1. Introduction
Triple-negative breast cancer (TNBC), accounting for 15–20% of all breast cancers (BC), is defined by the absence of oestrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) expression on cancer cells [
1]. It is a heterogeneous disease, including cancers with different gene expression and clinical features [
2,
3]. As compared to other BC subtypes, it has a worse prognosis, an increased risk of visceral metastasis and fewer therapeutic targets [
4,
5]. Treatment of early and locally advanced TNBC requires a multidisciplinary approach to reach better outcomes [
6]. Anthracycline and taxane-based neoadjuvant chemotherapy (NACT) is the most common choice in early and locally advanced TNBC due to the possibility to achieve a pathological complete response (pCR), which can impact on survival outcomes. In fact, patients who achieve a pCR have better disease-free survival (DFS) and overall survival (OS) compared to patients with residual disease (RD) [
7]. Several meta-analysis confirmed the prognostic role of pCR, especially in aggressive BC subtypes such as triple-negative and HER2 positive [
8,
9,
10]. The pCR is a primary endpoint in several studies and could be considered as a surrogate of long term survival outcomes [
11]. Despite TNBC is more sensitive to chemotherapy compared to other BC subtypes, only about 30% to 50% of patients will achieve a pCR after NACT [
12]. Therefore, the identification of new targets and therapeutic strategies is an urgent clinical need. If compared to other BC subtypes, TNBC appears to be more immunogenic [
13]. Moreover, accumulating data suggest that the antitumor activity of conventional chemotherapy is partly due to the enhancement of innate and adaptive immune-response through the triggering of immunogenic cell death (ICD) [
14]. Pre-treatment immune activation against cancer cells could influence the response to chemotherapy [
15]. Tumor infiltrating lymphocytes (TILs) are a prognostic biomarker in different types of cancer [
16]. In TNBC, high TILs have been associated with better prognosis and higher chance to achieve a pCR after NACT [
17,
18].
The interaction between Programmed cell Death protein 1 (PD-1) and its ligand Programmed Death-Ligand 1 (PD-L1) is one of the main mechanisms of immune escaping and the PD-1/PD-L1 axis represents a therapeutic target in several malignancies [
19]. PD-1 is a cell surface protein receptor expressed on both CD8+ cytotoxic and CD4+ helper T cells, B cells, natural killer (NK) cells, dendritic cells, and activated monocytes, while his ligand PD-L1 can be expressed on both immune and cancer cells [
20]. After engagement by its ligands, PD-1 indices exhausted T CD8+ and CD4+ lymphocytes and promotes T-regulatory cell proliferation and migration, allowing the immune response to be switched off [
21]. In BC, the prognostic role of PD-L1 is still uncertain, with limited and contrasting data [
22].
Recently, CD73 has been identified as a further molecular immunosuppressive element in TNBC [
23]. CD73 is an ectonucleotidase, expressed on the surface of cancer, stromal, and immune cells that converts adenosine monophosphate (AMP) to adenosine, a soluble immunosuppressive factor. By increasing extracellular adenosine, CD73 suppresses immune response through activation of high-affinity A2A and A2B adenosine receptors. Several types of human cancers overexpress CD73, which has been associated with a poor prognosis [
24]. In TNBC, CD73 overexpression seems to be associated with resistance to anthracycline-based chemotherapy and poor prognosis [
25,
26].
TILs, PD-L1, and CD73 are immune-related biomarkers that can be evaluated in neoplastic cells and tumor microenvironment. Previous studies from our group have shown that both TILs and CD73 can individually predict the response to NACT in TNBC B [
27,
28]. We now investigate if the contemporary expression of these biomarkers, along with PD-L1, combined in a tissue immune profile (TIP), can be more efficient at identifying patients prone to achieve a pCR.
2. Results
Clinical and pathological features of the 60 early TNBC patients in the study population are reported in
Table 1.
Median age was 49 (range 28–74). Fifty-nine patients (98.3%) had no special type ductal carcinoma, and 57 (95.0%) had a poorly differentiated (G3) carcinoma. Clinical stage at diagnosis was cT2 in the majority of patients (75.4%) with nodal involvement (cN+) in 46.7% of cases. Thirty-six (60%) patients had a clinical TNM II stage and 24 (40%) III stage. The percentage of Ki67 positive cells was ≥50% in 47 patients (78.4%). A pCR was achieved in 23 patients (38.0%). Thirty-one patients underwent conservative surgery while the remaining received mastectomy. Clinical node status was the only feature significantly associated with response to NACT (
p = 0.047,
Table 2).
