FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. FUCCI Images Cell-Cycle Dynamics of Cancer Cells in Real-Time
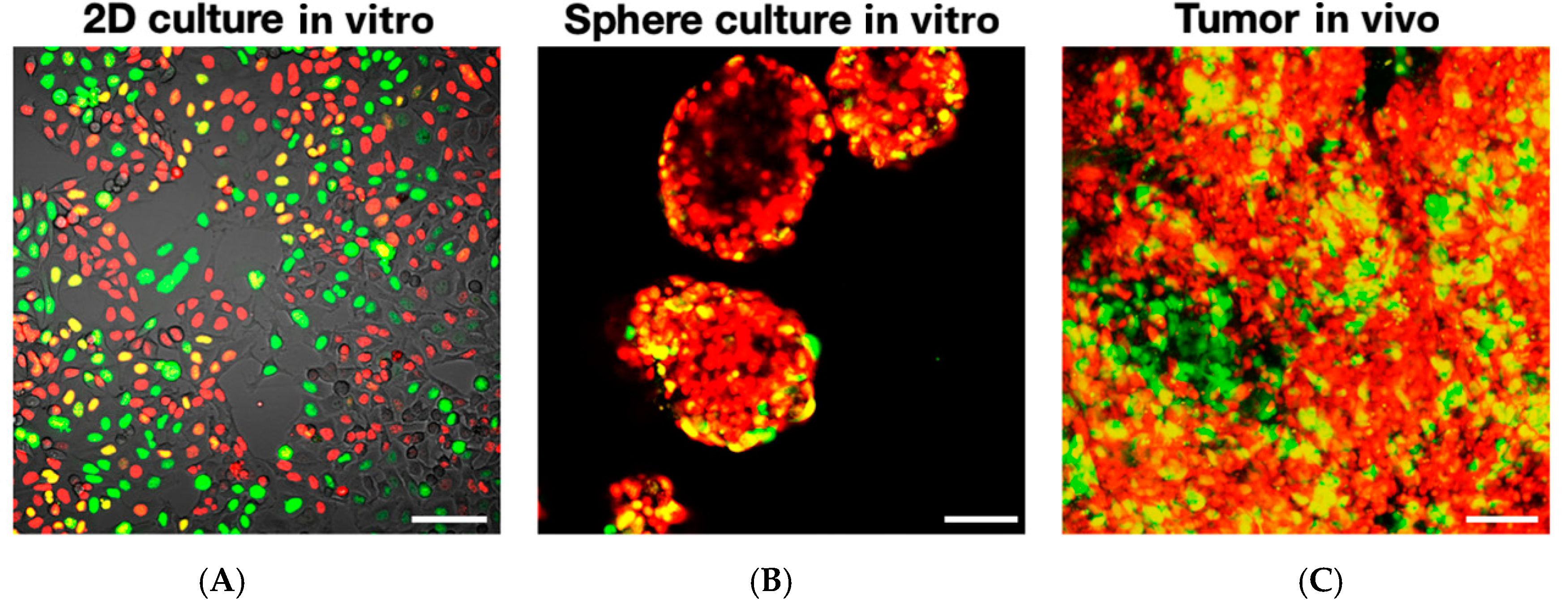
FUCCI Imaging Demonstrates Different Cell-Cycle Dynamics in 2D- and 3D-Cultured Cancer Cells
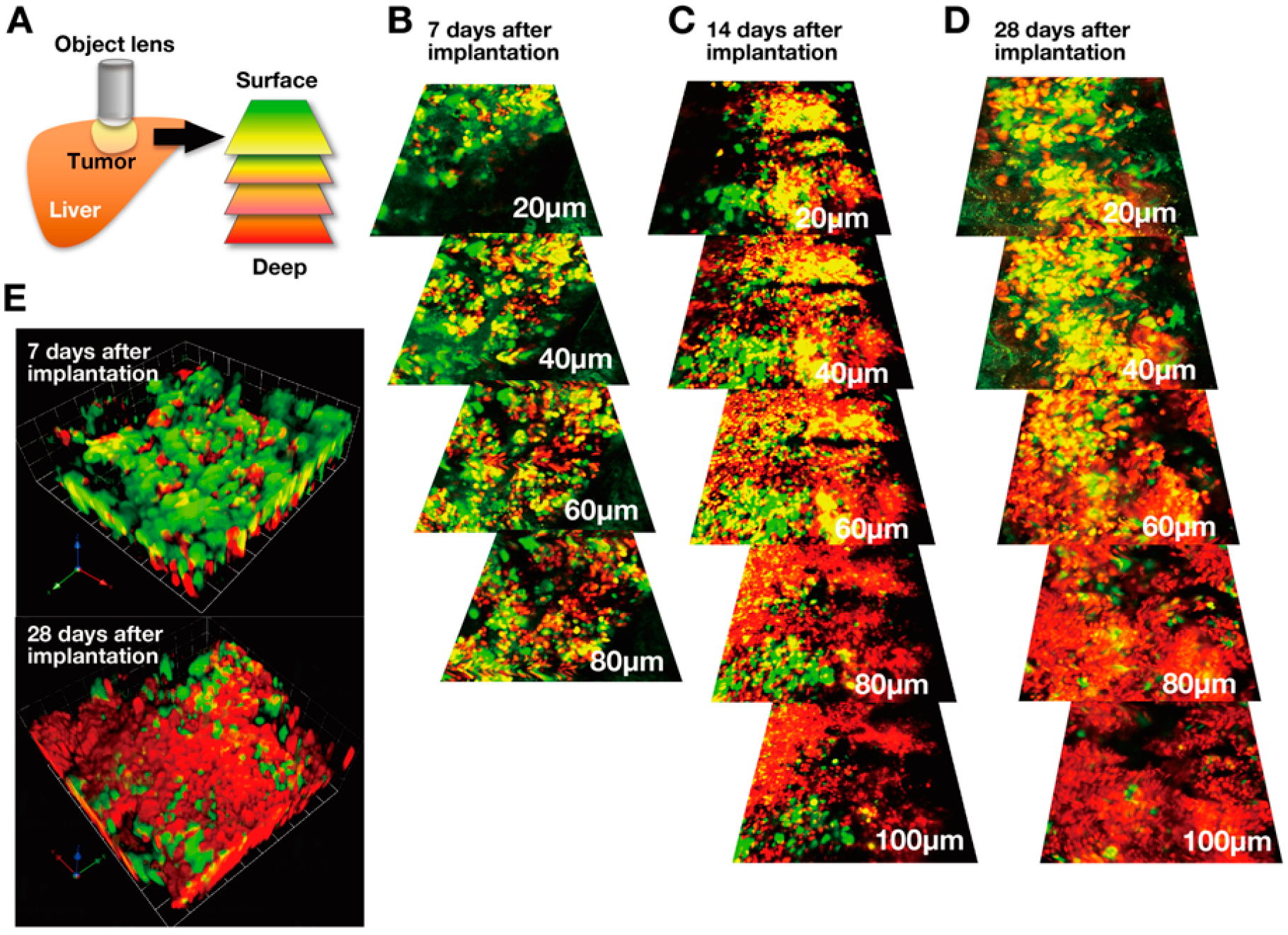
3. Real-time FUCCI Imaging of Cell-Cycle Dynamics of Cancer Cells in Solid Tumors
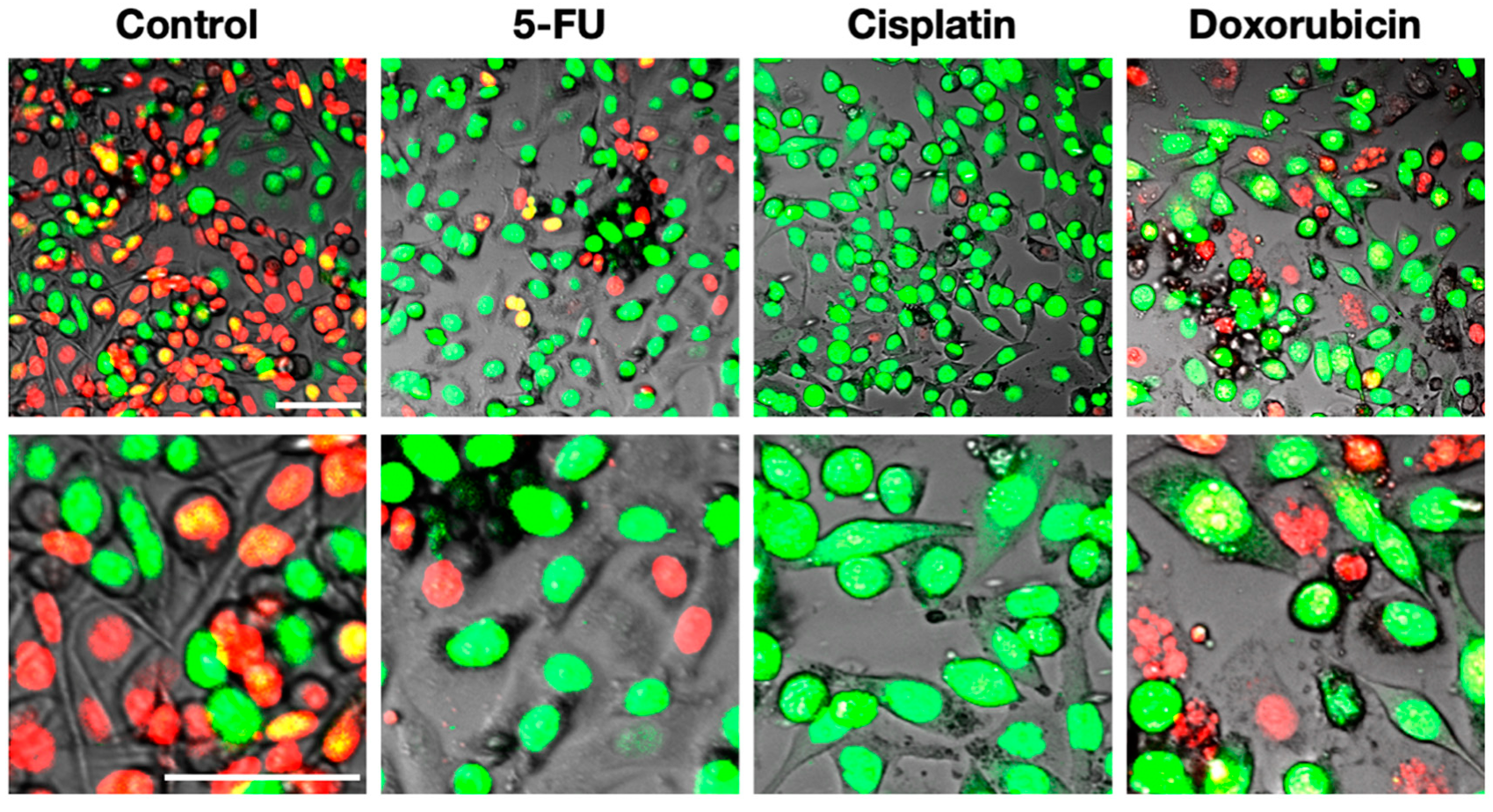
4. FUCCI Imaging of Cell-Cycle Phases of Cancer Cells during Chemotherapy and Radiotherapy In Vitro and In Vivo Identifies Resistant Cells
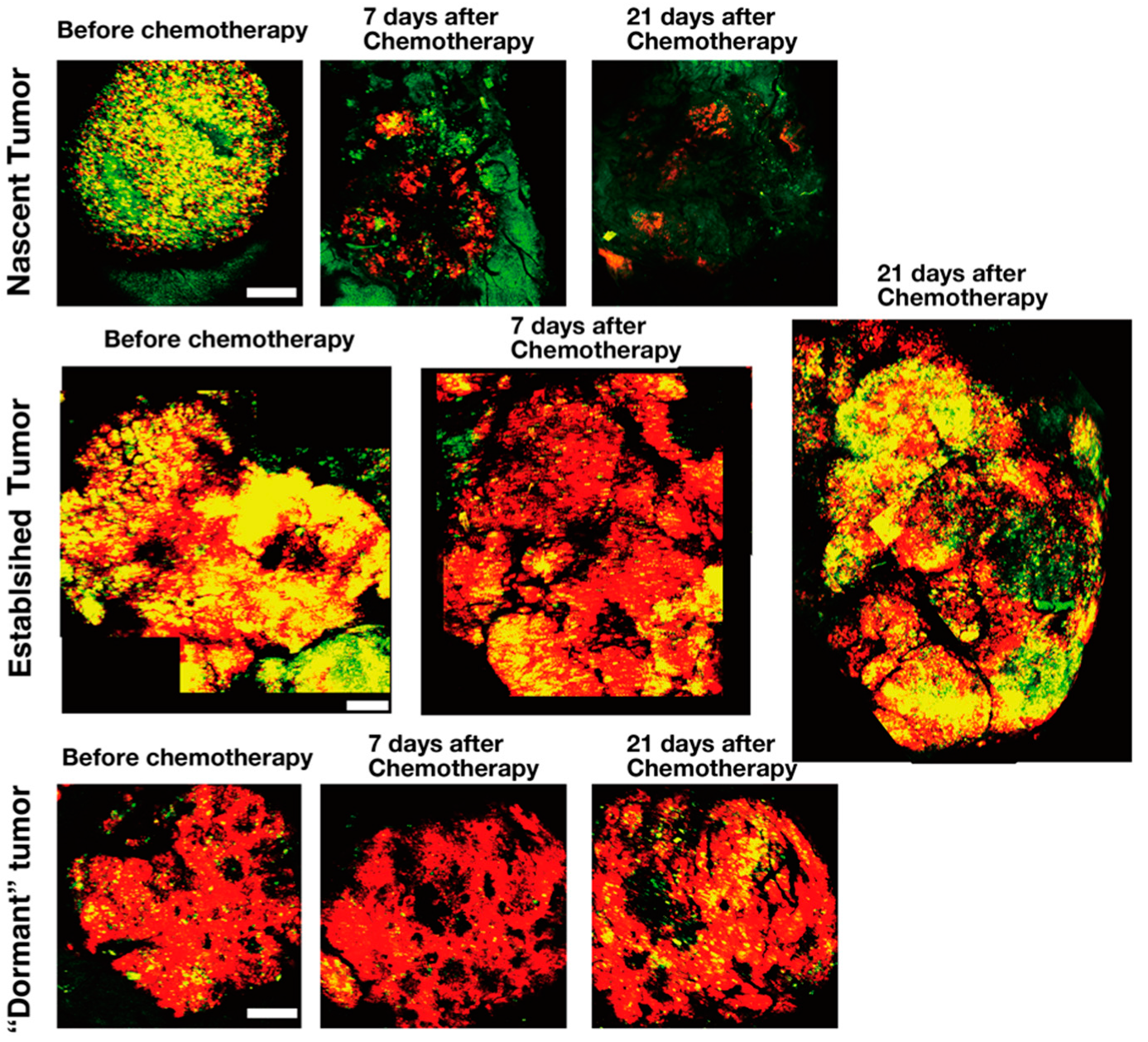
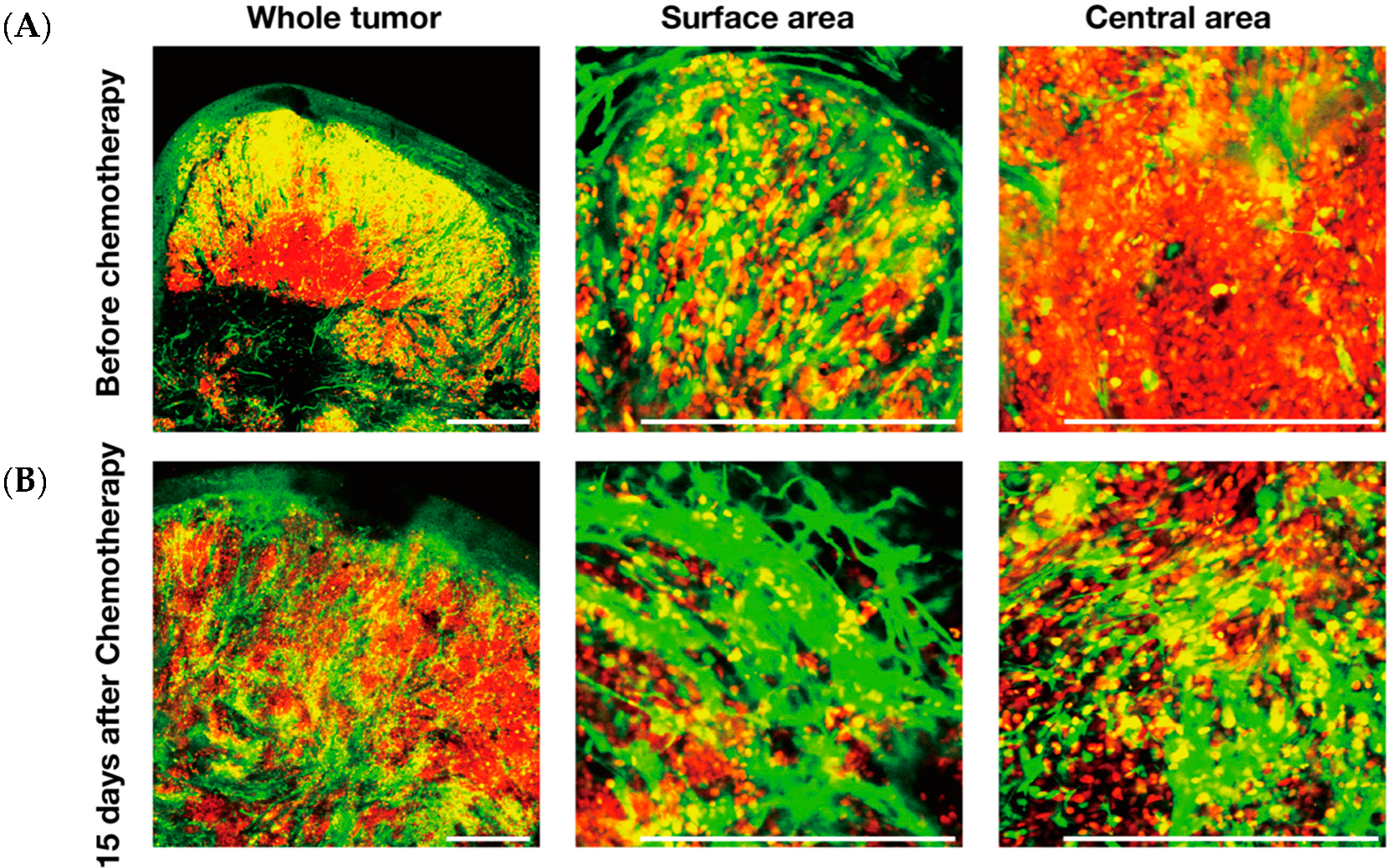
5. FUCCI Imaging Identified Angiogenesis Occurring Among Resistant Cells During Chemotherapy of Tumors
6. Use of FUCCI to Identify and Target Quiescent Cancer Cells In Vitro and In Vivo
6.1. FUCCI Imaging Can Identify and Target Quiescent Cancer Stem Cells
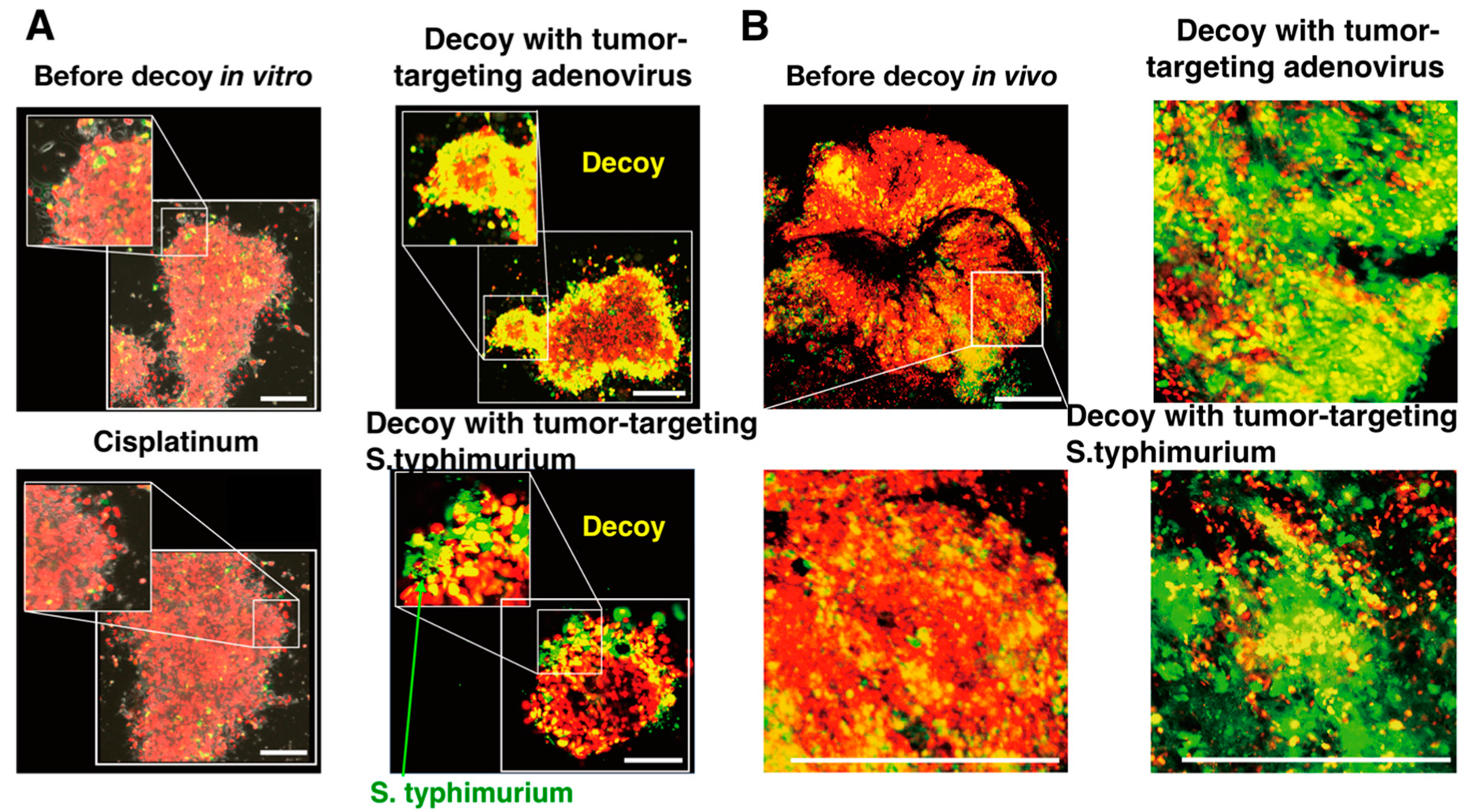
6.2. Decoy of Quiescent Cancer Stem Cells to Commence Cycling with a Tumor-Targeting Adenovirus, Demonstrated by FUCCI Imaging
6.3. Tumor-Targeting Salmonella Typhimurium Decoys Quiescent Cancer Cells in Tumors to Cycle and Become Chemosensitive, Shown by FUCCI Imaging
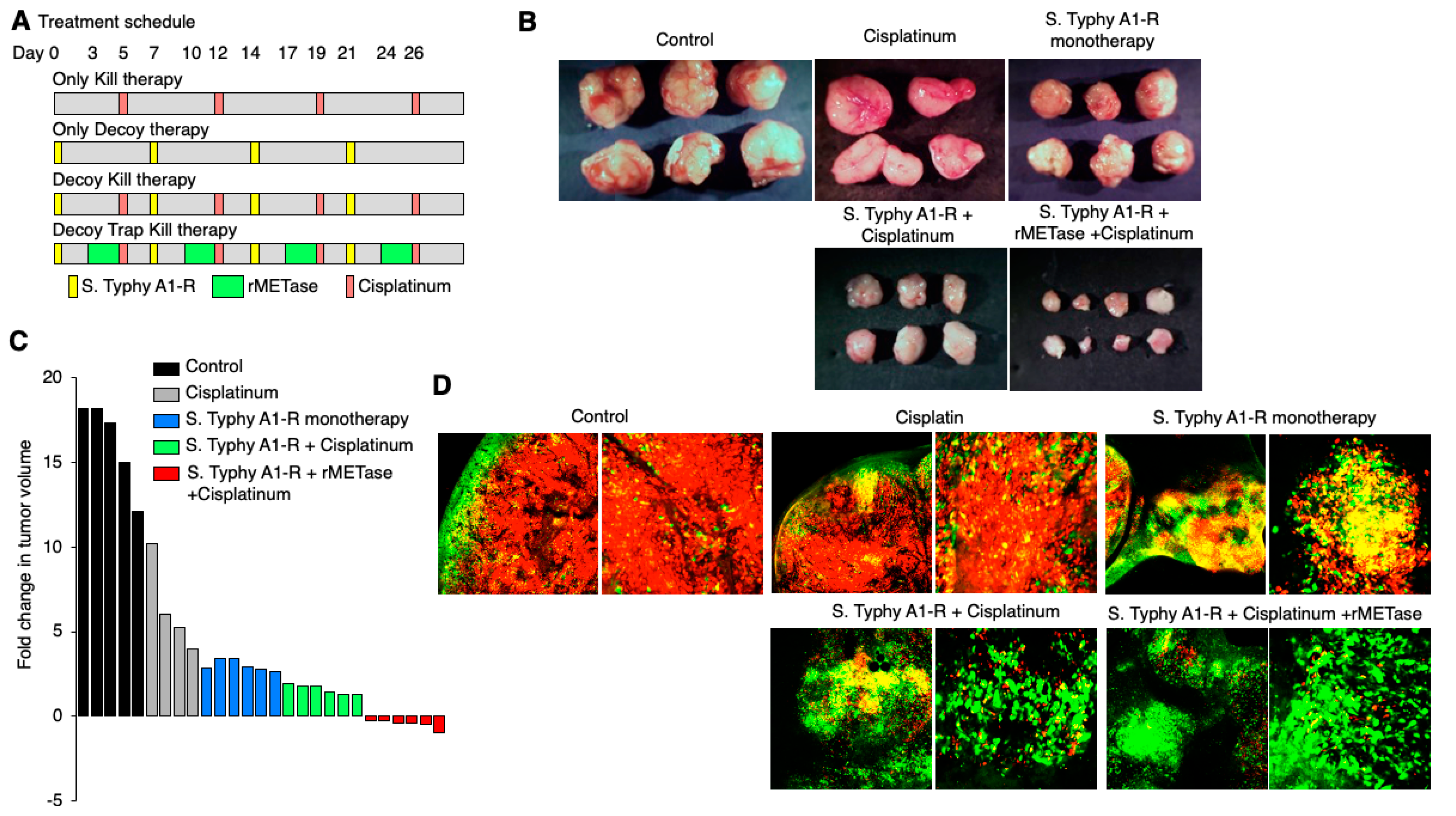
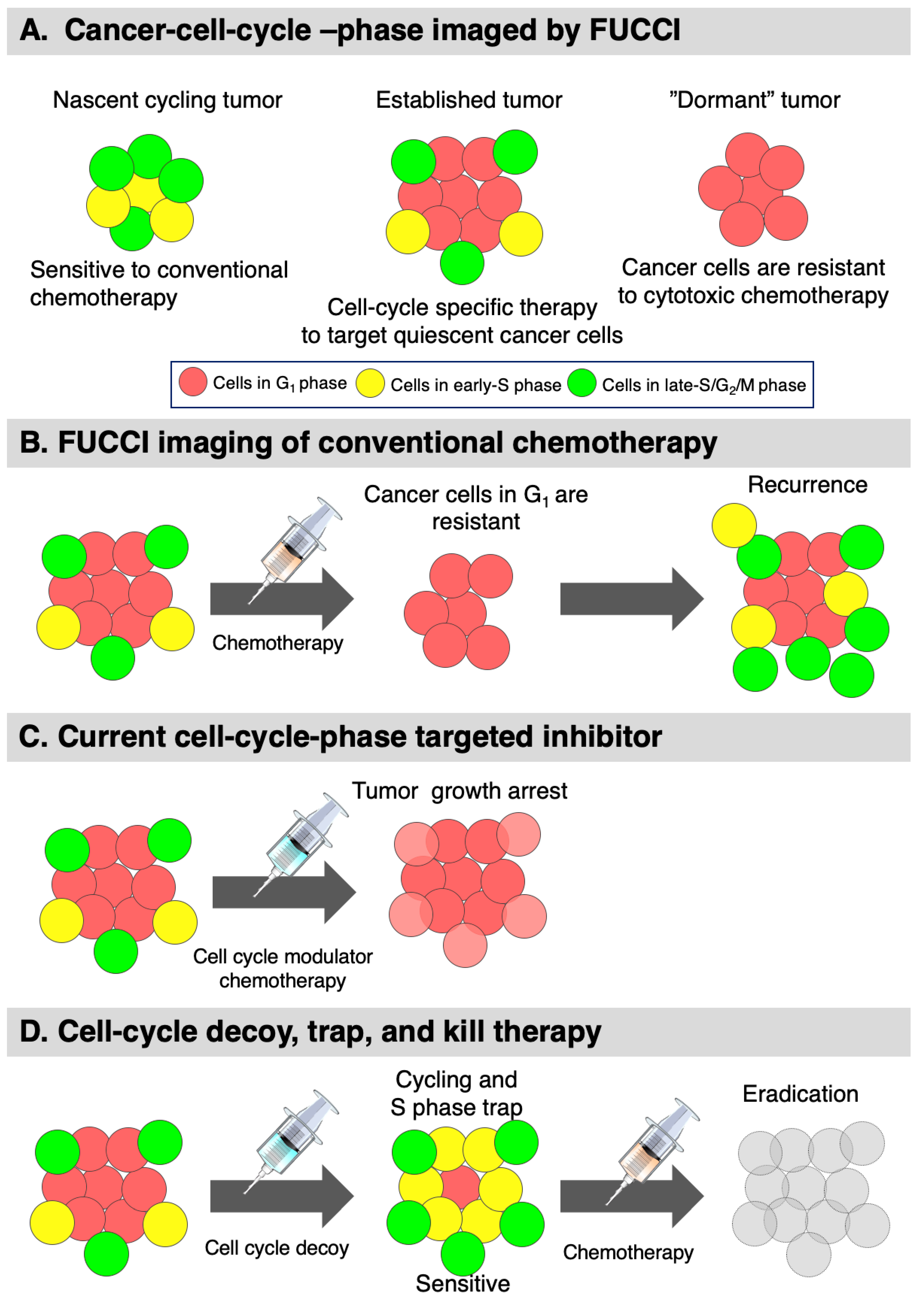
6.4. Decoy, Trap, and Kill Cancer Therapy Developed with FUCCI Imaging
6.5. FUCCI Imaging Demonstrates that Invading Cancer Cells Are Quiescent
