Implications of CLSPN Variants in Cellular Function and Susceptibility to Cancer
Abstract
:1. Introduction
2. Results
2.1. CLSPN Alterations in Breast Cancer and Glioma Patients
2.2. CLSPN Alterations in Cell Lines
2.3. CLSPN c.1574A>G Variant
2.4. Evaluation of the Impact of CLSPN Promoter c.-68C>T Mutation
2.5. Other CLSPN Variants: c.17G>A (p.Gly6Asp), c.2028+16G>A, c.2230T>C (p.Ser744Pro), c.3595-3597delGAA (p.Glu1199del) and c.3839C>T (p.Ser1280Leu)
3. Discussion
4. Material and Methods
4.1. Patients and Samples
4.2. Cell Lines
4.3. Antibodies
4.4. DNA Extraction
4.5. CLSPN Genotyping
4.6. Bioinformatics Tools
4.7. Luciferase Assay
4.8. Fluorescent Electrophoretic Mobility Shift Assay (fEMSA)
4.9. Minigene Splicing Assay
4.10. Transcripts Analysis by Reverse Transcriptase-PCR
4.11. Allelic Imbalance Analysis by Primer Extension Assay–SNaPshot
4.12. Functional Assays
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-K.; Choi, Y.-L.; Kwon, M.; Park, P.J. Mechanisms and Consequences of Cancer Genome Instability: Lessons from Genome Sequencing Studies. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 283–312. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; García-Muse, T. Causes of Genome Instability. Annu. Rev. Genet. 2013, 47, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Smits, V.A.J.; Cabrera, E.; Freire, R.; Gillespie, D.A. Claspin - checkpoint adaptor and DNA replication factor. FEBS J. 2019, 286, 441–455. [Google Scholar] [CrossRef] [Green Version]
- Azenha, D.; Lopes, M.C.; Martins, T.C. Claspin functions in cell homeostasis—A link to cancer? DNA Repair (Amst) 2017, 59, 27–33. [Google Scholar] [CrossRef]
- Azenha, D.; Lopes, M.C.; Martins, T.C. Claspin: From replication stress and DNA damage responses to cancer therapy. Adv. Protein Chem. Struct. Biol. 2019, 115, 203–246. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Chen, J. Claspin, a regulator of Chk1 in DNA replication stress pathway. DNA Repair (Amst) 2004, 3, 1033–1037. [Google Scholar] [CrossRef]
- Clarke, C.A.L.; Clarke, P.R. DNA-dependent phosphorylation of Chk1 and Claspin in a human cell-free system. Biochem. J. 2005, 388, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, A.; Dunphy, W.G. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell 2000, 6, 839–849. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Kumagai, A.; Lee, J.; Dunphy, W.G. Phosphorylated Claspin Interacts with a Phosphate-binding Site in the Kinase Domain of Chk1 during ATR-mediated Activation. J. Biol. Chem. 2003, 278, 46782–46788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guervilly, J.-H.; Macé-Aimé, G.; Rosselli, F. Loss of CHK1 function impedes DNA damage-induced FANCD2 monoubiquitination but normalizes the abnormal G2 arrest in Fanconi anemia. Hum. Mol. Genet. 2008, 17, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Li, K.; Stewart, G.S.; Elledge, S.J. Human Claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc. Natl. Acad. Sci. USA 2004, 101, 6484–6489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-F.; Shih, H.