The results of tissue biomarkers evaluation on the 60 pre-NACT biopsy specimens are reported in
Table 3.
Briefly, TILs were present in all pre-NACT biopsies with values ≥ 50% (H-TILs) in 17 samples (28.4%). The median expression value of CD73 on CC was 40%. Absence of CD73 immunostaining was recorded in seven out of 60 cases (11.6%). Twenty-nine biopsies (48.4%) were classified as high-CD73 with a mean expression score of 63.8% while in the remaining low CD73 group (51.6%) the mean expression value was 15.3%. Interestingly, the median value of CD73 was significantly lower in the H-TILs group compared to the L-TILs one (
p = 0.01). A positive PD-L1 staining on IC was recorded in 49 out of 60 pre-NACT biopsies (81.7%). The relation between each biomarker and the response to NACT is reported in
Table 4.
As expected, H-TILs on pre-NACT biopsies were associated with significantly higher rate of pCR (76.5% vs. 21.5%,
p = 0.001). Among PD-L1-positive patients, 18 (36.7%) had a pCR and 31 had a RD (63.3%). No significant difference in response to NACT was recorded between the two groups of patients (
p = 0.734). Patients with PD-L1 ≥ 1% had higher TILs compared to PD-L1 negative (
p = 0.02,
Figure 1). Finally, absent/low CD73 on neoplastic cells was significantly associated with pCR (54.8% vs. 20.6%,
p = 0.009).
All 60 patients were evaluable for TIP analysis. According to the established criteria, 12 (20.0%) were considered TIP positive (TIP+) and 48 (80.0%) TIP negative (TIP−). A pCR was achieved in eleven (91.7%) out of 12 TIP+ patients compared to 12 (25.0%) out of 48 TIP−. Using a univariate analysis, a positive TIP was significantly associated with pCR (
p < 0.0001,
Table 4). Using a logistic regression for age, tumor proliferation index evaluated by Ki67 and clinical lymph nodes involvement, TIP was confirmed as an independent predictive factor of pCR (OR 49.7 (6.30–392.4)),
p < 0.0001,
Table 5).
Pre-NACT clinical nodal stage was also confirmed as predictive factor (OR 0.11 (0.02–0.62),
p = 0.013). Finally, we compared the efficacy of TIP versus each single biomarker in predicting pCR by Akaike information criterion (AIC) and Bayesian information criterion (BIC). According to our analysis, the combined immune profile is more accurate in predicting pCR (AIC 68.3; BIC 74.5;
Table 6).
3. Discussion
Patients who achieve a pCR after NACT have a better prognosis compared to those with residual disease. However, only 30–40% of patients will achieve a pCR after standard treatments. Our study is in line with these data, reporting a pCR rate of 38%. In view of that, the identification of responder or resistant patients is an urgent clinical need. Moreover, novel biomarkers are envisaged, in light of the low predicting value of the clinical and pathological features currently available. In our study population, only cN status was an independent predictive factor of response to NACT. In particular, patients with clinical involvement of axillary lymph nodes had a significant lower pCR rate. Clinical TNM staging was not associated to pCR in our population. This result could be influenced by the relative small sample size; however, it is in line with several previous papers, highlighting the role of cancer biology over extension [
9,
29,
30]. A promising field of research is represented by the study of tumor microenvironment and immune background. Features of pre-existing immune activation in neoplastic tissue, such as TILs ≥50% (high TILs) have been associated with the highest probability to achieve a pCR in TNBC, which is the most immunogenic of breast cancer subtypes [
17]. The postulated mechanism could be an increased reaction to drug-mediated immunogenic death by a “ready-to-act” immune environment. A potential biomarker currently under active investigation is CD73, marker of immune suppression [
31]. While several studies confirmed the role of CD73 as a prognostic factor, its predictive role is still to be clarified [
26]. We previously reported a significant association between low level of CD73 expression by CC (below or equal to the median value in our population, 40%) and pCR in TNBC. A robust marker of pre-existing immune-activation against cancer cells is represented by PD-L1. In TNBC, PD-L1 expression by immune cells seems to be associated with response to various immunotherapy regimens [
32]. The cut-off of PD-L1 expression ≥1% on IC is currently used to define PD-L1 positivity in clinical practice [
33].
