Genetic Testing and Surveillance of Young Breast Cancer Survivors and Blood Relatives: A Cluster Randomized Trial
Abstract
:Simple Summary
Abstract
1. Introduction
Interventions
2. Results
2.1. (Cascade) Genetic Testing
2.2. Breast Cancer Surveillance/Screening
2.3. Effects for Black and White/Other Participants
2.4. Satisfaction with the Interventions
3. Discussion
4. Materials and Methods
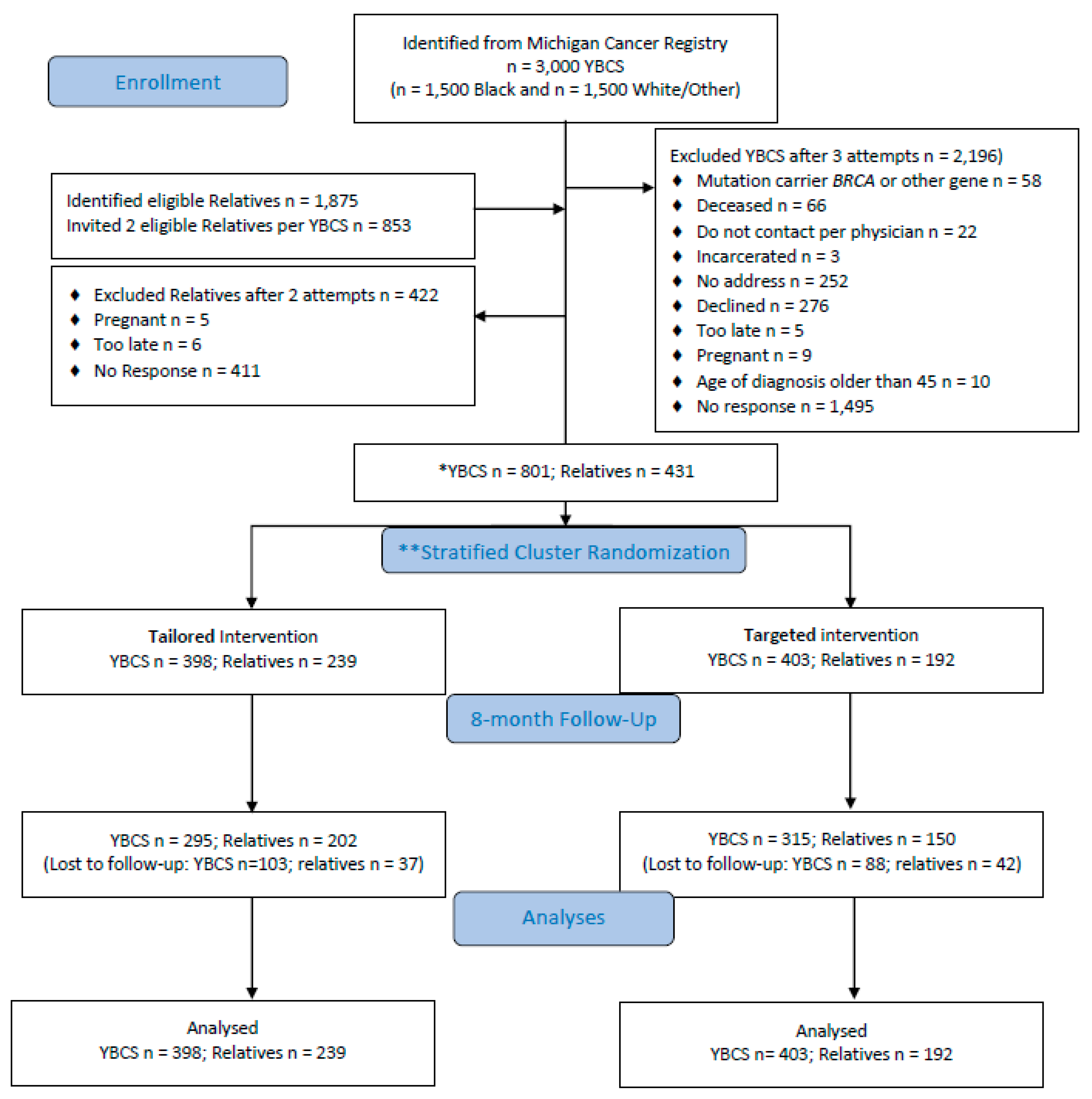
4.1. Design and Sample
4.2. Randomization and Masking
4.3. Data Collection and Measures
4.4. Sample Size and Power Evaluation
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Antecedents | Barriers | Subjective Norms | Family Trait | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YBCS * Outcomes Odds Ratio or Coefficient (p) | Intervention 1 Tailored 2 Targeted | Age | Race 0 White/Other 1 Black | Education ≤High school >High school | Caregiving 0 No, 1 Yes | Anxiety 0 No, 1 Yes | Depression: 0 No, 1 Yes | Comorbidities 0 No, 1 Yes | Income:≤$40,000, >$40,000 | Insurance 0 No, 1 Yes | Routine source of care 0 No, 1 Yes | Cost-related no access to care 0 No, 1 Yes | Distance genetic services | Perc. expect family (1–7) | Perc. expect providers (1–7) | Motivation comply family (1–7) | Motivation comply provider (1–7) | Family coherence (1–20) |
| Genetic testing and surveillance a | ||||||||||||||||||
| Had Genetic Testing | 0.4631 (0.0465) | 0.9829 (0.0478) | ||||||||||||||||
| CBE - NCCN** Guidelines | 0.9741 (0.0017) | |||||||||||||||||
| Mammography - NCCN** Guidelines1 | 0.3223 (0.0060) | 1.7535 (0.0185) | 0.6149 (0.0222) | |||||||||||||||
| Self-Efficacy b | ||||||||||||||||||
| Self-efficacy for genetic testing (1–7) | −0.4797 (0.0205) | −0.7221 (0.0037) | −0.3547 (0.0020) | |||||||||||||||
| Self-efficacy for CBE (1–7) | −0.6961 (0.0007) | −0.1369 (0.0070) | ||||||||||||||||
| Self-efficacy for mammography (1–7)1 | −0.8296 (0.0001) | 0.4030 (0.0457) | −0.1086 (0.0372) | 0.0987 (0.0225) | −0.1639 (0.0039) | |||||||||||||
| Intention b | ||||||||||||||||||
| Intention for genetic testing (1–7) | 0.0739 (0.0002) | 0.9838 (0.0000) | 1.0475 (0.0001) | 0.0139 (0.0000) | −0.4903 (0.0000) | |||||||||||||
| Intention for CBE (1–7) | −0.2229 (0.0076) | −0.1780 (0.0098) | ||||||||||||||||
| Intention for Mammography (1–7)1 | 1.0522 (0.0006) | −0.1703 (0.0323) | ||||||||||||||||
| Antecedents | Barriers | Subjective Norms | Family Trait | |||||||||||||||
| Relative Outcomes Odds Ratio or Coefficient (p) | Intervention 1 Tailored 2 Targeted | Age | Race 0 White/Other 1 Black | Education ≤High school >High school | Caregiving 0 No, 1 Yes | Anxiety 0 No, 1 Yes | Depression: 0 No, 1 Yes | Comorbidities 0 No, 1 Yes | Income ≤$40,000, >$40,000 | Insurance 0 No, 1 Yes | Routine source of care 0 No, 1 Yes | Cost-related no access to care 0 No; 1 Yes | Distance genetic services | Perc. expect family (1–7) | Perc. expect providers (1–7) | Motivation comply family (1–7) | Motivation comply provider (1–7) | Family coherence (1–20) |
| Genetic testing and surveillance a | ||||||||||||||||||
| Had Genetic Testing | 0.0983 (0.0468) | |||||||||||||||||
| CBE - NCCN** Guidelines | 0.3720 (0.0414) | |||||||||||||||||
| Mammography - NCCN** Guidelines 2 | 1.0040 (0.0019) | 2.9500 (0.0273) | 3.659 (0.0147) | 0.2252 (0.0039) | 1.8172 (0.0113) | |||||||||||||
| Self-Efficacyb | ||||||||||||||||||
| Self-efficacy for genetic testing (1–7) | −0.7305 (0.0210) | −0.3751 (0.0016) | ||||||||||||||||
| Self-efficacy for CBE (1–7) | 0.4329 (0.0414) | −0.5037 (0.0475) | −0.2106 (0.0146) | |||||||||||||||
| Self-efficacy for mammography (1–7)2 | −0.0431 (0.0329) | −0.8418 (0.0407) | −0.2897 (0.0337) | |||||||||||||||
| Intentionb | ||||||||||||||||||
| Intention for genetic testing (1–7) | −0.3532 (0.0048) | 0.3122 (0.0294) | ||||||||||||||||
| Intention for CBE (1–7) | −0.0088 (0.0005) | −0.2442 (0.0118) | ||||||||||||||||