-Y.; Shang, Z.; Matsunaga, S.; Chen, B.P. DNA-PKcs is required to maintain stability of Chk1 and Claspin for optimal replication stress response. Nucleic Acids Res. 2014, 42, 4463–4473. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, L.; Cupello, S.; Hossain, M.A.; Deem, B.; McLeod, M.; Raj, J.; Yan, S. APE2 promotes DNA damage response pathway from a single-strand break. Nucleic Acids Res. 2018, 46, 2479–2494. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Shao, H.; Lindsey-Boltz, L.; Sancar, A.; Modrich, P. Interactions of human mismatch repair proteins MutSalpha and MutLalpha with proteins of the ATR-Chk1 pathway. J. Biol. Chem. 2010, 285, 5974–5982. [Google Scholar] [CrossRef] [Green Version]
- Prætorius-Ibba, M.; Wang, Q.-E.; Wani, G.; El-Mahdy, M.A.; Zhu, Q.; Qin, S.; Wani, A.A. Role of Claspin in regulation of nucleotide excision repair factor DDB2. DNA Repair (Amst) 2007, 6, 578–587. [Google Scholar] [CrossRef]
- Sato, K.; Sundaramoorthy, E.; Rajendra, E.; Hattori, H.; Jeyasekharan, A.D.; Ayoub, N.; Schiess, R.; Aebersold, R.; Nishikawa, H.; Sedukhina, A.S.; et al. A DNA-Damage Selective Role for BRCA1 E3 Ligase in Claspin Ubiquitylation, CHK1 Activation, and DNA Repair. Curr. Biol. 2012, 22, 1659–1666. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.A.L.; Bennett, L.N.; Clarke, P.R. Cleavage of claspin by caspase-7 during apoptosis inhibits the Chk1 pathway. J. Biol. Chem. 2005, 280, 35337–35345. [Google Scholar] [CrossRef] [Green Version]
- Semple, J.I.; Smits, V.A.J.; Fernaud, J.-R.; Mamely, I.; Freire, R. Cleavage and degradation of Claspin during apoptosis by caspases and the proteasome. Cell Death Differ. 2007, 14, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Sar, F.; Lindsey-Boltz, L.A.; Subramanian, D.; Croteau, D.L.; Hutsell, S.Q.; Griffith, J.D.; Sancar, A. Human claspin is a ring-shaped DNA-binding protein with high affinity to branched DNA structures. J. Biol. Chem. 2004, 279, 39289–39295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uno, S.; Masai, H. Efficient expression and purification of human replication fork-stabilizing factor, Claspin, from mammalian cells: DNA-binding activity and novel protein interactions. Genes Cells 2011, 16, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Broderick, R.; Rainey, M.D.; Santocanale, C.; Nasheuer, H.P. Cell cycle-dependent formation of Cdc45-Claspin complexes in human cells is compromized by UV-mediated DNA damage. FEBS J. 2013, 280, 4888–4902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Gold, D.A.; Shevchenko, A.; Shevchenko, A.; Dunphy, W.G. Roles of replication fork-interacting and Chk1-activating domains from Claspin in a DNA replication checkpoint response. Mol. Biol. Cell 2005, 16, 5269–5282. [Google Scholar] [CrossRef] [Green Version]
- Serçin, O.; Kemp, M.G. Characterization of functional domains in human Claspin. Cell Cycle 2011, 10, 1599–1606. [Google Scholar] [CrossRef]
