RASAL1 and ROS1 Gene Variants in Hereditary Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Whole Exome Sequencing (WES) Analysis
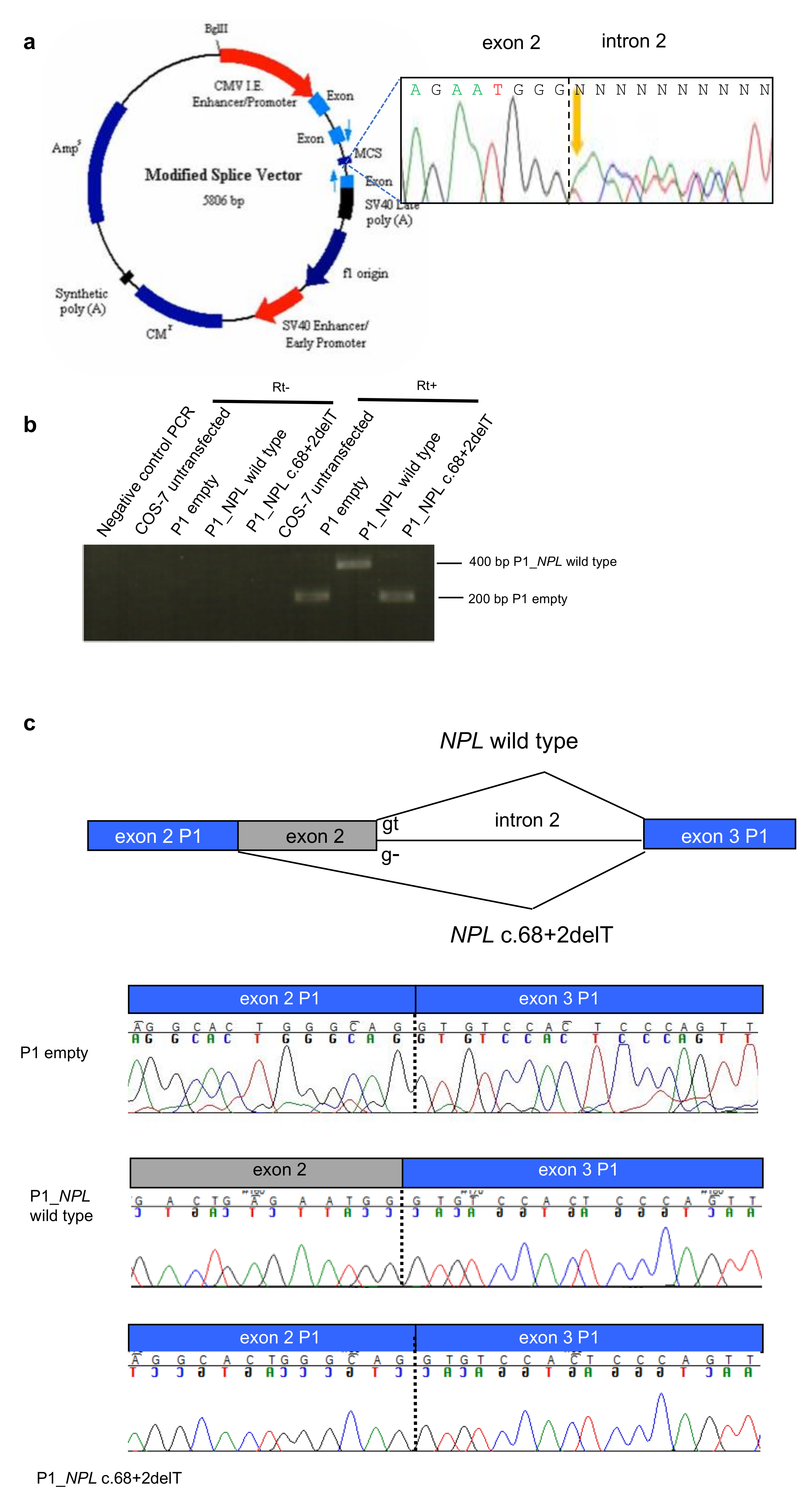
2.2. Splice Site Alterations in NPL and ROS1
2.3. Variant Screening in BC Patients Negative at BRCA1/BRCA2 Testing
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. WES Analysis
4.3. Splice Site Analysis
4.4. Target Gene Screening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, A.C.; Cunningham, A.P.; Peto, J.; Evans, D.G.; Lalloo, F.; Narod, S.A.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Southey, M.C.; et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: Updates and extensions. Br. J. Cancer 2008, 98, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Lindor, N.M. The Role of Risk-Reducing Surgery in Familial Breast and Ovarian Cancer. N. Engl. J. Med. 2016, 374, 454–468. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Michailidou, K.; Beesley, J.; Lindstrom, S.; Canisius, S.; Dennis, J.; Lush, M.J.; Maranian, M.J.; Bolla, M.K.; Wang, Q.; Shah, M.; et al. Genome-wide Association Analysis of More Than 120,000 Individuals Identifies 15 New Susceptibility Loci for Breast Cancer. Nat. Genet. 2015, 47, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Wendt, C.; Margolin, S. Identifying Breast Cancer Susceptibility Genes—A Review of the Genetic Background in Familial Breast Cancer. Acta Oncol. 2019, 58, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomarkers Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Beitsch, P.D.; Whitworth, P.W.; Hughes, K.; Patel, R.; Rosen, B.; Compagnoni, G.; Baron, P.; Simmons, R.; Smith, L.A.; Grady, I.; et al. Underdiagnosis of hereditary breast cancer: Are genetic testing guidelines a tool or an obstacle? J. Clin. Oncol. 2019, 37, 453–460. [Google Scholar] [CrossRef]
- Cybulski, C.; Carrot-Zhang, J.; Kluźniak, W.; Rivera, B.; Kashyap, A.; Wokołorczyk, D.; Giroux, S.; Nadaf, J.; Hamel, N.; Zhang, S.; et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat. Genet. 2015, 47, 643–646. [Google Scholar] [CrossRef]
