The FMS like Tyrosine Kinase 3 (FLT3) Is Overexpressed in a Subgroup of Multiple Myeloma Patients with Inferior Prognosis
Abstract
:1. Introduction
2. Results
2.1. FLT3 Protein Is Expressed in Bone Marrow Samples of MGUS, NDMM, and RRMM Patients
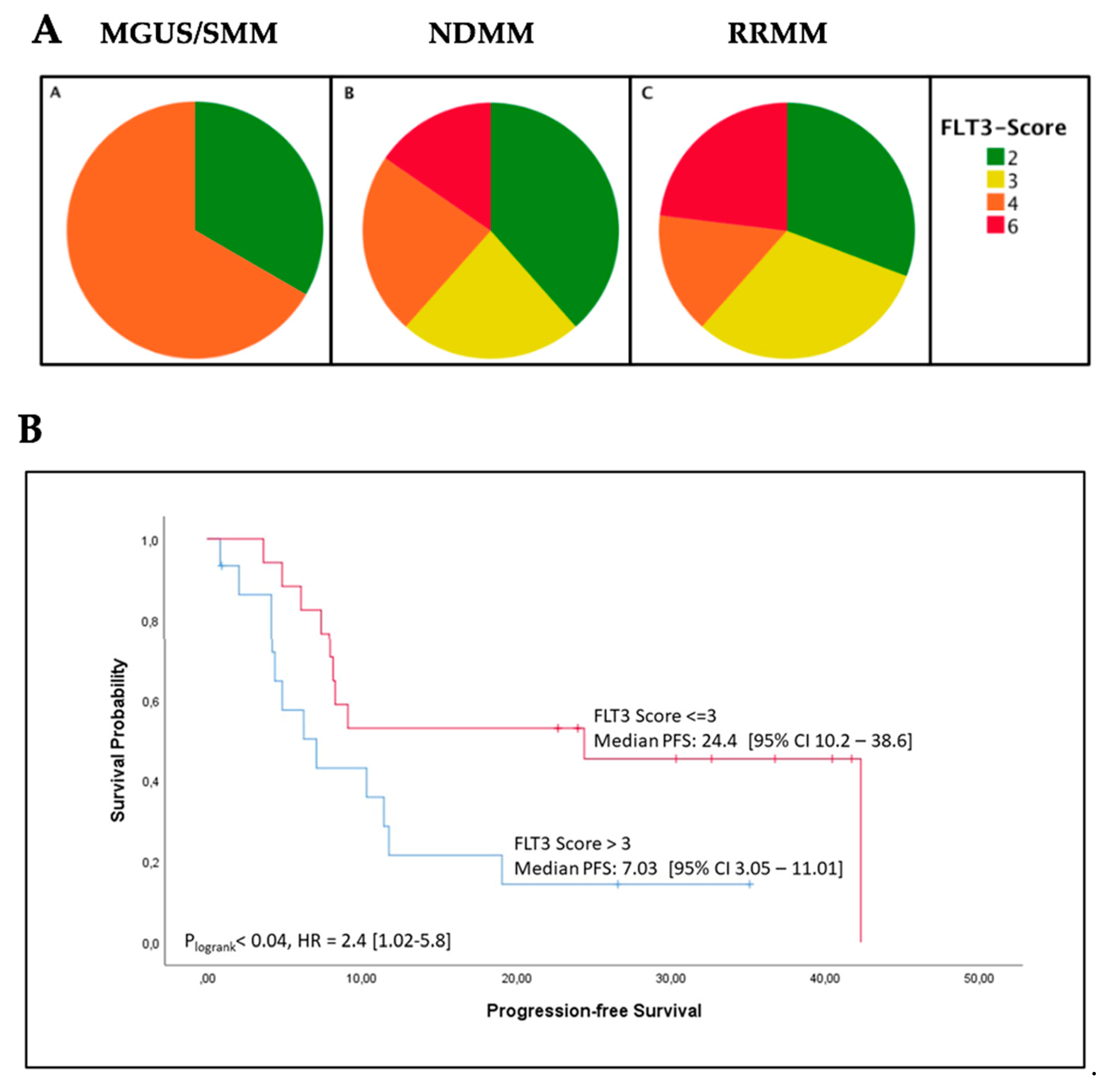
2.2. High FLT3 Protein (CD135) Expression in Myeloma Cells Correlates with Shorter Progression-Free Survival
2.3. High FLT3 Expression Scores in Bone Marrow Biopsies Correlate with Increased ß2-Microglobulin Levels
2.4. FLT3 Tyrosine Kinase Receptor Gene Expression Is Elevated in a Subgroup of MM Patients
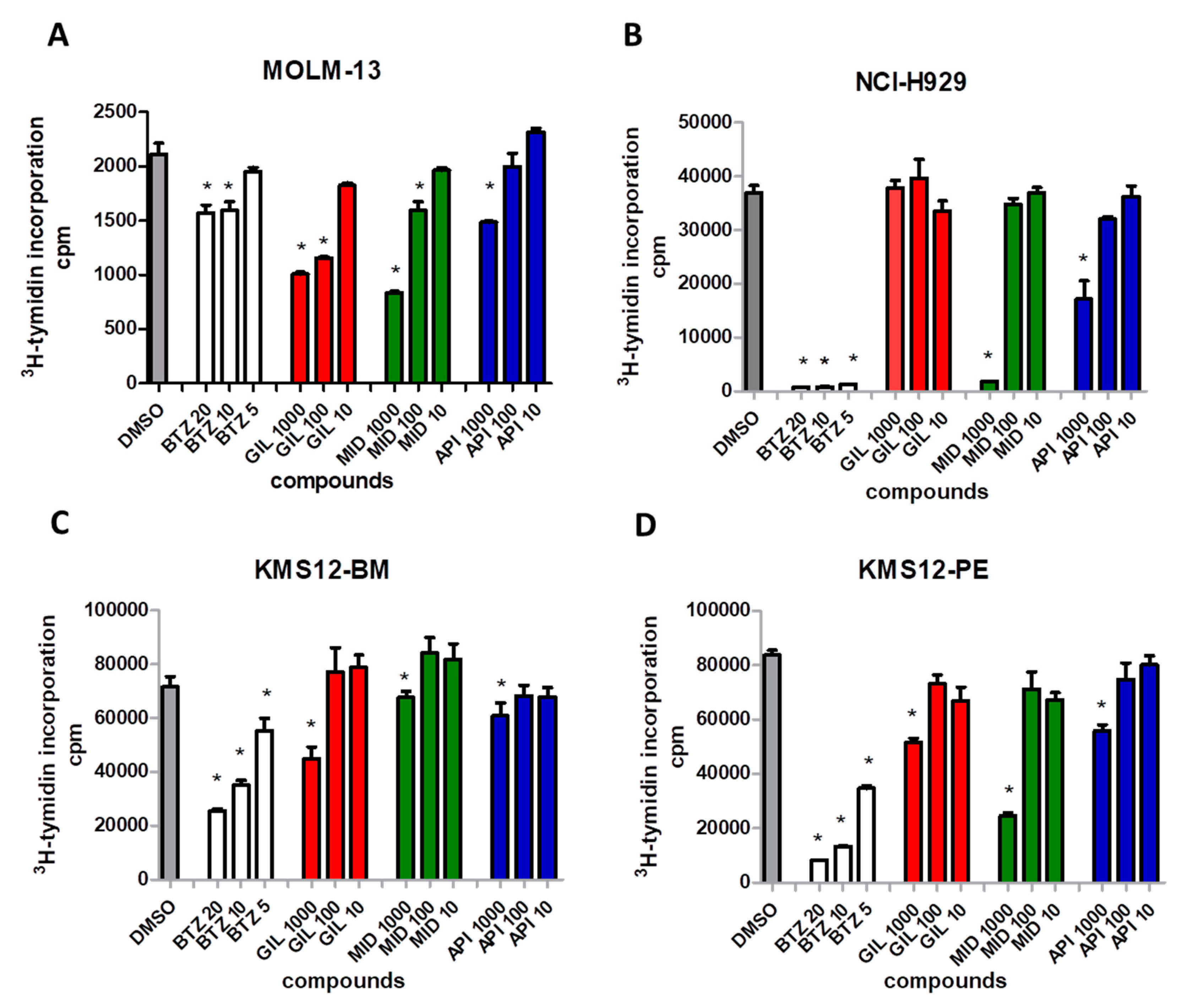
2.5. FLT3 Inhibitors Block Proliferation of MM Cell Lines and Induce Apoptosis of Primary MM Cells
3. Discussion
4. Materials and Methods
4.1. Patients and Cell Lines
4.2. Immunohistochemistry of Bone Marrow Biopsies
4.3. Scoring System for FLT3 Staining
4.4. Isolation of CD138 (Syndecan–1) Positive Cells from MM Patients
4.5. Subcellular Localization Studies of FLT3 in MM Cells
4.6. DNA Extraction and Next Generation Sequencing (NGS) for FLT3 Gene Mutations
4.7. RNA Extraction and Quantitative Multiplex Real-Time Analysis of FLT3 Gene Expression
4.8. 3′-RNA Whole Transcriptome Sequencing (WTS) of CD-138+-Purified MM Cells
4.9. 3. H-thymidine Proliferation Assay
4.10. Apoptosis Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Orlowski, R.Z.; Lonial, S. Integration of Novel Agents into the Care of Patients with Multiple Myeloma. Clin. Cancer Res. 2016, 22, 5443–5452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Björkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristinsson, S.Y.; Anderson, W.F.; Landgren, O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia 2014, 28, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Rajkumar, S.V. New Developments in Diagnosis, Prognosis, and Assessment of Response in Multiple Myeloma. Clin. Cancer Res. 2016, 22, 5428–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e418–e423. [Google