- Yang, C.-C.; Suzuki, M.; Yamakawa, S.; Uno, S.; Ishii, A.; Yamazaki, S.; Fukatsu, R.; Fujisawa, R.; Sakimura, K.; Tsurimoto, T.; et al. Claspin recruits Cdc7 kinase for initiation of DNA replication in human cells. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Petermann, E.; Helleday, T.; Caldecott, K.W. Claspin promotes normal replication fork rates in human cells. Mol. Biol. Cell 2008, 19, 2373–2378. [Google Scholar] [CrossRef] [Green Version]
- Scorah, J.; McGowan, C.H. Claspin and Chk1 regulate replication fork stability by different mechanisms. Cell Cycle 2009, 8, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Masai, H.; Yang, C.-C.; Matsumoto, S. Mrc1/Claspin: A new role for regulation of origin firing. Curr. Genet. 2017, 63, 813–818. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kanoh, Y.; Shimmoto, M.; Hayano, M.; Ueda, K.; Fukatsu, R.; Kakusho, N.; Masai, H. Checkpoint-Independent Regulation of Origin Firing by Mrc1 through Interaction with Hsk1 Kinase. Mol. Cell. Biol. 2017, 37, e00355-16. [Google Scholar] [CrossRef] [Green Version]
- Focarelli, M.L.; Soza, S.; Mannini, L.; Paulis, M.; Montecucco, A.; Musio, A. Claspin inhibition leads to fragile site expression. Genes. Chromosomes Cancer 2009, 48, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, X.; Gao, Y.; Lewis, T.; Barthelemy, J.; Leffak, M. Altered replication in human cells promotes DMPK (CTG) (n) (CAG) (n) repeat instability. Mol. Cell. Biol. 2012, 32, 1618–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith-Roe, S.L.; Patel, S.S.; Simpson, D.A.; Zhou, Y.C.; Rao, S.; Ibrahim, J.G.; Kaiser-Rogers, K.A.; Cordeiro-Stone, M.; Kaufmann, W.K. Timeless functions independently of the Tim-Tipin complex to promote sister chromatid cohesion in normal human fibroblasts. Cell Cycle 2011, 10, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Smith-Roe, S.L.; Patel, S.S.; Zhou, Y.; Simpson, D.A.; Rao, S.; Ibrahim, J.G.; Cordeiro-Stone, M.; Kaufmann, W.K. Separation of intra-S checkpoint protein contributions to DNA replication fork protection and genomic stability in normal human fibroblasts. Cell Cycle 2013, 12, 332–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, J.N.; Bergoglio, V.; Lin, Y.L.; Pillaire, M.J.; Schmitz, A.L.; Gilhodes, J.; Lusque, A.; Mazières, J.; Lacroix-Triki, M.; Roumeliotis, T.I.; et al. Overexpression of Claspin and Timeless protects cancer cells from replication stress in a checkpoint-independent manner. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Song, Y.-H.; Brannigan, B.W.; Wahrer, D.C.R.; Schiripo, T.A.; Harris, P.L.; Haserlat, S.M.; Ulkus, L.E.; Shannon, K.M.; Garber, J.E.; et al. Prevalence and Functional Analysis of Sequence Variants in the ATR Checkpoint Mediator Claspin. Mol. Cancer Res. 2009, 7, 1510–1516. [Google Scholar] [CrossRef] [Green Version]
- Erkko, H.; Pylkäs, K.; Karppinen, S.-M.; Winqvist, R. Germline alterations in the CLSPN gene in breast cancer families. Cancer Lett. 2008, 261, 93–97. [Google Scholar] [CrossRef]
- Wang, X.; Szabo, C.; Qian, C.; Amadio, P.G.; Thibodeau, S.N.; Cerhan, J.R.; Petersen, G.M.; Liu, W.; Couch, F.J. Mutational Analysis of Thirty-two Double-Strand DNA Break Repair Genes in Breast and Pancreatic Cancers. Cancer Res. 2008, 68, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Madeira, A.; Azenha, D.; Correia, L.; Goncalves, V.; Ferreira, M.; Lacerda, M.; Lopes, C.; Martins, T.C. 724 Claspin Mutations and Loss of Function May Contribute to Breast Carcinogenesis and Gliomagenesis. Eur. J. Cancer 2012, 48, S172. [Google Scholar] [CrossRef]