- Thompson, E.R.; Doyle, M.A.; Ryland, G.L.; Rowley, S.M.; Choong, D.Y.H.; Tothill, R.W.; Thorne, H.; kConFab; Barnes, D.R.; Li, J.; et al. Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet. 2012, 8, e1002894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genome Aggregation Database (gnomAD). Available online: http://gnomad.broadinstitute.org/ (accessed on 16 July 2020).
- Mendelian Clinically Applicable Pathogenicity Score (M-CAP). Available online: http://bejerano.stanford.edu/mcap/ (accessed on 16 July 2020).
- Prediction of Functional Effects of Human nsSNPs (PolyPhen-2). Available online: http://genetics.bwh.harvard.edu/pph2/ (accessed on 16 July 2020).
- Human Splicing Finder (HSF), v.3.1. Available online: http://www.umd.be/HSF3/index.html (accessed on 16 July 2020).
- Genotype-Tissue Expression (GTEx). Available online: https://commonfund.nih.gov/gtex (accessed on 16 July 2020).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 16 July 2020).
- Catalogue of Somatic Mutations in Cancer (COSMIC). Available online: https://cancer.sanger.ac.uk/cosmic (accessed on 16 July 2020).
- Ohta, M.; Seto, M.; Ijichi, H.; Miyabayashi, K.; Kudo, Y.; Mohri, D.; Asaoka, Y.; Tada, M.; Tanaka, Y.; Ikenoue, T.; et al. Decreased expression of the RAS-GTPase activating protein RASAL1 is associated with colorectal tumor progression. Gastroenterology 2009, 136, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Seto, M.; Ohta, M.; Ikenoue, T.; Sugimoto, T.; Asaoka, Y.; Tada, M.; Mohri, D.; Kudo, Y.; Ijichi, H.; Tateishi, K.; et al. Reduced expression of RAS protein activator like-1 in gastric cancer. Int. J. Cancer. 2011, 128, 1293–1302. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, R.; Wei, G.; Jiang, R.; Zheng, Y.; Tian, Y.; Chen, B.; Ye, C.; Xue, M.; Yu, C. RAS protein activator-like 1 is functionally involved in hypoxia resistance in breast cancer cells by targeting hypoxia inducible factor-1α. Oncol. Lett. 2017, 14, 3839–3845. [Google Scholar] [CrossRef] [Green Version]
- Bonora, E.; Evangelisti, C.; Bonichon, F.; Tallini, G.; Romeo, G. Novel Germline Variants Identified in the Inner Mitochondrial Membrane Transporter TIMM44 and Their Role in Predisposition to Oncocytic Thyroid Carcinomas. Br. J. Cancer 2006, 95, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- DesignStudio. Available online: http://designstudio.illumina.com/ (accessed on 16 July 2020).