In light of current evidence suggesting a relevant role of the immune microenvironment in the response to NACT we tested the predictive role of TILs, CD73 expression on CC and PD-L1 staining of IC in a sample of TNBC. According to our results, high TILs are strongly associated with pCR (
Table 4), in line with previous reports. Repeated statistical analysis on the present study sample confirmed a significant association between absent/low CD73 expression and pCR (
Table 4). We found that samples with low TILs had a significantly higher expression of CD73 compared to those with high TILs (
p = 0.02), as a possible consequence of an immunosuppressive microenvironment.
No significant association between PD-L1 and response to NACT was observed (
Table 4).
The landscape of biomarkers is characterized by a plethora of single factors used to predict the response to treatment or the toxicity of therapeutic regimens. However, the idea that a single biomarker could represent the entire dynamic and complex relationship between cancer (metabolism, immunity, genomic profile) and host (clinical features, pharmacogenomics, microbiome, immune system) seems to be too simplistic. In this context, the combination of different biomarkers could be a more promising approach.
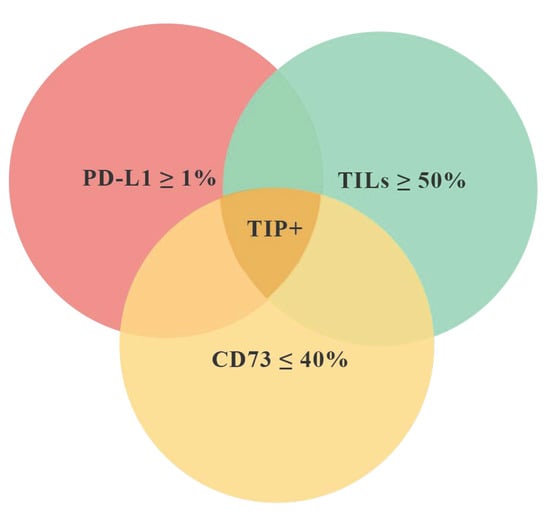
According to the main purpose of the study, we have identified a “favorable” tissue immune profile (TIP+) characterized by the simultaneous presence of three features: TILs ≥ 50%, CD73 ≤ 40% on CC, PD-L1 ≥ 1% on IC (
Figure 2). We then tested if the tissue immune profile could be more efficient than the single biomarkers at identifying patients more prone to achieve a pCR. In twelve patients (20.0% of the entire population), the pre-NACT samples met the inclusion criteria for TIP+. Eleven (91.7%) out of these 12 patients achieved a pCR. The difference of pCR rate between TIP+ and TIP− was statistically significant (
p < 0.0001,
Table 4). A multivariate analysis including clinical and pathological data confirmed the significant association between TIP+ and pCR (
p < 0.0001,
Table 5). Thus, a positive tissue immune profile is more efficient than each single biomarker in predicting a pCR (
Table 6).
This study has some limitations. Firstly, it is a retrospective, single-institute trial. The relatively small sample size of the population studied could be related to unexpected results, such as the borderline correlation that emerged between pCR and disease extension or the lack of association between PD-L1 and pCR. A prospective trial with a larger sample size is necessary to validate our results. Moreover, the benefit obtained using the profile over TILs alone (
Table 6) is significant but limited, emphasizing the need for validation on a larger sample. Finally, further evaluation will be needed to confirm the predictive value of TIP after addition of immunotherapy to chemotherapy backbone.
Recent clinical trials have shown promising activity of immune-checkpoint inhibitors on early TNBC, highlighting the need for new immune-related biomarkers. In Keynote 173 trial, the addition of pembrolizumab, an anti PD-1 agent, to standard chemotherapy produced a significant increase of pCR rate in an unselected population [
34]. In the phase III GEPAR NUEVO trial, the addition of durvalumab, an another anti PD-1 agent, resulted in an increased rate of pCR from 44% to 53% [
35]. In contrast, preliminary results from NeoTRIP trial show no significant difference in pCR adding atezolizumab to a backbone of chemotherapy with weekly carboplatin plus nab-paclitaxel in the same setting [
36]. Finally, the results from phase III Keynote-522 trial show that adding pembrolizumab to standard neoadjuvant chemotherapy increased the pCR rate and improve DFS, regardless of PD-L1 expression [
37]. Based on these results, it is expected that anti PD-1 agents will soon be introduced in neoadjuvant treatment of early TNBC. As a future perspective, an immune-profile, such as TIP, could improve the selection of patients who could benefit from combination strategies with anti PD-1 agents, considering the high cost and additional toxicities related to therapy escalation.