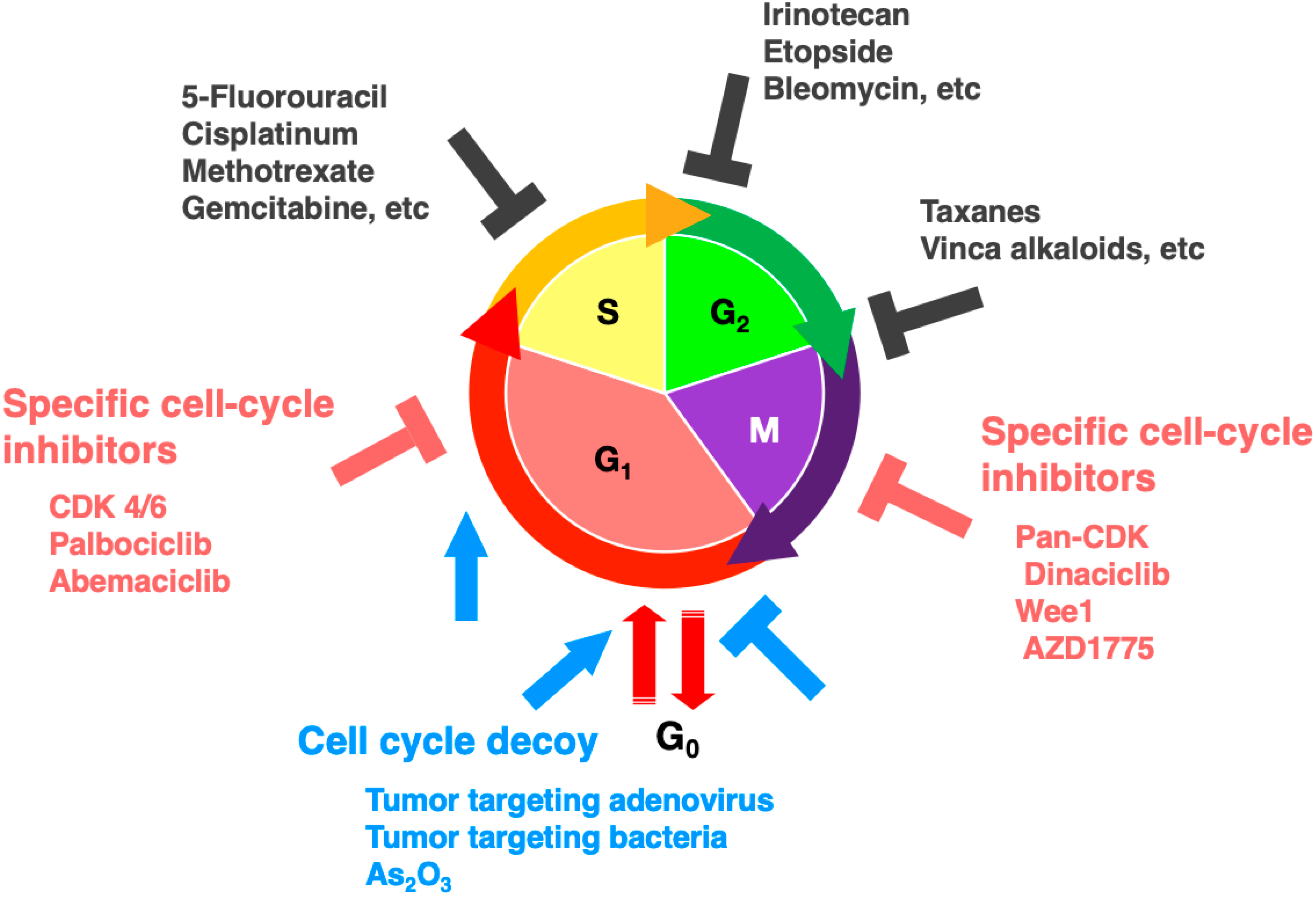
7. FUCCI Imaging to Evaluate Cell-Cycle-Specific Targeted Drugs
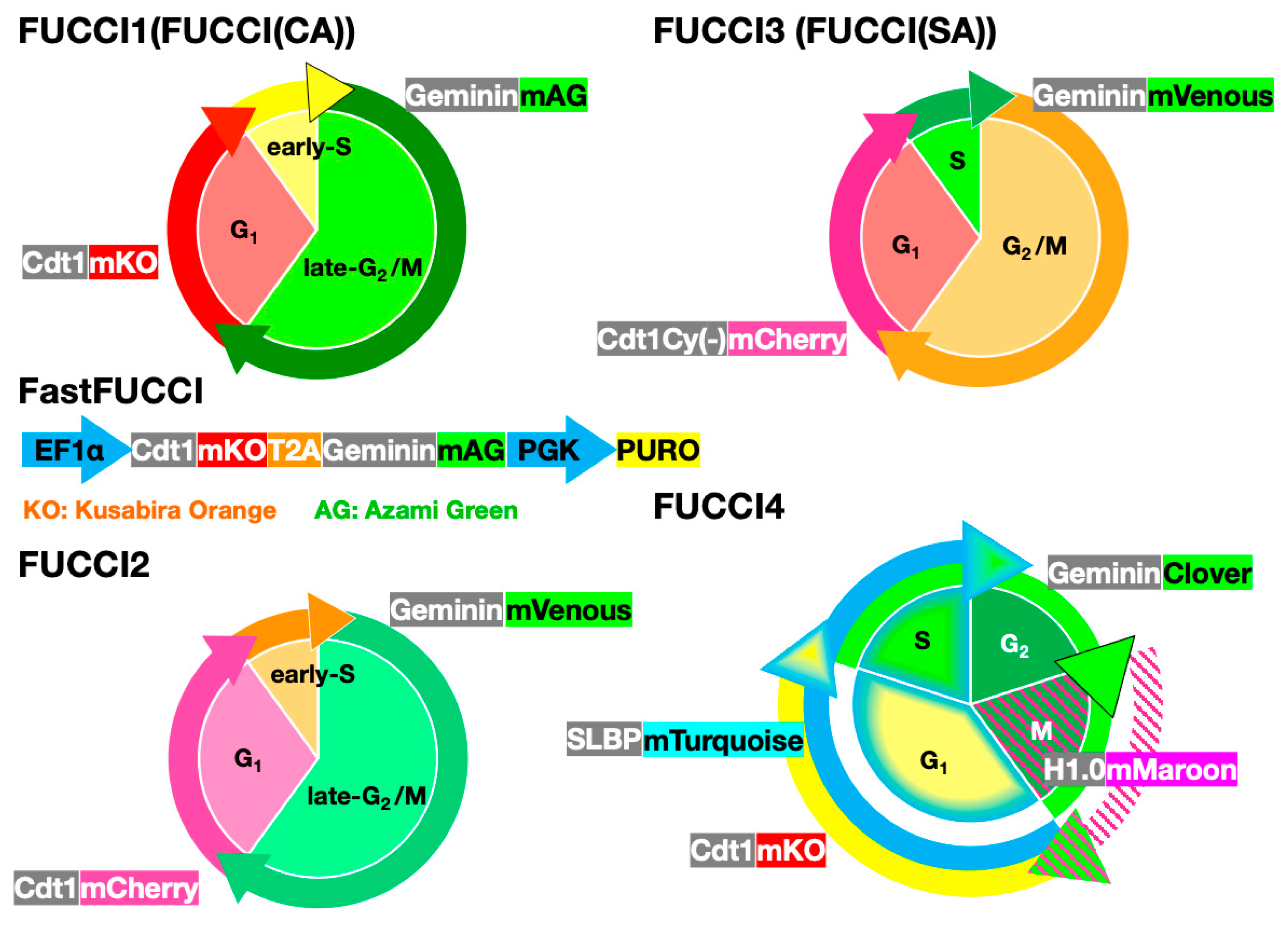
8. Comparison of FUCCI and Other Cell-Cycle Indicators
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK | cyclin-dependent kinase |
| CSC | cancer stem cell |
| FUCCI | fluorescence ubiquitination cell-cycle indicator |
| GFP | green fluorescent protein |
| RFP | red fluorescent protein |
References
- Malumbers, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Kastan, M.B.; Canman, C.E.; Leonard, C.J. p53, Cell cycle Control and Apoptosis: Implications for Cancer. Cancer Metastasis Rev. 1995, 14, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hannon, G.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Croix, B.S.; Florenes, V.A.; Rak, J.W.; Flanngan, M.; Bhattacharya, N.; Slingerland, J.M.; Kerbel, R.S. Impact of the cyclin-dependent kinase inhibitor p27kip1 on resistance of tumor cells to anticancer agents. Nat. Med. 1996, 2, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin/cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Tanyu, A.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; Imamura, T.; et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Zhang, Y.; Miwa, S.; Tome, Y.; Hiroshima, Y.; Uehara, F.; Yamamoto, M.; Suetsugu, A.; Kishimoto, H.; Tazawa, H.; et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle 2014, 13, 2110–2119. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Hoffman, R.M. Real-time determination of cell-cycle position of individual cells within live tumors using FUCCI cell-cycle imaging. Cells 2018, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Goss, P.E.; Chambers, A.F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 2010, 10, 871–877. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A.; Bragado, P.; Sosa, M.S. Targeting dormant cancer. Nat. Med. 2013, 19, 276–277. [Google Scholar] [CrossRef]
- Polzer, B.; Klein, C.A. The challenges of targeting minimal residual cancer. Nat. Med. 2013, 19, 274–275. [Google Scholar] [CrossRef]
- Holohan, C.; van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nature Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Ghiso, J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef] [Green Version]
- Pajic, M.; Blatter, S.; Guyader, C.; Gonggrijp, M.; Kersbergen, A.; Küçükosmanoğlu, A.; Sol, W.; Drost, R.; Jonkers, J.; Borst, P.; et al. Selected alkylating agents can overcome drug tolerance of G0-like tumors cells and eradicate BRCA1-deficient mammary tumors in mice. Clin. Cancer Res. 2017, 23, 7020–7033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaue-Sawano, A.; Kobayashi, T.; Ohtawa, K.; Miyawaki, A. Drug-induced cell cycle modulation leading to cell-cycle arrest, nuclear mis-segregation, or endoreplication. BMC Cell Biol. 2011, 12, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaue-Sawano, A.; Yo, M.; Komatsu, N.; Hiratsuka, T.; Kogure, T.; Hoshida, T.; Goshima, N.; Matsuda, M.; Miyoshi, H. Genetically Encoded Tools for Optical Dissection of the Mammalian Cell Cycle. Mol. Cell. 2017, 68, 626–640. [Google Scholar] [CrossRef]
- Oki, T.; Nishimura, K.; Kitaura, J.; Togami, K.; Maehara, A.; Izawa, K. A novel cell-cycle-indicator, mVenus-p27K-, identifies quiescent cells and visualizes G0-G1 transition. Sci. Rep. 2014, 4, 4012. [Google Scholar] [CrossRef] [Green Version]
- Bajar, B.T.; Lam, A.J.; Badiee, R.K.; Oh, Y.-H.; Chu, J.; Zhou, X.X.; Kim, N.; Kim, B.B.; Chung, M.; Yablonovitch, A.L. Fluorescent indicators for simultaneous reporting of all four cell cycle phases. Nat. Methods 2016, 13, 993–996. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.B.; Mascalchi, P.; Rodriguez, E.; Jodrell, D.I.; Richards, F.M.; Lyons, S.K. A quantitative FastFUCCI assay defines cell cycle dynamics at a single cell level. J. Cell Sci. 2017, 130, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; (Okayama University, Okayama, Japan); Hoffman, R.M.; (AntiCancer, Inc., San Diego, CA, USA). Personal communication, 2014.
- Hoffman, R.M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer 2005, 10, 796–806. [Google Scholar] [CrossRef]