Scholar] [CrossRef]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Landgren, O.; Morgan, G.J. Biologic frontiers in multiple myeloma: From biomarker identification to clinical practice. Clin. Cancer Res. 2014, 20, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Pawlyn, C.; Davies, F.E. Toward personalized treatment in multiple myeloma based on molecular characteristics. Blood 2019, 133, 660–675. [Google Scholar] [CrossRef] [Green Version]
- Vacca, A.; Ribatti, D. Bone marrow angiogenesis in multiple myeloma. Leukemia 2006, 20, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhri, B.; Vij, R. Clonal Evolution in Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, S130–S134. [Google Scholar] [CrossRef]
- Steiner, N.; Hajek, R.; Sevcikova, S.; Borjan, B.; Johrer, K.; Gobel, G.; Untergasser, G.; Gunsilius, E. High levels of FLT3-ligand in bone marrow and peripheral blood of patients with advanced multiple myeloma. PLoS ONE 2017, 12, e0181487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokonozaki, M.; Kanellou, P.; Pappa, C.A.; Vyzoukaki, R.; Sarantoulaki, S.; Stavroulaki, E.; Kyriakaki, S.; Alegakis, A.; Boula, A.; Alexandrakis, M.G. Serum Levels of Soluble FLT3 Ligand in Patients with Active Multiple Myeloma Constitute Marker of Bone Marrow Plasma Cell Proliferative Activity. Crit. Rev. Oncog. 2017, 22, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kokonozaki, M.; Tsirakis, G.; Devetzoglou, M.; Kyriakaki, S.; Antonakis, A.; Vyzoukaki, R.; Pappa, C.A.; Tzardi, M.; Alexandrakis, M.G. Potential role of FLT3-ligand in the angiogenic process of multiple myeloma. Leuk. Res. 2015, 39, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.J.; Paige, C.J.; Furlonger, C.; Lyman, S.D.; Rottapel, R. Flt3 ligand supports the differentiation of early B cell progenitors in the presence of interleukin-11 and interleukin-7. Eur. J. Immunol. 1996, 26, 1504–1510. [Google Scholar] [CrossRef]
- McKenna, H.J.; Stocking, K.L.; Miller, R.E.; Brasel, K.; De Smedt, T.; Maraskovsky, E.; Maliszewski, C.R.; Lynch, D.H.; Smith, J.; Pulendran, B.; et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000, 95, 3489–3497. [Google Scholar] [CrossRef]