| Intention for Mammography (1–7)2 | −0.0301 (0.0089) | −1.014 (0.0129) | −1.0914 (0.0258) | −0.0089 (0.0271) | −0.3514 (0.0411) | |||||||||||||
References
- King, M.C.; Levy-Lahad, E.; Lahad, A. Population-Based Screening forBRCA1andBRCA. JAMA 2014, 312, 1091–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, S.; Newman, L.A.; Partridge, A.H.; Rosenberg, S.S.M. Breast Cancer in Young Women. JAMA Oncol. 2015, 1, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, M.B.; Pilarski, R.; Axilbund, J.E.; Buys, S.S.; Crawford, B.; Friedman, S.; Garber, J.E.; Horton, C.; Kaklamani, V.G.; Klein, C.; et al. Genetic/familial high-risk assessment: Breast and ovarian, version 1. J. Natl. Compr. Cancer Netw. 2014, 12, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.L.; Donohue, K.A.; Alfano, C.M.; Day, J.M.; Herndon, J.E.; Paskett, E.D. Cancer surveillance behaviors and psychosocial factors among long-term survivors of breast cancer. Cancer Leuk. Group B Cancer 2009, 115, 480–488. [Google Scholar] [CrossRef]
- Sabatino, S.A.; Thompson, T.D.; Richardson, L.C.; Miller, J. Health Insurance and Other Factors Associated With Mammography Surveillance Among Breast Cancer Survivors. Med. Care 2012, 50, 270–276. [Google Scholar] [CrossRef]
- Shelby, R.A.; Scipio, C.D.; Somers, T.J.; Soo, M.S.; Weinfurt, K.P.; Keefe, F.J. Prospective Study of Factors Predicting Adherence to Surveillance Mammography in Women Treated for Breast Cancer. J. Clin. Oncol. 2012, 30, 813–819. [Google Scholar] [CrossRef]
- Wirtz, H.S.; Boudreau, D.M.; Gralow, J.R.; Barlow, W.E.; Gray, S.; Bowles, E.J.A.; Buist, D.S.M. Factors associated with long-term adherence to annual surveillance mammography among breast cancer survivors. Breast Cancer Res. Treat. 2014, 143, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Adams, I.; Christopher, J.; Williams, K.P.; Sheppard, V.B. What black women know and want to know about counseling and testing for brca1/2. J. Cancer Educ. 2015, 30, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Glenn, B.A.; Chawla, N.; Bastani, R. Barriers to genetic testing for breast cancer risk among ethnic minority women: An exploratory study. Ethn. Dis. 2012, 22, 267–273. [Google Scholar]
- Levy, D.E.; Byfield, S.D.; Comstock, C.B.; Garber, J.E.; Syngal, S.; Crown, W.H.; Shields, A.E. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet. Med. 2011, 13, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, V.B.; Graves, K.D.; Christopher, J.; Hurtado-De-Mendoza, A.; Talley, C.; Williams, K.P. African American Women’s Limited Knowledge and Experiences with Genetic Counseling for Hereditary Breast Cancer. J. Genet. Couns. 2013, 23, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Kolb, B.; Wallace, A.M.; Hill, D.; Royce, M. Disparities in cancer care among racial and ethnic minorities. Oncology 2006, 20, 1256–1261. [Google Scholar] [PubMed]
- Mai, P.L.; Vadaparampil, S.T.; Breen, N.; McNeel, T.S.; Wideroff, L.; Graubard, B.I. Awareness of cancer susceptibility genetic testing: The 2000, 2005, and 2010 National Health Interview Surveys. Am. J. Prev. Med. 2014, 46, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.; Brennan, K.; Brown, N.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassey, R.; Investigators, K.; O’Connor, M.; Ives, A.; Saunders, C.; O’Sullivan, S.; Hardcastle, S.J. Heightened perception of breast cancer risk in young women at risk of familial breast cancer. Fam. Cancer 2017, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.; Gold, R.; Levin, N.; Tainsky, M.A.; Berry-Bobovski, L. Communication and decision-making about seeking inherited cancer risk information: Findings from female survivor-relative focus groups. Psycho. Oncol. 2006, 15, 193–208. [Google Scholar] [CrossRef]
- Underhill-Blazey, M.; Habin, K.; Shannon, K.M. Perceptions of Cancer Risk, Cause, and Needs in Participants from Low Socioeconomic Background at Risk for Hereditary Cancer. Behav. Med. 2016, 43, 259–267. [Google Scholar] [CrossRef]