- Ke, S.; Shang, S.; Kalachikov, S.M.; Morozova, I.; Yu, L.; Russo, J.J.; Ju, J.; Chasin, L.A. Quantitative evaluation of all hexamers as exonic splicing elements. Genome Res. 2011, 21, 1360–1374. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, D.; Gaildrat, P.; Abuli, A.; Abdat, J.; Frébourg, T.; Tosi, M.; Martins, A. Functional Analysis of a Large set of BRCA2 exon 7 Variants Highlights the Predictive Value of Hexamer Scores in Detecting Alterations of Exonic Splicing Regulatory Elements. Hum. Mutat. 2013, 34, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Qin, X.; Wu, Z.; Shen, Q.; Yang, W.; Zhang, S.; Duan, J.; Liang, F.; Liu, C. Inhibitory effect of MyoD on the proliferation of breast cancer cells. Oncol. Lett. 2016, 11, 3589–3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Liu, Y.; Chen, L.; Zhu, H.; Di, G.H.; Ling, H.; Wu, J.; Shao, Z.M. Involvement of MyoD and c-myb in regulation of basal and estrogen-induced transcription activity of the BRCA1 gene. Breast Cancer Res. Treat. 2011, 125, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.Y.; Li, S.; Zhao, Z.; Li, J.; Cui, M.K.; Wang, E.H. Repression of ESR1 transcription by MYOD potentiates letrozole-resistance in ERα-positive breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 492, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, R.; Komori, H.; Ishida, S.; Okamura, N.; Nakayama, K.; Nakayama, K.I.; Ohtani, K. Identification of novel E2F1 target genes regulated in cell cycle-dependent and independent manners. Oncogene 2006, 25, 1786–1798. [Google Scholar] [CrossRef] [Green Version]
- Verlinden, L.; Vanden Bempt, I.; Eelen, G.; Drijkoningen, M.; Verlinden, I.; Marchal, K.; De Wolf-Peeters, C.; Christiaens, M.-R.; Michiels, L.; Bouillon, R.; et al. The E2F-Regulated Gene Chk1 Is Highly Expressed in Triple-Negative Estrogen Receptor/Progesterone Receptor/HER-2 Breast Carcinomas. Cancer Res. 2007, 67, 6574–6581. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Russell, P. DNA Binding Domain in the Replication Checkpoint Protein Mrc1 of Schizosaccharomyces pombe. J. Biol. Chem. 2004, 279, 53023–53027. [Google Scholar] [CrossRef] [Green Version]
- Chini, C.C.S.; Chen, J. Repeated Phosphopeptide Motifs in Human Claspin Are Phosphorylated by Chk1 and Mediate Claspin Function. J. Biol. Chem. 2006, 281, 33276–33282. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, A.; Dunphy, W.G. Repeated phosphopeptide motifs in Claspin mediate the regulated binding of Chk1. Nat. Cell Biol. 2003, 5, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Capalbo, L.; Glover, D.M.; Dunphy, W.G. Role for casein kinase 1 in the phosphorylation of Claspin on critical residues necessary for the activation of Chk1. Mol. Biol. Cell 2011, 22, 2834–2847. [Google Scholar] [CrossRef]