- Mackarehtschian, K.; Hardin, J.D.; Moore, K.A.; Boast, S.; Goff, S.P.; Lemischka, I.R. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity 1995, 3, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Lagana, A.; Beno, I.; Melnekoff, D.; Leshchenko, V.; Madduri, D.; Ramdas, D.; Sanchez, L.; Niglio, S.; Perumal, D.; Kidd, B.A.; et al. Precision Medicine for Relapsed Multiple Myeloma on the Basis of an Integrative Multiomics Approach. JCO Precis. Oncol. 2018, 1–17. [Google Scholar] [CrossRef]
- Dosil, M.; Wang, S.; Lemischka, I.R. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol. Cell Biol. 1993, 13, 6572–6585. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S. Inhibition of the MEK/MAPK signal transduction pathway strongly impairs the growth of Flt3-ITD cells. Am. J. Hematol. 2006, 81, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, F.; Towatari, M.; Kiyoi, H.; Tanimoto, M.; Kitamura, T.; Saito, H.; Naoe, T. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 2000, 19, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Via, M.C.; Solimando, A.G.; Garitano-Trojaola, A.; Bario, S.; Munawar, U.; Strifler, S.; Haertle, L.; Rhodes, N.; Teufel, E.; Vogt, C.; et al. CIC Mutation as a Molecular Mechanism of Acquired Resistance to Combined BRAF-MEK Inhibition in Extramedullary Multiple Myeloma with Central Nervous System Involvement. Oncologist 2020, 25, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliland, D.G.; Griffin, J.D. The roles of FLT3 in hematopoiesis and leukemia. Blood 2002, 100, 1532–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiede, C.; Steudel, C.; Mohr, B.; Schaich, M.; Schäkel, U.; Platzbecker, U.; Wermke, M.; Bornhäuser, M.; Ritter, M.; Neubauer, A.; et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002, 99, 4326–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallogly, M.M.; Lazarus, H.M.; Cooper, B.W. Midostaurin: A novel therapeutic agent for patients with FLT3-mutated acute myeloid leukemia and systemic mastocytosis. Adv. Hematol. 2017, 8, 245–261. [Google Scholar] [CrossRef]
