google Scholar] [CrossRef]
- Júlio, A.; Antunes, C.; Mineiro, R.; Raposo, M.; Caparica, R.; Araújo, M.; Rosado, C.; Fonte, P.; Santos de Almeida, T. Influence of two choline-based ionic liquids on the solubility of caffeine. J. Biomed. Biopharm. Res. 2018, 15, 96–102. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Rosado, C.; Santos de Almeida, T. Applicability of ionic liquids in topical drug delivery systems: A mini review. J. Pharmacol. Clin. Res. 2018, 4, 555649–555655. [Google Scholar] [CrossRef]
- Khezeli, T.; Ghaedi, M.; Daneshfar, A.; Bahrani, S.; Asfaram, A.; Soylak, M. Ionic liquids in separation and preconcentration of organic and inorganic species. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–318. [Google Scholar]
- Kumar, S.S.D.; Surianarayanan, M.; Vijayaraghavan, R.; Mandal, A.B.; Macfarlane, D.R. Curcumin loaded poly (2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid–In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic-liquid-based paclitaxel preparation: A new potential formulation for cancer treatment. Mol. Pharm. 2018, 15, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef]
- Fathi, M.; Zangabad, P.S.; Majidi, S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. BioImpacts 2017, 7, 269. [Google Scholar] [CrossRef]
- Chawla, A.; Sharma, P.; Pawar, P. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: Formulation, optimization and in vitro evaluation/Alginatne mikrosfere naproksen natrija obložene Eudragitom S-100: Priprava, optimizacija i in vitro vrednovanje. Acta Pharm. 2012, 62, 529–545. [Google Scholar] [CrossRef]
- Madhavi, M.; Madhavi, K.; Jithan, A. Preparation and in vitro/in vivo characterization of curcumin microspheres intended to treat colon cancer. J. Pharm. Bioallied Sci. 2012, 4, 164. [Google Scholar]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Lai, Y.-H. Synthesis, characterization, and biological evaluation of anti-her2 indocyanine green-encapsulated peg-coated plga nanoparticles for targeted phototherapy of breast cancer cells. PLoS ONE 2016, 11, e0168192. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.-l.; Nan, K.; Nie, G.; Chen, H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef]
- Mugaka, B.P.; Hu, Y.; Ma, Y.; Ding, Y. Surface modification of gold nanoparticles for targeted drug delivery. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 391–403. [Google Scholar]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Chmit, M.; Kanaan, H.; Habib, J.; Abbass, M.; Mcheik, A.; Chokr, A. Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon. Asian Pac. J. Trop Med. 2014, 7, S546–S552. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, M.R.; Warren, H.S.; Cowden, W.B.; Parish, C.R. Effects of the anti-inflammatory compounds castanospermine, mannose-6-phosphate and fucoidan on allograft rejection and elicited peritoneal exudates. Immunol. Cell Biol. 1994, 72, 367–374. [Google Scholar] [CrossRef]
- Phan, N.H.; Ly, T.T.; Pham, M.N.; Luu, T.D.; Vo, T.V.; Tran, P.H.; Tran, T.T. A comparison of fucoidan conjugated to paclitaxel and curcumin for the dual delivery of cancer therapeutic agents. Anticancer Agents Med. Chem. 2018, 18, 1349–1355. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Li, R.; Hsu, C.-H.; Lin, C.-W.; Chou, S.-C.; Tsai, M.-L.; Mi, F.-L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef]