- Condeelis, J.; Segall, J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 2003, 8, 921–930. [Google Scholar] [CrossRef]
- Kamb, A. What’s wrong with our cancer models? Nature Rev. Drug Discov. 2005, 4, 161–165. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Carragher, N. Live cell in vitro and in vivo imaging applications: Accelerating drug discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar]
- Conway, J.R.W.; Carragher, D.O.; Timpson, P. Developments in preclinical cancer imaging: Innovating the discovery of therapeutics. Nat. Rev. Cancer 2014, 14, 314–328. [Google Scholar] [CrossRef]
- Giedt, R.J.; Koch, P.D.; Weissleder, R. Single cell analysis of drug distribution by intravital imaging. PLoS ONE 2013, 8, e60988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurber, G.M.; Yang, K.S.; Reiner, T.; Kohler, R.H.; Sorger, P.; Mitchison, T.; Weissleder, R. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 2013, 4, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haass, N.K.; Beaumont, K.A.; Hill, D.S.; Anfosso, A.; Mrass, P.; Munoz, M.; Kinjo, I.; Weninger, W. Real-time cell cycle imaging during melanoma growth, invasion, and drug response. Pig. Cell. Melanoma Res. 2014, 27, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kaida, A.; Miura, M. Visualizing cell-cycle kinetics after hypoxia/reoxygenation in HeLa cells expressing fluorescent ubiquitination-based cell cycle indicator (Fucci). Exp. Cell. Res. 2015, 339, 389–396. [Google Scholar] [CrossRef]