- Liu, D.; Yang, C.; Bojdani, E.; Murugan, A.K.; Xing, M. Identification of RASAL1 as a major tumor suppressor gene in thyroid cancer. J. Natl. Cancer Inst. 2013, 105, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngeow, J.; Ni, Y.; Tohme, R.; Chen, F.S.; Bebek, G.; Eng, C. Germline alterations in RASAL1 in Cowden syndrome patients presenting with follicular thyroid cancer and in individuals with apparently sporadic epithelial thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1316–E1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povysil, G.; Petrovski, S.; Hostyk, J.; Aggarwal, V.; Allen, A.S.; Goldstein, D.B. Rare-variant collapsing analyses for complex traits: Guidelines and applications. Nat. Rev. Genet. 2019, 20, 747–759. [Google Scholar] [CrossRef]
- Parkin, D.M.; Boyd, L.; Walker, L.C. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105, S77–S81. [Google Scholar] [CrossRef]
- Madigan, M.P.; Ziegler, R.G.; Benichou, J.; Byrne, C.; Hoover, R.N. Proportion of breast cancer cases in the United States explained by well-established risk factors. J. Natl. Cancer Inst. 1995, 87, 1681–1685. [Google Scholar] [CrossRef] [Green Version]
- Tamimi, R.M.; Spiegelman, D.; Smith-Warner, S.A.; Wang, M.; Pazaris, M.; Willett, W.C.; Eliassen, A.H.; Hunter, D.J. Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am. J. Epidemiol. 2016, 184, 884–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer. Version 4. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf (accessed on 16 July 2020).
- Litton, J.K.; Burstein, H.J.; Turner, N.C. Molecular Testing in Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e1–e7. [Google Scholar] [CrossRef]
- Sood, R.; Bonner, T.I.; Makalowska, I.; Stephan, D.A.; Robbins, C.M.; Connors, T.D.; Morgenbesser, S.D.; Su, K.; Faruque, M.U.; Pinkett, H.; et al. Cloning and characterization of 13 novel transcripts and the human RGS8 gene from the 1q25 region encompassing the hereditary prostate cancer (HPC1) locus. Genomics 2001, 73, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.Y.; Zheng, Q.C.; Zhao, Y.S.; Zhang, H.X. Homology modeling and molecular dynamics study on N-acetylneuraminate lyase. J. Mol. Model. 2009, 15, 323–328. [Google Scholar] [CrossRef]
- Takata, K.; Tomida, J.; Reh, S.; Swanhart, L.M.; Takata, M.; Hukriede, N.A.; Woodl, R.A. Conserved overlapping gene arrangement, restricted expression, and biochemical activities of DNA polymerase ν (POLN). J. Biol Chem. 2015, 290, 24278–24293. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Zhang, X.; Li, N.; Zhang, Y.; Jing, P.; Chang, N. ALK and ROS1 as targeted therapy paradigms and clinical implications to overcome crizotinib resistance. Oncotarget 2016, 7, 12289–12304. [Google Scholar] [CrossRef] [Green Version]
- Eom, M.; Lkhagvadorj, S.; Oh, S.S.; Han, A.; Park, K.H. ROS1 expression in invasive ductal carcinoma of the breast related to proliferation activity. Yonsei Med. J. 2013, 54, 650–657. [Google Scholar] [CrossRef]