| Type of Cancer | Type of Study | Cell Type/Animal Model | Anticancer Effects | References |
|---|---|---|---|---|
| Breast | In vitro In vivo | Human TNBC cells (MDA-MB-231 and MDA-MB-468) Female athymic Foxn1nu/Foxn1C mice | ↓c-met/ HGF, ↓paxillin, ↓Rac-1 ↓mTOR, ↓Akt, ↓tumor volume | [101] |
| Breast | In vitro | Human breast cancer cells (MCF-7) | ↓Proliferation, ↑apoptosis, ↑cell cycle arrest, ↑PTEN, ↑p53 ↑p21 | [102] |
| Breast | In vitro In vivo | Human breast cancer cells (MCF-7) Female Swiss albino mice | ↑Apoptosis, ↓tumor volume, ↓CEA, ↓cholesterol, ↓FAS, ↓MDA, ↑GSH, ↑caspase-3, ↑caspase-7 | [103] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) and breast cancer cells (MCF-7) | ↑Chemosensitivity, ↓MDR, ↓P-gp ↓BCRP | [104] |
| Breast | In vitro | Human breast cancer cells (MCF-7) | ↑Chemosensitivity | [105] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) | ↓VEGF, ↓angiogenesis | [106] |
| Breast | In vitro | Human breast cancer cells (MCF-7) | ↑Apoptosis, ↑cell cycle arrest | [107] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) and Human breast cancer cells (MCF-7) | ↑Apoptosis, ↑p53, ↑Bax, ↓Bcl-2, ↓VEGF | [108] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) and human breast cancer cells (MCF-7) | ↓Proliferation, ↑apoptosis, ↑ROS | [109] |
| Lung | In vitro | Human lung cancer cells (A549) | ↑Cytotoxicity, ↑GSK-3β, ↑TNF-α | [110] |
| Lung | In vitro | Human lung cancer cells (A549) | ↓Migration, ↓fibronectin, ↓collagen type I and IV, ↑ROS, ↓superoxide | [29] |
| Lung | In vitro | Human lung cancer cells (A549) | ↓Single strand DNA break, ↓ROS | [111] |
| Lung | In vivo | C57BL/6 female mice | ↓Lung tumor nodules, ↑life span | [112] |
| Lung | In vivo | Albino Swiss mice | ↓Lung tumor nodules, ↓growth, ↓invasion index | [113] |
| Lung | In vitro | Human lung cancer cells (A549) | ↑Autophagy, ↑Beclin1, ↑Atg5/12, ↑LC3-II, ↓NF-κB, ↓TNF-α | [114] |
| Lung | In vitro | Human lung cancer cells | ↓Proliferation ↓cell cycle, ↓NF-κB, ↓p38 | [115] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↑Apoptosis, ↑caspase-3, ↑caspase-8, ↑caspase-9 ↑PARP, ↓Bcl-2, ↑Bax | [116] |
| Colon | In vitro In vivo | Human colon cancer cells (SW480) nu/nu mice | ↓Tumor growth ↓angiogenesis, ↓VEGF | [117] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↑Cytotoxicity, ↓mitochondrial membrane potential, ↑lipid peroxidation, ↓SOD ↓CAT ↓GPx | [118] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↓Adhesion, ↓migration, ↑ROS, ↓superoxide | [29] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↑Apoptosis, ↓Bcl-2, ↑Bax, ↑caspase-3, ↑caspases-8, ↑caspase-9, ↑p53, ↓NF-kB, ↓IKK-α, ↓IKK-β, ↓MAPK | [119] |
| Colon | In vitro | Human colon cancer cells (SW480) | ↑Apoptosis, ↑cell cycle arrest, ↓metabolism | [120] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↓ cell viability | [121] |
| Colon | In vitro | Human colon cancer cells (LoVo) | ↑Apoptosis, ↑cell cycle arrest | [107] |
| Colon | In vitro | Human colon cancer cells (Caco2) | ↓DNA damage | [122] |
| Colon | In vitro | Human colon cancer cells (Caco2) | No effect on DNA repair | [123] |