- Chittajallu, D.R.; Florian, S.; Kohler, R.; Iwamoto, Y.; Orth, J.D.; Weissleder, R.; Danuse, G.; Mitchison, T.J. In vivo cell-cycle profiling in xenograft tumors by quantitative intravital microscopy. Nat. Methods 2015, 12, 577–585. [Google Scholar] [CrossRef]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914. [Google Scholar] [CrossRef]
- Onozato, Y.; Kaida, A.; Harada, H.; Miura, M. Radiosensitivity of quiescent and proliferating cells grown as multicellular tumor spheroids. Cancer Sci. 2017, 108, 704–712. [Google Scholar] [CrossRef]
- Bouchard, G.; Bouvette, G.; Therriault, H.; Bujold, R.; Saucier, C.; Paquette, B. Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br. J. Cancer 2013, 109, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Nomalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Amoh, Y.; Yang, M.; Li, L.; Reynoso, J.; Bouvet, M.; Moossa, A.R.; Katsuoka, K.; Hoffman, R.M. Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 2005, 65, 5352–5357. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Takehara, K.; Tazawa, H.; Kishimoto, H.; Urata, Y.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Cell-cycle-dependent drug-resistant quiescent cancer cells induce tumor angiogenesis after chemotherapy as visualized by real-time FUCCI imaging. Cell Cycle 2017, 16, 406–414. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Condeels, J.S.; Oktay, M.H. Chemotherapy-induced metastasis: Mechanisms and translational opportunities. Clin. Exp. Metastasis. 2018, 35, 269–284. [Google Scholar] [CrossRef]