- Nickless, A.; Bailis, J.M.; You, Z. Control of gene expression through the nonsense-mediated RNA decay pathway. Cell Biosci. 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Bailey, M.H.; Porta-Pardo, E.; Thorsson, V.; Colaprico, A.; Bertrand, D.; Gibbs, D.L.; Weerasinghe, A.; Huang, K.; Tokheim, C.; et al. Perspective on Oncogenic Processes at the End of the Beginning of Cancer Genomics. Cell 2018, 173, 305–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukarieh, O.; Gaildrat, P.; Hamiet, M.; Drouet, A.; Baert-Desurmont, S.; Frébourg, T.; Tosi, M.; Martins, A. Exonic splicing mutations are more prevalent than currently estimated and can be predicted using in silico tools. PLoS Genet. 2016, 12, e1005756. [Google Scholar] [CrossRef] [Green Version]
- Rheinbay, E.; Parasuraman, P.; Grimsby, J.; Tiao, G.; Engreitz, J.M.; Kim, J.; Lawrence, M.S.; Taylor-Weiner, A.; Rodriguez-Cuevas, S.; Rosenberg, M.; et al. Recurrent and functional regulatory mutations in breast cancer. Nature 2017, 547, 55–60. [Google Scholar] [CrossRef]
- Fraile-Bethencourt, E.; Valenzuela-Palomo, A.; Díez-Gomez, B.; Infante, M.; Durán, M.; Marcos, G.; Lastra, E.; Gómez-Barrero, S.; Velasco, E.A. Genetic dissection of the BRCA2 promoter and transcriptional impact of DNA variants. Breast Cancer Res. Treat. 2018, 171, 53–63. [Google Scholar] [CrossRef]
- Benevolo, M.; Musio, A.; Vocaturo, A.; Donà, M.; Rollo, F.; Terrenato, I.; Carosi, M.; Pescarmona, E.; Vocaturo, G.; Mottolese, M. Claspin as a Biomarker of Human Papillomavirus-Related High Grade Lesions of Uterine Cervix. J. Transl. Med. 2012, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Tsimaratou, K.; Kletsas, D.; Kastrinakis, N.; Tsantoulis, P.; Evangelou, K.; Sideridou, M.; Liontos, M.; Poulias, I.; Venere, M.; Salmas, M.; et al. Evaluation of Claspin as a Proliferation Marker in Human Cancer and Normal Tissues. J. Pathol. 2007, 211, 331–339. [Google Scholar] [CrossRef]
- Allera-Moreau, C.; Rouquette, I.; Lepage, B.; Oumouhou, N.; Walschaerts, M.; Leconte, E.; Schilling, V.; Gordien, K.; Brouchet, L.; Delisle, M.B.; et al. DNA Replication Stress Response Involving PLK1, CDC6, POLQ, RAD51 and CLASPIN Upregulation Prognoses the Outcome of Early/mid-Stage Non-Small Cell Lung Cancer Patients. Oncogenesis 2012, 1, e30. [Google Scholar] [CrossRef]
- Choi, S.; Yang, H.; Lee, S.; Ki, J.-H.; Nam, D.-H.; Yoo, H. TopBP1 and Claspin Contribute to the Radioresistance of Lung Cancer Brain Metastases. Mol. Cancer 2014, 13, 211. [Google Scholar] [CrossRef] [Green Version]
- Kauffmann, A.; Rosselli, F.; Lazar, V.; Winnepenninckx, V.; Mansuet-Lupo, A.; Dessen, P.; vanden Oord, J.J.; Spatz, A.; Sarasin, A. High expression of DNA repair pathways is associated with metastasis in melanoma patients. Oncogene 2007, 27, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Sarasin, A.; Dessen, P. DNA repair pathways and human metastatic malignant melanoma. Curr. Mol. Med. 2010, 10, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.E.; Kenoyer, A. Signal Transduction and Nuclear Responses in Staphylococcus Aureus-Induced Expression of Human Beta-Defensin 3 in Skin Keratinocytes. Infect. Immun. 