- Brevik, T.B.; Laake, P.; Bjørkly, S. Effect of culturally tailored education on attendance at mammography and the Papanicolaou test. Health Serv. Res. 2020, 55, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Champion, V.L.; Skinner, C.S.; Hui, S.; Monahan, P.; Juliar, B.; Daggy, J.K.; Menon, U. The effect of telephone versus print tailoring for mammography adherence. Patient Educ. Couns. 2007, 65, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Champion, V.L.; Springston, J.K.; Zollinger, T.W.; Saywell, R.M.; Monahan, P.O.; Zhao, Q.; Russell, K.M. Comparison of three interventions to increase mammography screening in low income African American women. Cancer Detect. Prev. 2006, 30, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Copeland, V.C.; Kim, Y.J.; Eack, S.M. Effectiveness of Interventions for Breast Cancer Screening in African American Women: A Meta-Analysis. HealTH Serv. Res. 2017, 53, 3170–3188. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-R.; Lee, J.-E.; Kim, J.; Hedlin, H.K.; Song, H.; Kim, M.T. A Meta-Analysis of Interventions to Promote Mammography Among Ethnic Minority Women. Nurs. Res. 2009, 58, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohl, S.J.; Moyer, A. Tailored interventions to promote mammography screening: A meta-analytic review. Prev. Med. 2007, 45, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernon, S.W.; McQueen, A.; Tiro, J.A.; Del Junco, D.J. Interventions to Promote Repeat Breast Cancer Screening With Mammography: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2010, 102, 1023–1039. [Google Scholar] [CrossRef]
- Kinney, A.Y.; Steffen, L.E.; Brumbach, B.H.; Kohlmann, W.; Du, R.; Lee, J.-H.; Gammon, A.; Butler, K.; Buys, S.S.; Stroup, A.M.; et al. Randomized Noninferiority Trial of Telephone Delivery of BRCA1/2 Genetic Counseling Compared With In-Person Counseling: 1-Year Follow-Up. J. Clin. Oncol. 2016, 34, 2914–2924. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.D.; Valdimarsdottir, H.B.; Peshkin, B.N.; Mandelblatt, J.; Nusbaum, R.; Huang, A.-T.; Chang, Y.; Graves, K.; Isaacs, C.; Wood, M.; et al. Randomized Noninferiority Trial of Telephone Versus In-Person Genetic Counseling for Hereditary Breast and Ovarian Cancer. J. Clin. Oncol. 2014, 32, 618–626. [Google Scholar] [CrossRef]
- Pasick, R.J.; Joseph, G.; Stewart, S.L.; Kaplan, C.; Lee, R.; Luce, J.; Davis, S.; Marquez, T.; Nguyen, T.; Guerra, C. Effective Referral of Low-Income Women at Risk for Hereditary Breast and Ovarian Cancer to Genetic Counseling: A Randomized Delayed Intervention Control Trial. Am. J. Public Health 2016, 106, 1842–1848. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behaviour: Reactions and reflections. Psychol. Health 2011, 26, 1113–1127. [Google Scholar] [CrossRef]
- Tadros, A.; Arditi, B.; Weltz, C.; Port, E.R.; Margolies, L.R.; Schmidt, H. Utility of surveillance MRI in women with a personal history of breast cancer. Clin. Imaging 2017, 46, 33–36. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Duquette, D.; Yang, J.J.; Mendelsohn-Victor, K.; Anderson, B.; Nikolaidis, C.; Mancewicz, E.; Northouse, L.L.; Duffy, S.; Ronis, D.; et al. Recruiting families at risk for hereditary breast and ovarian cancer from a statewide cancer registry: A methodological study. Cancer Causes Control. 2017, 28, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.P.; Kreuter, M.; Resnicow, K.; Fishbein, M.; Dijkstra, A. Understanding tailoring in communicating about health. Health Educ. Res. 2008, 23, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuter, M.W.; Farrell, D.W.; Olevitch, L.R.; Brennan, L.K. Tailoring Health Messages; Informa UK Limited: London, UK, 2013. [Google Scholar]