- D’Alterio, G.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 2020, 60, 351–361. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Lytle, N.K.; Barber, A.G.; Reya, T. Stem cell fate in cancer growth, progression and therapy resistance. Nature Rev. Cancer 2018, 18, 669–680. [Google Scholar] [CrossRef]
- Yano, S.; Tazawa, H.; Hashimoto, Y.; Shirakawa, Y.; Kuroda, S.; Nishizaki, M.; Kishimoto, H.; Uno, F.; Nagasaka, T.; Urata, Y.; et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells in S/G2/M phases. Clin. Cancer Res. 2013, 19, 6495–6505. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Kagawa, S.; Kobayashi, N.; Shirakiya, Y.; Umeoka, T.; Teraishi, F.; Taki, M.; Kyo, S.; Tanaka, N.; Fujiwara, T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004, 10, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Nemunaitis, J.; Tong, A.W.; Nemunaitis, M.; Senzer, N.; Phadke, A.P.; Bedell, C.; Adams, N.; Zhang, Y.A.; Maples, P.B.; Chen, S.; et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010, 18, 429–434. [Google Scholar] [CrossRef]
- Yano, S.; (Okayama University, Okayama, Japan); Hoffman, R.M.; (AntiCancer, Inc., San Diego, CA, USA). Personal communication, 2017.