| Colon | In vivo | Female CF1 mice | ↓Focal areas of dysplasia, ↓hyperproliferation | [124] |
| Colon | In vivo | Male F344 rats | ↓Aberrant crypt foci, ↑apoptosis | [125] |
| Colon | In vivo | Male F344 rats | No effect | [126] |
| Colon | In vitro | Human colon cancer cells (HCT-8) | No effect | [127] |
| Colon | In vivo | Male F344 rats | No effect | [128] |
| Brain | In vitro | Human glioblastoma cell line (GL-15) | ↓Proliferation, ↑apoptosis, ↓ERK ↑GFAP | [129] |
| Brain | In vitro | Human glioblastoma cell line (GL-15) | ↓Invasion, ↓angiogenesis, ↓VEGF, ↓TGF-β1 | [130] |
| Brain | In vitro | Human glioblastoma cell line (GL-15) | ↓Proliferation, ↓invasion, ↓MMP-2, ↑fibronectin, ↑laminin | [131] |
| Brain | In vitro | Human neuroblastoma cells (LAN-5) | ↑Apoptosis, ↓cell cycle, ↓TNF-α, ↓Bcl-2, ↑Bax | [132] |
| Brain | In vitro | Human glioma cells (CHME) | ↑p53, ↑caspase-3, ↑caspase-9, ↑cytochrome c, ↑Bax, ↓Bcl-2, ↑ROS ↓mitochondrial membrane potential | [133] |
| Brain | In vitro In vivo | Human glioblastoma cells (U87-MG, D54-MG, and U251-MG) BALB/c athymic mice | ↑Cytotoxicity, ↑apoptosis, ↓ JNK, ↓autophagy, ↑caspase-3, | [134] |
| Leukemia | In vitro | Human leukemic cells (U937, HL-60, KG1, and KG1a) | ↑Cytotoxicity, ↑apoptosis, ↓GSK-3β, ↑Akt | [135] |
| Leukemia | In vivo | Human leukemia HL-60 cells induced leukemia in BALB/c mice | ↓Tumor weight, ↓tumor volume | [136] |
| Leukemia | In vivo | Murine leukemia WEHI-3 cells induced leukemia in BALB/c mice | ↓Proliferation, ↓macrophage phagocytosis | [137] |
| Leukemia | In vitro | Human leukemic cells (THP-1) | ↑Autophagy, ↓NF-κB, ↓TNF-α | [114] |
| Leukemia | In vitro | Human promyelocytic leukemia cells (HL-60) | ↓Angiogenesis, ↓VEGF, ↓AP-1, ↓IGF-1R/IRS-1 | [138] |
| Leukemia | In vitro | Human acute myeloid leukemia cells (KG1) | ↑Cytotoxicity, ↑antioxidant activity | [139] |
| Leukemia | In vitro | human myelogenous leukemia cells (K562) | ↓Single strand DNA break, ↓ROS | [140] |
| Leukemia | In vitro | human myelogenous leukemia cells (K562) | ↑Apoptosis | [141] |
| Leukemia | In vitro | human promyeloleukemic cells (HL-60) | No effect | [142] |
| Leukemia | In vitro | Murine leukemia cells (L1210) | No effect | [143] |
| Multiple myeloma | In vitro | Human multiple myeloma cells (RPMI8226) | ↑Cytotoxicity, ↑antioxidant activity | [139] |
| Multiple myeloma | In vitro | Human multiple myeloma cells (ARH–77) | ↑Cytotoxicity, ↓mitochondrial and lysosomal activity | [144] |
| Lymphoma | In vitro | Dalton’s lymphoma cells | ↑Apoptosis, ↓Bcl-xL, ↓c-FLIP, ↓GST, ↓GR | [145] |
| Liver | In vitro | Rat hepatoma cells (HTC) | ↓Proliferation, ↓cell viability | [146] |
| Liver | In vitro | Human liver cancer cells (HEPG2) | ↓Proliferation, ↑apoptosis, ↓CYP3A4, ↓CYP1A1, ↑NQO1, ↑GSTP1 | [147] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↓ROS, ↓MDA | [148] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↓GSH | [149] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↑Cytotoxicity | [150] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↓DNA damage | [122] |
| Liver | In vitro | human hepatoma cell line (HepG2) | No effect | [123] |
| Liver | In vivo | Wistar albino rats | ↑Membrane bound ATPases | [151] |
| Liver | In vivo | Wistar rats | ↓PARP, ↓DNA polymerase β, ↓DNA ligase | [152] |