- Kreuter, M.W.; Wray, R.J. Tailored and targeted health communication: Strategies for enhancing information relevance. Am. J. Health Behav. 2003, 27, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, C.; Ming, C.; Pedrazzani, C.; Van Der Horst, T.; Kaiser-Grolimund, A.; Ademi, Z.; Bührer-Landolt, R.; Bürki, N.; Caiata-Zufferey, M.; Champion, V.; et al. Challenges and Opportunities for Cancer Predisposition Cascade Screening for Hereditary Breast and Ovarian Cancer and Lynch Syndrome in Switzerland: Findings from an International Workshop. Public Heal. Genom. 2018, 21, 121–132. [Google Scholar] [CrossRef]
- Ayanian, J.Z.; Ehrlich, G.M.; Grimes, D.R.; Levy, H. Economic effects of medicaid expansion in michigan. N. Engl. J. Med. 2017, 376, 407–410. [Google Scholar] [CrossRef]
- Cragun, D.; Weidner, A.; Lewis, C.; Bonner, D.; Kim, J.; Vadaparampil, S.T.; Pal, T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 2017, 123, 2497–2505. [Google Scholar] [CrossRef] [Green Version]
- Brach, C.; Fraser, I. Can cultural competency reduce racial and ethnic health disparities? A review and conceptual model. Med. Care Res. Rev. 2000, 57 (Suppl. 1), 181–217. [Google Scholar] [CrossRef]
- Kreuter, M.W.; Lukwago, S.N.; Bucholtz, D.C.; Clark, I.M.; Sanders-Thompson, V. Achieving Cultural Appropriateness in Health Promotion Programs: Targeted and Tailored Approaches. Heal. Educ. Behav. 2003, 30, 133–146. [Google Scholar] [CrossRef]
- Resnicow, K.; Baranowski, T.; Ahluwalia, J.S.; Braithwaite, R.L. Cultural sensitivity in public health: Defined and demystified. Ethn. Dis. 1999, 9, 10–21. [Google Scholar]
- Jones, T.; Duquette, D.; Underhill, M.; Ming, C.; Mendelsohn-Victor, K.E.; Anderson, B.; Milliron, K.J.; Copeland, G.; Janz, N.K.; Northouse, L.L.; et al. Surveillance for cancer recurrence in long-term young breast cancer survivors randomly selected from a statewide cancer registry. Breast Cancer Res. Treat. 2018, 169, 141–152. [Google Scholar] [CrossRef]
- Jones, T.; Lockhart, J.S.; Mendelsohn-Victor, K.E.; Duquette, D.; Northouse, L.L.; Duffy, S.A.; Donley, R.; Merajver, S.D.; Milliron, K.J.; Roberts, J.S.; et al. Use of Cancer Genetics Services in African-American Young Breast Cancer Survivors. Am. J. Prev. Med. 2016, 51, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, C.; Duquette, D.; Mendelsohn-Victor, K.E.; Anderson, B.; Copeland, G.; Milliron, K.J.; Merajver, S.D.; Janz, N.K.; Northouse, L.L.; Duffy, S.A.; et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet. Med. 2018, 21, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Bonham, V.L.; Citrin, T.; Modell, S.M.; Franklin, T.H.; Bleicher, E.W.B.; Fleck, L.M. Community-based dialogue: Engaging communities of color in the United states’ genetics policy conversation. J. Heal. Politi. Policy Law 2009, 34, 325–359. [Google Scholar] [CrossRef] [Green Version]
- Bonner, D.; Cragun, D.; Bs, M.R.; Vadaparampil, S.T.; Pal, T. Recruitment of a Population-Based Sample of Young Black Women with Breast Cancer through a State Cancer Registry. Breast J. 2015, 22, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Thomas, M.; Mac Eachern, M. Using registries to recruit subjects for clinical trials. Contemp. Clin. Trials 2014, 41, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Millar, M.M.; Kinney, A.Y.; Camp, N.J.; Cannon-Albright, L.A.; Hashibe, M.; Penson, D.F.; Kirchhoff, A.C.; Neklason, D.W.; Gilsenan, A.W.; Dieck, G.S.; et al. Predictors of Response Outcomes for Research Recruitment Through a Central Cancer Registry: Evidence From 17 Recruitment Efforts for Population-Based Studies. Am. J. Epidemiol. 2019, 188, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Katapodi, M.C.; Northouse, L.L.; Schafenacker, A.M.; Duquette, D.; Duffy, S.A.; Ronis, D.L.; Anderson, B.; Janz, N.K.; McLosky, J.; Milliron, K.J.; et al. Using a state cancer registry to recruit young breast cancer survivors and high-risk relatives: Protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer 2013, 13, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Questionnaire. Available online: https://www.cdc.gov/brfss/questionnaires/pdf-ques/2001brfss.pdf (accessed on 18 January 2019).