- Acquaviva, J.; Wong, R.; Charest, A. The Multifaceted Roles of the Receptor Tyrosine Kinase ROS in Development and Cancer. Biochim. Biophys. Acta. 2009, 1795, 37–52. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.A.; Khoo, C.; Solomon, B.J. Targeting ROS1 Rearrangements in Non-small Cell Lung Cancer: Crizotinib and Newer Generation Tyrosine Kinase Inhibitors. Drugs 2019, 79, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Doebele, R.C. Molecular Pathways: ROS1 Fusion Proteins in Cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charest, A.; Wilker, E.W.; McLaughlin, M.E.; Lane, K.; Gowda, R.; Coven, S.; McMahon, K.; Kovach, S.; Feng, Y.; Yaffe, M.B.; et al. ROS Fusion Tyrosine Kinase Activates a SH2 Domain-Containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian Target of Rapamycin Signaling Axis to Form Glioblastoma in Mice. Cancer Res. 2006, 66, 7473–7481. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.T.; Riely, G.J.; Bang, Y.J.; Kim, D.W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roys, A.; Chang, X.; Liu, Y.; Xu, X.; Wu, Y.; Zuo, D. Resistance mechanisms and potent-targeted therapies of ROS1-positive lung cancer. Cancer Chemother. Pharmacol. 2019, 84, 679–688. [Google Scholar] [CrossRef]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef] [Green Version]
- Bajrami, I.; Marlow, R.; Van de Ven, M.; Brough, R.; Pemberton, H.N.; Frankum, J.; Song, F.; Rafiq, R.; Konde, A.; Krastev, D.B.; et al. E-Cadherin/ROS1 Inhibitor Synthetic Lethality in Breast Cancer. Cancer Discov. 2018, 8, 498–515. [Google Scholar] [CrossRef] [Green Version]
- McCoach, C.E.; Le, A.T.; Gowan, K.; Jones, K.; Schubert, L.; Doak, A.; Estrada-Bernal, A.; Davies, K.D.; Merrick, D.T.; Bunn, P.A.J.; et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin. Cancer Res 2018, 2, 3334–3347. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, F.; Loriot, Y.; Kuo, M.S.; Mahjoubi, L.; Lacroix, L.; Planchard, D.; Besse, B.; Farace, F.; Auger, N.; Remon, J.; et al. Crizotinib-Resistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin. Cancer Res. 2016, 22, 5983–5991. [Google Scholar] [CrossRef] [Green Version]
- Drilon, A.; Ou, S.I.; Cho, B.C.; Kim, D.W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Vacher, S.; Boulai, A.; Bernard, V.; Baulande, S.; Bohec, M.; Bièche, I.; Lerebours, F.; Callens, C. Targeted next-generation sequencing identifies clinically relevant somatic mutations in a large cohort of inflammatory breast cancer. Breast Cancer Res. 2018, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Diquigiovanni, C.; Bergamini, C.; Evangelisti, C.; Isidori, F.; Vettori, A.; Tiso, N.; Argenton, F.; Costanzini, A.; Iommarini, L.; Anbunathan, H.; et al. Mutant MYO1F alters the mitochondrial network and induces tumor proliferation in thyroid cancer. Int. J. Cancer 2018, 143, 1706–1719. [Google Scholar] [CrossRef] [Green Version]
- Tournier, I.; Vezain, M.; Martins, A.; Charbonnier, F.; Baert-Desurmont, S.; Olschwang, S.; Wang, Q.; Buisine, M.P.; Soret, J.; Tazi, J.; et al. A large fraction of unclassified variants of the mismatch repair genes MLH1 and MSH2 is associated with splicing defects. Hum. Mutat. 2008, 29, 1412–1424. [Google Scholar] [CrossRef]
- Van der Klift, H.M.; Jansen, A.M.; Van der Steenstraten, N.; Bik, E.C.; Tops, C.M.J.; Devilee, P.; Wijnen, J.T. Splicing analysis for exonic and intronic mismatch repair gene variants associated with Lynch syndrome confirms high concordance between minigene assays and patient RNA analyses. Mol. Genet. Genomic Med. 2015, 3, 327–345. [Google Scholar] [CrossRef]
- Fenne, B.M. Truncated TrkB: Beyond a Dominant Negative Receptor. Cytokine Growth Factor Rev. 2012, 23, 15–24. [Google Scholar] [CrossRef]