| Gastric | In vitro | Human gastric cancer cells (SGC-7901) | ↑Apoptosis, ↑caspase-3, ↑caspase-7, ↑caspase-9, ↓Bcl-2/Bax, ↑p38MAPK, ↑G0/G1 arrest | [153] |
| Prostate | In vitro | Human prostatic cancer cells (PC3) | ↓Proliferation, ↑apoptosis, ↓Bcl-2, ↑p53 | [28] |
| Prostate | In vitro | Human prostate cancer cells (LNCaP) | No effect | [154] |
| Oral | In vitro | Drug resistance oral carcinoma cells (KBCHR8–5) | ↓Wnt/GSK-3β/β-catenin pathway, ↓P-gp | [105] |
| Cervical | In vitro | cervical cancer cells (HeLa) | ↓Proliferation, ↓growth | [155] |
| Ovarian | In vitro | ovarian cancer cells (OVCAR-3) | ↓Proliferation, ↓VEGF | [156] |
| Melanoma | In vitro | melanoma cells (B16F-10) | ↓Angiogenesis, ↓VEGF, ↓IL-1β, ↑TNF-α | [157] |
| Nanoformulation Model | Type of Cancer | Type of Study | Cell Type/Animal Model | Outcomes | References |
|---|---|---|---|---|---|
| Folic acid-conjugated keratin NPs | Breast | In vitro | Huma breast cancer cells (MCF-7) | ↑Apoptosis, ↓migration, ↑ROS, ↓mitochondrial membrane potential | [230] |
| Nanoemulsions | Prostate | In vitro | Human prostatic cancer cells (PC3) | ↑Apoptosis, ↑ROS | [233] |
| Ionic liquids-NPs | Renal | In vitro | Human renal cancer cells (786-O) | ↑Cytotoxicity, ↑sub-G1 population, ↑solubility | [234] |
| Eudragit S100 nanospheres | Colon | In vitro | Human colon cancer cells (HCT 116) | ↑Cytotoxicity ↑solubility | [235] |
| Keratin NPs | Cervical | In vitro | Human cervical cancer cells (Hela) | ↑Cytotoxicity | [236] |
| PLGA NPs | Liver | In vivo | Albino male Wistar rats | ↓IL-1β, ↓TNF-α, ↓IL-6 ↓NF-κB, ↑SOD, ↑CAT, ↑GSH, ↑GPx, ↑membrane-bound enzymes | [237] |
| PLGA nanospheres | Breast | In vitro | Human TNBC cells (MDA-MB-231) | ↓Proliferation, ↑apoptosis, ↑ROS | [238] |
| Chitosan NPs | Breast | In vitro | Human TNBC cells (MDA-MB-231) | ↑Apoptosis, ↑cell cycle arrest | [239] |
| ZnO NPs | Breast | In vitro | Human breast cancer cells (MCF-7) | ↑Cytotoxicity | [240] |
| Chitosan/copper oxide nanocomposites | Lung | In vitro | Human lung cancer cells (A549) | ↑Cytotoxicity, ↑apoptosis | [241] |
| Fucoidan NPs | Cervical | In vitro | Human cervical cancer cells (Hela) | ↑DNA fragmentation, ↑cell cycle arrest, ↑ROS, ↓mitochondrial membrane potential | [242] |
| Chitosan/poly (acrylic acid) nanogel | Liver | In vivo | Male albino rats | ↓proliferation, ↓angiogenesis, ↓VEGF, ↑Bax, ↓Bcl-2, ↑p53, ↑caspase-3 | [243] |
| Nanosized polymeric micelles | Leukemia | In vitro | human myelogenous leukemia cells (K562) | Low cytotoxicity | [244] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. https://doi.org/10.3390/cancers12082276
Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers. 2020; 12(8):2276. https://doi.org/10.3390/cancers12082276
Chicago/Turabian StyleNouri, Zeinab, Sajad Fakhri, Keyvan Nouri, Carly E. Wallace, Mohammad Hosein Farzaei, and Anupam Bishayee. 2020. "Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach" Cancers 12, no. 8: 2276. https://doi.org/10.3390/cancers12082276
APA StyleNouri, Z., Fakhri, S., Nouri, K., Wallace, C. E., Farzaei, M. H., & Bishayee, A. (2020). Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers, 12(8), 2276. https://doi.org/10.3390/cancers12082276