- Larrosa-Garcia, M.; Baer, M.R. FLT3 Inhibitors in Acute Myeloid Leukemia: Current Status and Future Directions. Mol. Cancer 2017, 16, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Li, C.; Zhu, X. FLT3 inhibitors in acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 133. [Google Scholar] [CrossRef]
- IMWG. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Jöhrer, K.; Obkircher, M.; Neureiter, D.; Parteli, J.; Zelle-Rieser, C.; Maizner, E.; Kern, J.; Hermann, M.; Hamacher, F.; Merkel, O.; et al. Antimyeloma activity of the sesquiterpene lactone cnicin: Impact on Pim-2 kinase as a novel therapeutic target. J. Mol. Med. 2012, 90, 681–693. [Google Scholar] [CrossRef]





| Parameter | MGUS/SMM (n = 3) | % | NDMM (n = 26) | % | RRMM (n = 13) | % |
|---|---|---|---|---|---|---|
| Age, years Median (range) | 69 (48–76) | 74 (45–88) | 66 (41–88) | |||
| Sex | ||||||
| Female | 0 | 0 | 12 | 46.2 | 6 | 46.2 |
| Male | 3 | 100 | 14 | 53.8 | 7 | 53.8 |
| ISS Stage | ||||||
| No Stage | 3 | 100 | 1 | 3.8 | 0 | 0 |
| I | 0 | 0 | 5 | 19.2 | 3 | 23.1 |
| II | 0 | 0 | 3 | 11.5 | 4 | 30.8 |
| III | 0 | 0 | 17 | 65.4 | 6 | 46.2 |
| Type of heavy chain (serum) | ||||||
| IgG | 2 | 66.7 | 12 | 46.2 | 6 | 46.2 |
| IgM | 0 | 0 | 0 | 0 | 2 | 15.4 |
| IgA | 1 | 33.3 | 5 | 19.2 | 2 | 15.4 |
| IgD | 0 | 0 | 1 | 3.8 | 0 | 0 |
| Light chain only | 0 | 0 | 8 | 30.8 | 3 | 23.1 |
| Type of light chain (serum) | ||||||
| Kappa | 3 | 100 | 16 | 61.5 | 8 | 61.5 |
| Lambda | 0 | 0 | 9 | 34.6 | 5 | 38.5 |
| Mixed gradient | 0 | 0 | 1 | 3.8 | 0 | 0 |
| ß2–microglobulin > UNV | 1 | 33.3 | 22 | 88 | 11 | 84.6 |
| LDH > UNV | 1 | 33.3 | 8 | 30.8 | 4 | 30.8 |
| Creatinine ≥ 1.3 mg/dL | 1 | 33.3 | 12 | 46.2 | 3 | 23.1 |
| Serum calcium > UNV | 0 | 0 | 3 | 11.5 | 0 | 0 |
| Hemoglobin ≤ 12 g/dL | 2 | 66.7 | 22 | 84.6 | 11 | 84.6 |
| Platelets < 100,000/mm3 | 1 | 33.3 | 4 | 15.4 | 6 | 46.2 |
| Osteolytic bone lesions | 0 | 0 | 22 | 84.6 | 13 | 100 |