- Durand, M.-A.; Witt, J.; Joseph-Williams, N.; Newcombe, R.G.; Politi, M.C.; Sivell, S.; Elwyn, G. Minimum standards for the certification of patient decision support interventions: Feasibility and application. Patient Educ. Couns. 2015, 98, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Northouse, L.L.; Walker, J.; Schafenacker, A.; Mood, D.; Mellon, S.; Galvin, E.; Harden, J.; Freeman-Gibb, L. A Family-Based Program of Care for Women With Recurrent Breast Cancer and Their Family Members. Oncol. Nurs. Forum 2002, 29, 1411–1419. [Google Scholar] [CrossRef]
- Bliss, R.L.; Katz, J.N.; Wright, E.A.; Losina, E. Estimating Proximity to Care. Med. Care 2012, 50, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.; McLosky, J.; Wasilevich, E.; Lyon-Callo, S.; Duquette, D.; Copeland, G. Barriers and Facilitators for Utilization of Genetic Counseling and Risk Assessment Services in Young Female Breast Cancer Survivors. J. Cancer Epidemiol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Kuchenbacker, K.; Engel, C.; Zachariae, S.; Rhiem, K.; Meindl, A.; Rahner, N.; Dikow, N.; Plendl, H.; Debatin, I.; et al. Evaluating the performance of the breast cancer genetic risk models boadicea, ibis, brcapro and claus for predicting brca1/2 mutation carrier probabilities: A study based on 7352 families from the german hereditary breast and ovarian cancer consortium. J. Med. Genet. 2013, 50, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.H.; Brinton, L.A.; Byar, D.P.; Corle, D.K.; Green, S.B.; Schairer, C.; Mulvihill, J.J. Projecting Individualized Probabilities of Developing Breast Cancer for White Females Who Are Being Examined Annually. J. Natl. Cancer Inst. 1989, 81, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Rockhill, B.; Spiegelman, N.; Byrne, C.; Hunter, D.J.; Colditz, G.A. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J. Natl. Cancer Inst. 2001, 93, 358–366. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, H.I.; Thompson, A.I.; McCubbin, M.A. Family Assessment: Resiliency, Coping and Adaptation: Inventories for Research and Practice; University of Wisconsin-Madison: Madison, WI, USA, 1996. [Google Scholar]
- Rakowski, W.; Andersen, M.R.; Stoddard, A.M.; Urban, N.; Al, E. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychol. 1997, 16, 433–442. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights: Breast Cancer, Version 1. J. Natl. Compr. Cancer Netw. 2017, 15, 433–451. [Google Scholar] [CrossRef]
- Chu, A.; Cui, J.; Dinov, I.D. SOCR Analyses: Implementation and Demonstration of a New Graphical Statistics Educational Toolkit. J. Stat. Softw. 2009, 30, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hintze, J. Pass 2008 User’s Guide; Number Cruncher Statistical Software Google Scholar: Kaysville, UT, USA, 2008. [Google Scholar]
- Wright, C.; Sim, J. Intention-to-treat approach to data from randomized controlled trials: A sensitivity analysis. J. Clin. Epidemiol. 2003, 56, 833–842. [Google Scholar] [CrossRef]
- Christou, N.; Dinov, I.D. Confidence Interval Based Parameter Estimation—A New SOCR Applet and Activity. PLoS ONE 2011, 6, e19178. [Google Scholar] [CrossRef]
- Dinov, I.D. Data Science and Predictive Analytics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Dinov, I.D.; Heavner, B.D.; Tang, M.; Glusman, G.; Chard, K.; Darcy, M.; Madduri, R.; Pa, J.; Spino, C.; Kesselman, C.; et al. Predictive Big Data Analytics: A Study of Parkinson’s Disease Using Large, Complex, Heterogeneous, Incongruent, Multi-Source and Incomplete Observations. PLoS ONE 2016, 11, e0157077. [Google Scholar] [CrossRef]
- Toga, A.W.; Dinov, I.D. Sharing big biomedical data. J. Big Data 2015, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosse, S.D.; Rogowski, W.; Ross, L.; Cornel, M.; Dondorp, W.; Khoury, M. Population Screening for Genetic Disorders in the 21st Century: Evidence, Economics, and Ethics. Public Health Genom. 2010, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Evans, J.P. A public health perspective on a national precision medicine cohort: Balancing long-term knowledge generation with early health benefit. JAMA 2015, 313, 2117–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, K.L.; Rivers, S.E.; Latimer, A.E.; Salovey, P. Targeting or tailoring? Maximizing resources to create effective health communications. Mark. Health Serv. 2008, 28, 32. [Google Scholar]
| Adapted TPB * | Tailored Intervention | Targeted Intervention | ||
|---|---|---|---|---|
| Booklet 1—Surveillance and Genetic Testing ** | ||||