- Hu, J.; Jo, M.; Cavenee, W.K.; Furnari, F.; VandenBerg, S.R.; Gonias, S.L. Crosstalk Between the Urokinase-Type Plasminogen Activator Receptor and EGF Receptor Variant III Supports Survival and Growth of Glioblastoma Cells. Proc. Natl Acad. Sci. USA 2011, 108, 15984–15989. [Google Scholar] [CrossRef] [Green Version]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Servizio Sanità Pubblica; Regione Emilia-Romagna. Contributo n. 91/2016: “Protocollo Assistenziale Nelle Donne a Rischio Ereditario di Tumore Della Mammella e/o Ovaio”; Regione Emilia-Romagna: Bologna, Italy, 2016; ISSN 2464-9252. [Google Scholar]
- Zuntini, R.; Ferrari, S.; Bonora, E.; Buscherini, F.; Bertonazzi, B.; Grippa, M.; Godino, L.; Miccoli, S.; Turchetti, D. Dealing with BRCA1/2 unclassified variants in a cancer genetics clinic: Does cosegregation analysis help? Front. Genet. 2018, 9, 378. [Google Scholar] [CrossRef]
- Diquigiovanni, C.; Bergamini, C.; Diaz, R.; Liparulo, I.; Bianco, F.; Masin, L.; Baldassarro, V.A.; Rizzardi, N.; Tranchina, A.; Buscherini, F.; et al. A novel mutation in SPART gene causes a severe neurodevelopmental delay due to mitochondrial dysfunction with complex I impairments and altered pyruvate metabolism. FASEB J. 2019, 33, 11284–11302. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Family ID | Genomic Coordinates (hg19) | Gene | Functional Effect | dbSNP | PolyPhen-2 Scores | M-CAP Score | MAF gnomAD (European) a |
|---|---|---|---|---|---|---|---|
| ITA_BC01 | 6: g.117639349A > C | ROS1 (NM_002944) | c.6005 + 2T > G | rs200482357 | -- | -- | 0.000008850 |
| ITA_BC02 | 12: g.113543542A > G | RASAL1 (NM_001193521) | p.Phe602Leu | rs142556970 b | HumDiv = 0.136 (benign) HumVar = 0.217 (benign) | 0.207 (Possibly pathogenic) | 0.002692452 |
| ITA_BC02 | 4: g.2209742T > G | POLN (NM_181808) | p.Asp229Ala | rs747058815 | HumDiv = 1.000 (Probably damaging) HumVar = 0.993 (Probably damaging) | 0.032 (Possibly pathogenic) | 3.53638 × 10−5 |
| ITA_BC03 | 1:g.182763575GT > G | NPL (NM_030769) | c.68 + 2delT | rs755026414 | -- | -- | 0.000425763 |
| Patient ID | chr6 (hg19) | cDNA NM_002944 | Functional Effect | dbSNP | PolyPhen-2 Scores | M-CAP Score | MAF gnomAD (European) a |
|---|---|---|---|---|---|---|---|
| BC14623 BC12674 | g.117746850C > T | c.-32C > T | 5′UTR | rs376700858 | -- | -- | 0/127478 (p < 0.00001) b |
| BC12132 d BC11298 BC15651 | g.117730797C > T | c.237G > A | p.Ser79= (removing ESE) | rs55736087 | -- | -- | 0.004862 628/129166 (p = 0.1371) b |
| BC10557 BC10536 | g.117715381A > G | c.1108T > C | p.Ser370Pro | rs56274823 | HumDiv = 0.917 Possibly damaging HumVar = 0.446 Benign | 0.086 Possibly pathogenic | 0.003072 390/126944 (p = 0.1937) b |
| BC9762 | g.117710875C > T | c.1397G > A | p.Arg466Gln | rs140178288 | HumDiv = 0.957 Probably damaging HumVar = 0.246 Benign | 0.048 Possibly pathogenic | 0.000007752 1/129000 (p = 0.004) b |
| BC9631 | g.117710828G > T | c.1444C > A | p.Leu482Met | rs757273336 | HumDiv = 1.000 Probably damaging HumVar = 0.998 Probably damaging | 0.075 Possibly pathogenic | 0/113636 (p = 0.0023) b |
| BC14139 | g.117710578G > A | c.1694C > T | p.Ser565Leu | rs142303126 | HumDiv = 0.830 Possibly damaging HumVar = 0.124 Benign | 0.042 Possibly pathogenic | 0.0005425 70/129034 (p = 0.1342) b |
| BC11084 | g.117709072T > C | c.1885A > G | p.Ile629Val (inserting splice donor site) | rs142877218 | HumDiv = 0.000 Benign HumVar = 0.001 Benign | 0.027 Possibly pathogenic | 0.00009297 12/129076 (p = 0.026) b |