| Cytogenetic risk | ||||||
| Standard risk | 1 | 33.3 | 3 | 11.5 | 1 | 7.7 |
| High risk | 1 | 33.3 | 11 | 42.3 | 5 | 38.5 |
| Not available | 1 | 33.3 | 12 | 46.2 | 7 | 53.8 |
| Therapy lines | ||||||
| Therapy not started | 3 | 100 | 22 | 84.6 | 0 | 0 |
| 1st line | 0 | 0 | 4 | 15.4 | 4 | 30.8 |
| 2nd line | 0 | 0 | 0 | 0 | 1 | 7.7 |
| 3rd line | 0 | 0 | 0 | 0 | 2 | 15.4 |
| 4th line | 0 | 0 | 0 | 0 | 3 | 23.1 |
| 5th line | 0 | 0 | 0 | 0 | 2 | 15.4 |
| 7th line | 0 | 0 | 0 | 0 | 1 | 7.7 |
| Therapy at the time of sample | ||||||
| No active therapy | 3 | 100 | 22 | 84.6 | 3 | 23.1 |
| PI-based | 0 | 0 | 1 | 3.9 | 3 | 23.1 |
| IMiD-based | 0 | 0 | 0 | 0 | 5 | 38.5 |
| PI and IMiD | 0 | 0 | 3 | 11.5 | 1 | 7.7 |
| Others | 0 | 0 | 0 | 0 | 1 | 7.7 |
| Parameter | FES ≤ 3 (n = 17) | % | FES > 3 (n = 15) | % |
|---|---|---|---|---|
| Age, years Median (range) | 69 (41–85) | 71 (49–88) | ||
| Stage of disease | ||||
| NDMM | 11 | 64.7 | 10 | 66.7 |
| RRMM | 6 | 35.3 | 5 | 33.3 |
| Sex | ||||
| Female | 7 | 41.2 | 8 | 53.3 |
| Male | 10 | 58.8 | 7 | 46.7 |
| ISS Stage | ||||
| I | 7 | 41.2 | 1 | 6.7 |
| II | 3 | 17.6 | 3 | 20 |
| III | 7 | 41.2 | 11 | 73.3 |
| Type of heavy chain (serum) | ||||
| IgG | 7 | 41.2 | 7 | 46.7 |
| IgM | 1 | 5.9 | 1 | 6.7 |
| IgA | 3 | 17.6 | 4 | 26.7 |
| IgD | 1 | 5.9 | 0 | 0 |
| Light chain only | 5 | 29.4 | 3 | 20 |
| Type of light chain (serum) | ||||
| Kappa | 8 | 47.1 | 11 | 73.3 |
| Lambda | 9 | 52.9 | 4 | 26.7 |
| ß2-microglobulin > UNV | 13 | 76.5 | 14 | 93.3 |
| LDH > UNV | 3 | 17.6 | 3 | 20 |
| Creatinine ≥ 1.3 mg/dL | 5 | 29.4 | 7 | 46.7 |
| Serum calcium > UNV | 2 | 11.8 | 1 | 6.7 |
| Hemoglobin ≤ 12 g/dL | 13 | 76.5 | 13 | 86.7 |
| Platelets < 100,000/mm3 | 2 | 11.8 | 6 | 40 |
| Osteolytic bone lesions | 15 | 88.2 | 14 | 93.3 |
| Cytogenetic risk | ||||
| Standard risk | 6 | 35.3 | 8 | 53.3 |
| High risk | 11 | 64.7 | 5 | 33.3 |
| Not available | 0 | 0 | 2 | 13.3 |
| Therapy line | ||||
| Therapy not started | 9 | 52.9 | 8 | 53.3 |