| Knowledge | Risk factors and cancer genetics | Risk factors and cancer genetics | ||
| Breast cancer surveillance | Breast cancer surveillance | |||
| Self-efficacy screening and genetic services | Genetic counseling, cost | Genetic counseling, cost | ||
| CBE and Mammography, sources for low cost screening | CBE and Mammography, sources for low cost screening | |||
| Certified genetic services in MI | Certified genetic services in MI | |||
| Booklet 2—Family Support ** | ||||
| Subjective norms | Cancer and open family communication | |||
| Family support in illness | ||||
| Tailored Letter | Targeted Letter | |||
| YBCS | Relatives | YBCS ** | Relatives | |
| Knowledge | Surveillance according to guidelines for follow-up care | Screening according to guidelines for breast cancer | NCCN guidelines for follow-up care | NCCN guidelines for screening |
| Attitudes | Barriers/facilitators to follow-up care | Barriers/facilitators to screening | Increased risk - early age of cancer onset | Increased risk - family history |
| Barriers/facilitators to genetic services | Barriers/facilitators to genetic services | Suggest genetic evaluation | Suggest genetic evaluation | |
| Fear of cancer recurrence | Gail and Claus risk scores | |||
| Genetic literacy, breast cancer risk factors, inheritance | Genetic literacy, breast cancer risk factors, inheritance | |||
| Subjective norms | Family communication | Family communication | ||
| Family support in illness | Family support in illness | |||
| YBCS * | Demographics | Baseline n = 801 | Follow-Up n = 610 | ||
|---|---|---|---|---|---|
|
Tailored n = 398 |
Targeted n = 403 |
Tailored n = 295 |
Targeted n = 315 | ||
| Antecedents | Age (range 25–64) | 51.58 ± 5.73 | 50.65 ± 5.76 | 51.76 ± 5.64 | 51.17 ± 5.51 |
| Race (Black %) | 162 (40.70%) | 162 (40.20%) | 98 (33.22%) | 116 (36.83%) | |
| Education ≤ High School | 85 (21.36%) | 103 (25.56%) | 65 (22.03%) | 78 (24.76%) | |
| Caregiving responsibilities | 120 (30.15%) | 141 (34.99%) | 71 (24.07%) | 89 (28.25%) | |
| Anxiety | 102 (25.63%) | 122 (30.27%) | 80 (27.12%) | 94 (29.84%) | |
| Depression | 109 (27.39%) | 116 (28.78%) | 91 (30.85%) | 91 (28.89%) | |
| Comorbidities | 252 (63.32%) | 277 (68.73%) | 190 (64.41%) | 211 (66.98%) | |
| Barriers ** | Income ≤ $40,000 | 118 (29.65%) | 124 (30.77%) | 90 (30.51%) | 95 (30.16%) |
| No insurance | 30 (7.54%) | 22 (5.46%) | 15 (5.08%) | 17 (5.40%) | |
| No routine source of care | 23 (5.78%) | 33 (8.19%) | 20 (6.78%) | 16 (5.08%) | |
| Cost-related lack of access | 73 (18.34%) | 71 (17.62%) | 42 (14.24%) | 43 (13.65%) | |
| Mean distance to closest genetic center (miles) |
18.58 ± 26.48 (0–147.6) |
19.51 ± 27.38 (0–147.6) |
18.58 ± 26.45 (0–147.6) |
19.24 ± 27.10 (0–147.6) | |
| RELATIVES | Demographics | Baseline n = 431 | Follow-Up n = 352 | ||
| Tailored n = 239 | Targeted n = 192 | Tailored n = 202 | Targeted n = 150 | ||
| Antecedents | Age (range 25–64) | 43.64 ± 12.05 | 43.00 ± 11.69 | 43.45 ± 12.14 | 43.23 ± 11.86 |
| Race (Black %) | 46 (19.25%) | 41 (21.35%) | 33 (16.34%) | 32 (21.33%) | |
| Education ≤ High School | 40 (16.74%) | 32 (16.67%) | 33 (16.34%) | 27 (18.00%) | |
| Caregiving responsibilities | 105 (43.93%) | 80 (41.67%) | 87 (43.07%) | 58 (38.67%) | |
| Anxiety | 72 (30.13%) | 43 (22.40%) | 55 (27.22%) | 34 (22.67%) | |
| Depression | 62 (25.94%) | 49 (25.52%) | 54 (26.73%) | 42 (28.00%) | |
| Comorbidities | 138 (57.74%) | 92 (47.92%) | 115 (56.93%) | 76 (50.67%) | |
| Barriers ** | Income ≤ $40,000 | 65 (27.20%) | 70 (36.46%) | 63 (31.19%) | 55 (36.67%) |
| No insurance | 33 (13.81%) | 23 (11.98%) | 16 (7.92%) | 16 (10.67%) | |
| No routine source of care | 30 (12.55%) | 16 (8.33%) | 20 (9.90%) | 9 (6.00%) | |
| Cost-related lack of access | 52 (21.76%) | 30 (15.63%) | 42 (20.79%) | 28 (18.67%) | |
| Mean distance to closest genetic center (miles) |
21.16 ± 31.09 (0–196.7) |
25.44 ± 33.41 (0–195.9) |
21.16 ± 31.09 (0–196.7) |
25.69 ± 33.65 (0–195.9) | |
| Outcomes for YBCS * Tailored n = 398 Targeted n = 403 | Baseline | Follow-Up ** | Tailored vs. Targeted p Value A (95% CI) | Change from Baseline to Follow-Up p Value B (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Tailored | Targeted | Tailored | Targeted | Tailored | Targeted | ||
| Had Genetic Testing | 79 (19.85%) | 107 (26.55%) | 99 (24.87%) | 127 (31.52%) | 1.00 (−0.030–0.031) | ≤0.001 b (0.031–0.077) | <0.001 b (0.031–0.076) |