2006, 74, 6847–6854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaildrat, P.; Killian, A.; Martins, A.; Tournier, I.; Frébourg, T.; Tosi, M. Use of Splicing Reporter Minigene Assay to Evaluate the Effect on Splicing of Unclassified Genetic Variants. Methods Mol. Biol. 2010, 653, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mamely, I.; van Vugt, M.A.; Smits, V.A.J.; Semple, J.I.; Lemmens, B.; Perrakis, A.; Medema, R.H.; Freire, R. Polo-like Kinase-1 Controls Proteasome-Dependent Degradation of Claspin during Checkpoint Recovery. Curr. Biol. 2006, 16, 1950–1955. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Castro, A.J.; Freire, R. Rad9B Responds to Nucleolar Stress through ATR and JNK Signalling, and Delays the G1-S Transition. J. Cell Sci. 2012, 125, 1152–1164. [Google Scholar] [CrossRef] [Green Version]







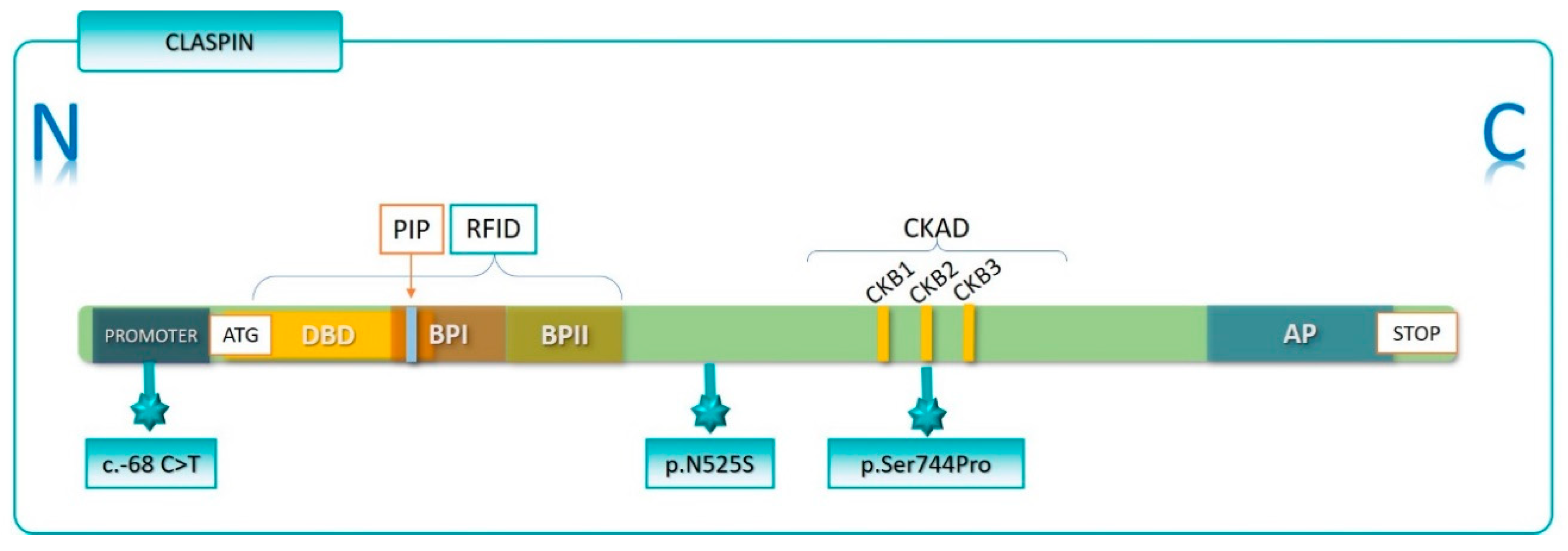

| Variant | Rs Number | Gene Location | aa Change | fBC | sBC | Glioma | Controls |
|---|---|---|---|---|---|---|---|
| c.-68C>T | rs372789882 | Promoter | n.a. | 2/147 | 1/66 | 3/53 | 1/79 |
| c.17G>A | rs61751002 | Exon 1 | p.Gly6Asp | 16/147 | 7/66 | 6/53 | 8/79 |
| c.1574A>G | rs7537203 | Exon 8 | p.Asn525Ser | 36/147 | 10/66 | 14/53 | 28/79 |
| c.2028+16G>A | rs535638 | Intron 10 | n.a. | 74/147 | 56/66 | 52/53 | 68/79 |
| c.2230T>C | rs753369867 | Exon 12 | p.Ser744Pro | 2/147 | 1/66 | 1/53 | 0/79 |
| c.3595-3597delGAA | rs200760879 | Exon 22 | p.Glu1199del | 16/133 | * | 9/53 | 17/79 |
| c.3839C>T | rs35490896 | Exon 24 | p.Ser1280Leu | 19/133 | * | 9/53 | 19/79 |
| c.1574A>G, p.Asn525Ser | c.2028+16G>A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypic (n, %) and Allelic Frequencies (%) | ||||||||||||
| Group | n | AA | AG | GG | A | G | GG | GA | AA | G | A | |
| Control | 79 | 51 (64.6) | 20 (25.3) | 8 (10.1) | 122 (77.2) | 36 (22.8) | 11 (13.9) | 19 (24.1) | 49 (62.0) | 41 (25.9) | 117 (74.1) | |
| Breast Cancer | Familial | 147 | 111 (75.5) | 32 (21.8) | 4 (2.7) | 254 (86.4) | 40 (13.6) | 73 (49.6) | 32 (21.8) | 42 (28.6) | 178 (60.5) | 116 (39.5) |
| p value | p < 0.05 | p < 0.05 | p < 0.0001 | p < 0.0001 | ||||||||
| Sporadic | 66 | 56 (84.9) | 9 (13.6) | 1 (1.5) | 121 (91.7) | 11 (8.3) | 10 (15.1) | 12 (18.2) | 44 (66.7) | 32 (24.2) | 100 (75.8) | |
| p value | p < 0.05 | p < 0.01 | 0.6916 | 0.7866 | ||||||||
| Glioma | 53 | 39 (73.6) | 13 (24.5) | 1 (1.9) | 91 (85.8) | 15 (14.2) | 1 (1.9) | 14 (26.4) | 38 (71.7) | 16 (15.1) | 90 (84.9) | |
| p value | 0.1699 | 0.1112 | 0.0615 | p < 0.05 | ||||||||
| CLSPN Variants | |||||||
|---|---|---|---|---|---|---|---|
| Cell Line | c.-68C>T | c.17G>A | c.1574A>G | c.2028+16G>A | c.2230T>C | c.3595_3597del | c.3839C>T |
| HEK293 | WT | WT | WT | AA | WT | WT | WT |
| HeLa | WT | WT | AG | GA | WT | WT | CT |
| RKO | WT | WT | WT | AA | WT | WT | WT |
| U2 OS | WT | AA | AG | GA | WT | WT | WT |
| U87-MG | WT | WT | WT | AA | WT | WT | WT |
| DLD-1 | WT | WT | WT | AA | WT | WT | WT |
| Cancer Type | Number | Gender (Female/Male) | Age (Years, Mean ± SD) | Additional Information |
|---|---|---|---|---|