| BC13285 | g.117704600C > G | c.2376G > C | p.Met792Ile (removing ESE) | rs140511382 | HumDiv = 0.279 Benign HumVar = 0.081 Benign | -- | 0.00002323 3/129128 (p = 0.0081) b |
| BC11925 | g.117704565G > T | c.2411C > A | p.Thr804Asn | rs200615700 | HumDiv = 0.999 Probably damaging HumVar = 0.922 Probably damaging | 0.085 Possibly pathogenic | 0.0003331 43/129096 (p = 0.0854) b |
| BC12188 | g.117678020T > A | c.3913A > T | p.Ile1305Phe | -- | HumDiv = 0.553 Possibly damaging HumVar = 0.110 Benign | 0.058 Possibly pathogenic | Novel |
| BC14203 BC17333 BC18206 | g.117642495C > T | c.5704G > A | p.Glu1902Lys | rs9489124 | HumDiv = 0.923 Probably damaging HumVar = 0.122 Benign | -- | 0.0009854 127/128884 (p = 0.0024) b |
| BC14840 | g.117642468C > G | c.5731G > C | p.Gly1911Arg | -- | HumDiv = 0.997 Probably damaging HumVar = 0.925 Benign | 0.033 Possibly pathogenic | Novel |
| BC14177 c | g.117639349A > C | c.6005 + 2T > G | Altered donor splice site | rs200482357 | -- | -- | 0.000008850 1/112990 (p = 0.0046) b |
| BC12132 d | g.117622137C > T | c.6733G > A | p.Gly2245Ser | rs142264513 | HumDiv = 0.196 Benign HumVar = 0.063 Benign | 0.032 Possibly pathogenic | 0.001065 124/116398 (p = 0.2451) b |
| BC13141 BC13389 BC16373 | g.117631301C > T | c.6377G > A | p.Arg2126Gln | rs199882276 | HumDiv = 1.000 Probably damaging HumVar = 0.998 Probably damaging | 0.096 Possibly pathogenic | 0.0006670 86/128930 (p = 0.0008) b |
| Patient ID | chr.12 (hg19) | cDNA NM_001193520 | Functional Effect | dbSNP | PolyPhen-2 Scores | M-CAP Score | MAF gnomAD (European) a |
|---|---|---|---|---|---|---|---|
| BC14290 | g.113128174G > A | c.127C > T | p.Ala43Thr | -- | HumDiv = 0.986 Probably damaging HumVar = 0.969 Probably damaging | -- | Novel |
| BC14401 | (1) g.113565948C > T (2) g.113127862T > A | (1) c.158G > A (2) c.248A > T | (1) p.Trp53Ter (2) p.Ile83Asn | rs139329607 | (2) HumDiv = 0.880 Possibly damaging HumVar = 0.718 Possibly damaging | -- | (1) 0.000008797 1/113678 (p = 0.0046) b (2) Novel |
| BC17895 | g.113565663G > A | c.252C > T | p.Ile84= | rs61759864 | -- | -- | 0.001356 175/129046 (p = 0.3004) b |
| BC18101 | g.113546005C > T | c.1400G > A | p.Ser466Asn | -- | HumDiv = 0.494 Possibly damaging HumVar = 0.474 Possibly damaging | 0.099 Possibly pathogenic | Novel |
| BC16851 | g.113553568 delA | c.875 delT | p.Leu292CysfsTer5 | -- | -- | -- | Novel |
| Individuals | ROS1 | RASAL1 | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | Total | Cases | Controls | Total | |
| Mutated | 16 | 11 | 27 | 4 | 4 | 8 |
| Not mutated | 115 | 186 | 301 | 127 | 193 | 320 |
| Total | 131 | 197 | 328 | 131 | 197 | 328 |
| p = 0.0401 a | p = 0.7179 a | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isidori, F.; Bozzarelli, I.; Ferrari, S.; Godino, L.; Innella, G.; Turchetti, D.; Bonora, E. RASAL1 and ROS1 Gene Variants in Hereditary Breast Cancer. Cancers 2020, 12, 2539. https://doi.org/10.3390/cancers12092539
Isidori F, Bozzarelli I, Ferrari S, Godino L, Innella G, Turchetti D, Bonora E. RASAL1 and ROS1 Gene Variants in Hereditary Breast Cancer. Cancers. 2020; 12(9):2539. https://doi.org/10.3390/cancers12092539
Chicago/Turabian StyleIsidori, Federica, Isotta Bozzarelli, Simona Ferrari, Lea Godino, Giovanni Innella, Daniela Turchetti, and Elena Bonora. 2020. "RASAL1 and ROS1 Gene Variants in Hereditary Breast Cancer" Cancers 12, no. 9: 2539. https://doi.org/10.3390/cancers12092539