| 1st line | 4 | 23.5 | 4 | 26.7 |
| 2nd line | 1 | 5.9 | 0 | 0 |
| 3rd line | 0 | 0 | 1 | 6.7 |
| 4th line | 2 | 11.8 | 1 | 6.7 |
| 5th line | 1 | 5.9 | 0 | 0 |
| 7th line | 0 | 0 | 1 | 6.7 |
| Therapy at the time of sample | ||||
| No active therapy | 11 | 64.7 | 9 | 60 |
| PI-based | 1 | 5.9 | 3 | 20 |
| IMiD-based | 2 | 11.8 | 1 | 6.7 |
| PI and IMiD | 2 | 11.8 | 2 | 13.3 |
| Others | 1 | 5.9 | 0 | 0 |
| Variable | FES ≤ 3 (n = 17) | FES > 3 (n = 15) | p Value | |
|---|---|---|---|---|
| PFS (months) | Median (95% CI) | 24.4 [10.2–38.6] | 7.03 [3.05–11.01] | 0.04 |
| ß2-microglobulin (mg/L) | Median (95% CI) | 4.3 [2.6–10.3] | 11.4 [3.7–14.6] | 0.005 |
| ISS stage III | N/% | 7/41% | 11/73% | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, N.; Jöhrer, K.; Plewan, S.; Brunner-Véber, A.; Göbel, G.; Nachbaur, D.; Wolf, D.; Gunsilius, E.; Untergasser, G. The FMS like Tyrosine Kinase 3 (FLT3) Is Overexpressed in a Subgroup of Multiple Myeloma Patients with Inferior Prognosis. Cancers 2020, 12, 2341. https://doi.org/10.3390/cancers12092341
Steiner N, Jöhrer K, Plewan S, Brunner-Véber A, Göbel G, Nachbaur D, Wolf D, Gunsilius E, Untergasser G. The FMS like Tyrosine Kinase 3 (FLT3) Is Overexpressed in a Subgroup of Multiple Myeloma Patients with Inferior Prognosis. Cancers. 2020; 12(9):2341. https://doi.org/10.3390/cancers12092341
Chicago/Turabian StyleSteiner, Normann, Karin Jöhrer, Selina Plewan, Andrea Brunner-Véber, Georg Göbel, David Nachbaur, Dominik Wolf, Eberhard Gunsilius, and Gerold Untergasser. 2020. "The FMS like Tyrosine Kinase 3 (FLT3) Is Overexpressed in a Subgroup of Multiple Myeloma Patients with Inferior Prognosis" Cancers 12, no. 9: 2341. https://doi.org/10.3390/cancers12092341
APA StyleSteiner, N., Jöhrer, K., Plewan, S., Brunner-Véber, A., Göbel, G., Nachbaur, D., Wolf, D., Gunsilius, E., & Untergasser, G. (2020). The FMS like Tyrosine Kinase 3 (FLT3) Is Overexpressed in a Subgroup of Multiple Myeloma Patients with Inferior Prognosis. Cancers, 12(9), 2341. https://doi.org/10.3390/cancers12092341