| CBE according to NCCN *** Guidelines | 342 (85.92%) | 333 (82.63%) | 361 (90.70%) | 356 (88.33%) | 0.66 (−0.040–0.023) | <0.001 b (0.029–0.074) | <0.001 b (0.037–0.084) |
| Mammography according to NCCN *** Guidelines1 | 298 (87.64%) | 292 (87.16%) | 315 (92.65%) | 302 (90.15%) | 0.17 (−0.009–0.055) | <0.001 b (0.029–0.079) | 0.002b (0.014–0.054) |
| Outcomes for Relatives Tailored n = 239 Targeted n = 192 | Baseline | Follow-Up ** | Tailored vs. Targeted p Value A (95% CI) | Change from Baseline to Follow-Up p Value B (95% CI) | |||
| Tailored | Targeted | Tailored | Targeted | Tailored | Targeted | ||
| Had Genetic Testing | 9 (0.04%) | 4 (0.02%) | 17 (0.07%) | 5 (0.03%) | 0.08 a (−0.001–0.058) | 0.008b (0.015–0.065) | 1b (0.000–0.029) |
| CBE according to NCCN *** Guidelines | 179 (74.89%) | 146 (76.04%) | 204 (85.36%) | 161 (83.85%) | 0.44 (−0.032–0.085) | <0.001 (0.069–0.151) | <0.001b (0.044–0.125) |
| Mammography according to NCCN *** Guidelines 2 | 109 (69.87%) | 87 (71.31%) | 126 (80.77%) | 96 (78.69%) | 0.43 (−0.039–0.110) | <0.001b (0.065–0.168) | 0.004b (0.034–0.135) |
| Outcomes for YBCS * Black n = 324 White/Other n = 447 | Baseline | Follow-Up ** | Black vs. White/Other p Value A (95% CI) | Change from Baseline to Follow-Up p Value B (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Black | White/Other | Black | White/Other | Black | White/Other | ||
| Had Genetic Testing | 52 (16.05%) | 134 (28.09%) | 68 (20.99%) | 158 (33.12%) | 0.92 (−0038–0.054) | <0.001 b (0.028–0.079) | <0.001 b (0.035–0.079) |
| CBE according to NCCN *** Guidelines | 268 (82.72%) | 407 (85.32%) | 286 (88.27%) | 431 (90.36%) | 1 (−0.033–0.036) | <0.001 b (0.033–0.086) | <0.001 b (0.035–0.079) |
| Mammography according to NCCN *** Guidelines 1 | 244 (83.28%) | 346 (90.58%) | 259 (88.40%) | 360 (94.24%) | 0.46 (−0.020–0.049) | <0.001 b (0.029–0.083) | <0.001 b (0.020–0.061) |
| Outcomes for Relatives Black n = 87 White/Other n = 344 | Baseline | Follow-Up ** | Black vs. White/Other p Value A (95% CI) | Change from Baseline to Follow-Up p Value B (95% CI) | |||
| Black | White/Other | Black | White/Other | Black | White/Other | ||
| Had Genetic Testing | 2 (2.30%) | 11 (3.20%) | 4 (4.60%) | 18 (5.23%) | 1.00 a (−0.035–0.039) | 0.5b (0.003–0.081) | 0.016b (0.008–0.041) |
| CBE according to NCCN *** Guidelines | 63 (72.41%) | 262 (76.16%) | 71 (81.61%) | 294 (85.47%) | 1.00 (−0.076–0.068) | 0.008b (0.041–0.173) | <0.001 (0.064–0.129) |
| Mammography according to NCCN *** Guidelines 2 | 39 (65.00%) | 157 (72.02%) | 45 (75.00%) | 177 (81.19%) | 1.00 (−0.085–0.102) | 0.031b (0.038–0.205 | <0.001b (0.057–0.138) |
| I Discussed the Information in the Booklet(s) and Letter with… (Multiple Choice) | Count | ||||||
|---|---|---|---|---|---|---|---|
| No one | 324 | ||||||
| Not a biological relative (spouse, in laws, friend) | 323 | ||||||
| First degree relatives (mother, father, sister, brother, children) | 700 | ||||||
| Second degree relative (grandmother, grandfather, grandchildren, aunts, uncles, nephews, nieces) | 163 | ||||||
| First cousins | 65 | ||||||
| Healthcare provider (oncologist, genetic specialist, nurse, primary care provider) | 124 | ||||||
| Other | 5 | ||||||
| The Brochures and Letter I Received… (1–7) (Mean Score) | Overall | YBCS ** | Relatives | Tailored | Targeted | Black | White/Other |
| …provided me with new information | 4.84 | 4.77 | 4.94 | 4.81 | 4.87 | 5.07 | 4.74 |
| …provided helpful information | 5.15 | 5.16 | 5.14 | 5.14 | 5.17 | 5.36 | 5.07 |
| …were overall easy to understand, important, useful, and interesting * | 5.04 | 5.05 | 5.04 | 5.06 | 5.02 | 5.35 | 4.93 |
| …helped me talk with my healthcare provider about my breast cancer risk | 4.26 | 4.24 | 4.32 | 4.28 | 4.25 | 4.74 | 4.07 |
| …helped me talk with my provider about ways to lower my cancer risk | 4.23 | 4.21 | 4.25 | 4.22 | 4.23 | 4.70 | 4.02 |
| I Would Like to Get More Information about… (1–7) (Mean score) | Overall | YBCS ** | Relatives | Tailored | Targeted | Black | White/Other |
| …risk factors for breast cancer | 4.87 | 4.67 | 5.22 | 4.87 | 4.88 | 5.39 | 4.66 |
| …importance of family history for cancer risk | 4.90 | 4.71 | 5.22 | 4.83 | 4.98 | 5.46 | 4.67 |
| …genetic counseling and genetic testing | 4.83 | 4.73 | 5.02 | 4.75 | 4.92 | 5.47 | 4.57 |
| …where to get genetic counseling and testing | 4.70 | 4.58 | 4.90 | 4.67 | 4.74 | 5.39 | 4.41 |
| …breast cancer screening | 4.86 | 4.71 | 5.10 | 4.86 | 4.86 | 5.43 | 4.63 |
| …low cost breast cancer screening | 4.52 | 4.37 | 4.75 | 4.37 | 4.68 | 5.29 | 4.20 |