| Familial Breast Cancer | 147 | 128/19 | 39.4 ± 11 | Co-morbidities: bilateral (16), Ovary cancer (4), Medullary thyroid cancer (4), Colon cancer (3), Melanoma (1), Uterus/endometrial cancer (3), Larynx cancer (1), Gastric cancer (1), Prostate cancer (1) |
| Sporadic Breast Cancer | 66 | 66/0 | >50 | |
| Glioma | 53 | 24/29 | 57.2 ± 14.7 | Grade: I (0); II (11); III (9); IV (33) Types: Oligo (9); Astro (38); Mix (4); Ependimoma (2) |
| Oligonucleotide | Sense Strand Sequence |
|---|---|
| c.-68C>T (C) | ggagacggcgggagcCgctgctctccggctg |
| c.-68C>T (T) | ggagacggcgggagcTgctgctctccggctg |
| Variant | Exon (s) | Sequence | Amplicon (bp) |
|---|---|---|---|
| c.1574A>G | Exon 7 + exon 8 | Fw–TCACTAggatccGCTTTTTTGTACTTAGCTCC Rv–TGATAGacgcgtGCTTAGATCATTCTGATACC | 1200 |
| c.1574A>G | Exon 8 | Fw–TCACTAggatccCATGAACATTTAGTTTTGTAGC Rv–TGATAGacgcgtGCTTAGATCATTCTGATACC | 911 |
| c.2028+16G>A | Exon 10 | Fw–TCACTAggatccCTGAGTAGTATACTATCTAGG Rv–TGATAGacgcgtTACAGATATTCAGTGGTACTG | 652 |
| c.2230T>C | Exon 11 + exon 12 | Fw–TCACTAggatccGGGAAAATTATGTTGATAATGG Rv–TGATAGacgcgtAAACTGCAAAAAATAGACCAAG | 687 |
| c.3595_3597del | Exon 22 | Fw–TCACTAggatccGTGTTTTGAGAAGGCTATACC Rv–TGATAGacgcgtGAACATAAAGTAAAACCAGCC | 549 |
| c.3839C>T | Exon 23 + exon 24 | Fw–TCACTAggatccGTGTCTCTTCTTGGAGCC Rv–TGATAGacgcgtGAAAGATAAACTTTCTCGGC | 871 |
| c.3839C>T | Exon 24 | Fw–TCACTAggatccTTAATGTCAAAGGAGTCTGC Rv–TGATAGacgcgtGAAAGATAAACTTTCTCGGC | 503 |
| Variant | Primers Sequence | Primer Location | Amplicon (bp) |
|---|---|---|---|
| c.1574 A>G (exon 8) | Fw-TAAACCCCGGCCCACTTGCC | Exon 2 | 822 |
| Rv–AGCTTTTCACCTGGTTTTGTGTGGC | Exon 9/10 | ||
| c.2028+16 G>A (intron 10) | Fw-GCCACACAAAACCAGGTGAAAAGCT | Exon 9/10 | 607 |
| Rv-GGCTGATAGGATGGAATCGTGG | Exon 13 | ||
| c.3839 C>T | Fw-GATGAGGCAGAGGTGTCAGG | Exon 19 | 855 |
| Rv-TTAGCTCTCCAAATATTTGAAGATGC | Exon 25 |
| Fragment | Primer Sequence (5′ to 3′) | Size (bp) | |
|---|---|---|---|
| A | Fw 1 | ATGACAGGCGAGGTGGGTTC | 1004 |
| Rv 1 | TTCAATAGTGCCATGGCATTTCC | ||
| B | Oligo 1 | GCCATGGCACTATTGAAAACTGGAAGCCTTGAAGCAGCGTTTCTGGAAGCATGCTAA | 59 |
| Oligo 2 | CGGTACCGTGATAACTTTTGACCTTCGGAACTTCGTCGCAAAGACCTTCGTACGATTT | ||
| Overlap PCR | Fw 1 | ATGACAGGCGAGGTGGGTTC | 1044 |
| Rv 2 | TTAGCATGCTTCCAGAAACGCTG | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azenha, D.; Hernandez-Perez, S.; Martin, Y.; Viegas, M.S.; Martins, A.; Lopes, M.C.; Lam, E.W.-F.; Freire, R.; Martins, T.C. Implications of CLSPN Variants in Cellular Function and Susceptibility to Cancer. Cancers 2020, 12, 2396. https://doi.org/10.3390/cancers12092396
Azenha D, Hernandez-Perez S, Martin Y, Viegas MS, Martins A, Lopes MC, Lam EW-F, Freire R, Martins TC. Implications of CLSPN Variants in Cellular Function and Susceptibility to Cancer. Cancers. 2020; 12(9):2396. https://doi.org/10.3390/cancers12092396
Chicago/Turabian StyleAzenha, Diana, Santiago Hernandez-Perez, Yuse Martin, Marta S. Viegas, Alexandra Martins, Maria C. Lopes, Eric W. -F. Lam, Raimundo Freire, and Teresa C. Martins. 2020. "Implications of CLSPN Variants in Cellular Function and Susceptibility to Cancer" Cancers 12, no. 9: 2396. https://doi.org/10.3390/cancers12092396