| …family communication in breast cancer | 4.26 | 4.18 | 4.41 | 4.13 | 4.41 | 5.04 | 3.95 |
| …family support in breast cancer | 4.22 | 4.14 | 4.36 | 4.11 | 4.34 | 4.98 | 3.91 |
| I would suggest the study to other women like me | 5.77 | 5.81 | 5.70 | 5.77 | 5.77 | 6.05 | 5.66 |
| The study was important | 6.16 | 6.16 | 6.16 | 6.22 | 6.10 | 6.37 | 6.08 |
| I benefited from taking part in the study | 5.57 | 5.51 | 5.67 | 5.61 | 5.53 | 5.97 | 5.40 |
| Instrument | YBCS | Relative | |||
|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | ||
| Demographics | |||||
| Age, Race, Education | Behavioral risk factors surveillance system [49] | √ | √ | ||
| Income, Insurance | Behavioral risk factors surveillance system [49] | √ | √ | ||
| Routine source of care | Coordination of medical care (multiple choices) | √ | √ | ||
| Cost-related lack of access | High out-of-pocket costs (yes/no) | √ | √ | √ | √ |
| Distance—genetic services | Great Circle Distance Formula [52] | √ | √ | ||
| Caregiving responsibilities | Lives with children under 18 years old and/or with elderly parents | √ | √ | ||
| Health history | |||||
| Anxiety, Depression, Comorbidities | Anxiety, Depression, and 11 chronic conditions associated with mobility (yes/no) [53] | √ | √ | √ | √ |
| Cancer and family history | Behavioral risk factors surveillance system (validated) [49] | √ | √ | √ | √ |
| Surgery | American Society of Clinical Oncology (ASCO) guidelines [4] | √ | √ | ||
| Reproductive history | Risk factors associated w/the Gail and the Claus models [54,55,56] | √ | √ | ||
| Family characteristics | |||||
| Family coherence | Family Hardiness Index, 20 items, 7-point Likert scale [57] | √ | √ | ||
| Facilitators and barriers | |||||
| Barriers for mammography | Decisional balance scale for mammography, 20 items, 7-point Likert scale [58] | √ | √ | √ | √ |
| Perceived expectations of healthcare providers/family members | 1 item, 7-point Likert scale “Do you believe that your healthcare providers/relatives want you to get (genetic testing) to find cancer at an early stage?” | √ | √ | ||
| Motivation to comply with recommendations from healthcare providers/family members | 1 item, 7-point Likert scale “How often do you try to do what your healthcare providers/relatives want you to do about finding cancer at an early stage?” | √ | √ | ||
| Genetic services and breast cancer surveillance | |||||
| Genetic services (testing) | NCCN Guidelines [59] | √ | √ | √ | √ |
| Cancer surveillance (CBE, mammography) | NCCN Guidelines [59] | √ | √ | √ | √ |
| Self-efficacy (genetic testing, CBE, mammography) | 1 item, 7-point Likert scale “During the next 12 months how confident do you feel in your ability to ask your healthcare provider for (genetic testing/CBE, mammography).” | √ | √ | √ | √ |
| Intention (genetic testing, CBE, mammography) | 1 item, 7-point Likert scale “During the next 12 months how likely are you to ask your healthcare provider if (genetic testing/CBE/mammography) is a right test for you.” | √ | √ | √ | √ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katapodi, M.C.; Ming, C.; Northouse, L.L.; Duffy, S.A.; Duquette, D.; Mendelsohn-Victor, K.E.; Milliron, K.J.; Merajver, S.D.; Dinov, I.D.; Janz, N.K. Genetic Testing and Surveillance of Young Breast Cancer Survivors and Blood Relatives: A Cluster Randomized Trial. Cancers 2020, 12, 2526. https://doi.org/10.3390/cancers12092526
Katapodi MC, Ming C, Northouse LL, Duffy SA, Duquette D, Mendelsohn-Victor KE, Milliron KJ, Merajver SD, Dinov ID, Janz NK. Genetic Testing and Surveillance of Young Breast Cancer Survivors and Blood Relatives: A Cluster Randomized Trial. Cancers. 2020; 12(9):2526. https://doi.org/10.3390/cancers12092526
Chicago/Turabian StyleKatapodi, Maria C., Chang Ming, Laurel L. Northouse, Sonia A. Duffy, Debra Duquette, Kari E. Mendelsohn-Victor, Kara J. Milliron, Sofia D. Merajver, Ivo D. Dinov, and Nancy K. Janz. 2020. "Genetic Testing and Surveillance of Young Breast Cancer Survivors and Blood Relatives: A Cluster Randomized Trial" Cancers 12, no. 9: 2526. https://doi.org/10.3390/cancers12092526
APA StyleKatapodi, M. C., Ming, C., Northouse, L. L., Duffy, S. A., Duquette, D., Mendelsohn-Victor, K. E., Milliron, K. J., Merajver, S. D., Dinov, I. D., & Janz, N. K. (2020). Genetic Testing and Surveillance of Young Breast Cancer Survivors and Blood Relatives: A Cluster Randomized Trial. Cancers, 12(9), 2526. https://doi.org/10.3390/cancers12092526