- Zhao, M.; Yang, M.; Li, X.M.; Jiang, P.; Baranov, E.; Li, S.; Xu, M.; Penman, S.; Hoffman, R.M. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2005, 102, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Zhang, Y.; Zhao, M.; Hiroshima, Y.; Miwa, S.; Uehara, F.; Kishimoto, H.; Tazawa, H.; Bouvet, M.; Fujiwara, T.; et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014, 13, 3958–3963. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Li, S.; Han, Q.; Tan, Y.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget 2014, 5, 8729–8736. [Google Scholar] [CrossRef]
- Yano, S.; Takehara, K.; Zhao, M.; Tan, Y.; Han, Q.; Li, S.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging. Cell Cycle 2016, 15, 1715–1723. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Miwa, S.; Mii, S.; Hiroshima, Y.; Uehara, F.; Yamamoto, M.; Kishimoto, H.; Tazawa, H.; Bouvet, M.; Fujiwara, T.; et al. Invading cancer cells are predominantly in G0/G1 resulting in chemoresistance demonstrated by real-time FUCCI imaging. Cell Cycle 2014, 13, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, T.; Omori, T.; Nakamura, H.; Sugano, M.; Neri, S.; Fujii, S.; Hashimoto, H.; Tsuboi, M.; Ochiai, A.; Ishii, G. Spatiotempral characteristics of fibroblasts-dependent cancer invasion. J. Can. Res. Clin. Oncol. 2019, 145, 371–381. [Google Scholar] [CrossRef]
- Schmidt, E.E.; Ichimura, K.; Reifenberger, G.; Collins, V.P. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1996, 54, 6321–6324. [Google Scholar]
- Yu, Q.; Sicinska, E.; Geng, Y.; Ahnstrom, M.; Zagozdzon, A.; Kong, Y.; Gardner, H.; Kiyokawa, H.; Harris, L.N.; Stal, O.; et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006, 9, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Reddy, H.K.; Mettus, R.V.; Rane, S.G.; Grana, X.; Litvin, J.; Reddy, E.P. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005, 65, 10174–10178. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.G.; Deshpande, A.; Enos, M.; Hinds, E.A.; Hu, G.F.; Chang, R.; Guo, Z.; Dose, M.; Mao, C.; Tsichlis, P.N.; et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 2009, 69, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016, 6, 353–367. [Google Scholar] [CrossRef] [Green Version]
- VanArsdale, T.; Boshoff, C.; Arndt, K.T.; Abraham, R.T. Molecular pathways: Targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin. Cancer Res. 2015, 21, 2905–2910. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.L.; Thangavel, C.; McClendon, A.K.; Reed, C.A.; Knudsen, E.S. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene 2010, 29, 4018–4032. [Google Scholar] [CrossRef] [Green Version]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK4/6 inhibitors as single agent in advanced solid tumors. Front. Oncol. 2018, 12, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwapisz, D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: Palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 2017, 166, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival With Ribociclib Plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Watanabe, N.; Broome, M.; Hunter, T. Regulation of the human WEE1 Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995, 14, 1878–1891. [Google Scholar] [CrossRef]
- Nojima, H.; Homma, H.; Onozata, Y.; Kaida, A.; Harada, H.; Miura, M. Differential properties of mitosis-associated events following CHK1 and WEE1 inhibitor treatments in human tongue carcinoma cells. Exp. Cell Res. 2020, 386, 111720. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin- dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.C.; Kolb, E.A.; Sanmpson, V.B. Development of Chemotherapy with Cell Cycle Inhibitors for Adult and Pediatric Cancer Therapy. Cancer Res. 2018, 78, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, T.; Sulliva, K.F.; Wahl, G.M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998, 8, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Catala, M.; Lamontagne, B.; Larose, S.; Ghazal, G.; Elena, S.A. Cell cycle-dependent nuclear localization of yeast RNase III for efficient cell division. Mol. Biol. Cell 2004, 15, 3015–3030. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, A.; Nakayama, Y.; Kinjo, M. Efficient and dynamic nuclear localization of green fluorescent protein via RNA binding. Biochem. Biophys. Res. Commun. 2015, 463, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Niimi, A.; Brown, S.; Sabbioneda, S.; Kannouche, P.L.; Scott, A.; Yasui, A.; Green, C.M.; Lehmann, A.R. Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16125–16130. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, H.; Rahn, H.P.; Weinzierl, P.; Sporbert, A.; Cremer, T.; Zink, D.; Cardoso, M.C. Dynamics of DNA replication factories in living cells. J. Cell Biol. 2000, 149, 271–280. [Google Scholar] [CrossRef]
- Yamamoto, N.; Jiang, P.; Yang, M.; Xu, M.; Yamauchi, K.; Tsuchiya, H.; Tomita, K.; Wahl, G.M.; Moossa, A.R.; Hoffman, R.M. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res. 2004, 64, 4251–4256. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Yamauchi, K.; Yang, M.; Tsuji, K.; Xu, M.; Maitra, A.; Bouvet, M.; Hoffman, R.M. Tumor cells genetically labeled with GFP in the nucleus and RFP in the cytoplasm for imaging cellular dynamics. Cell Cycle 2006, 5, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Yang, M.; Jiang, P.; Yamamoto, N.; Xu, M.; Amoh, Y.; Tsuji, K.; Bouvet, M.; Tsuchiya, H.; Tomita, K.; et al. Real-time in vivo dual-color imaging of intracapillary cancer cell and nucleus deformation and migration. Cancer Res. 2005, 65, 4246–4252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Kimura, H.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Kishimoto, H.; Hasegawa, A.; Bouvet, M.; Hoffman, R.M. Comparison of cancer-cell seeding, viability and deformation in the lung, muscle and liver, visualized by subcellular real-time imaging in the live mouse. Anticancer Res. 2011, 31, 3665–3672. [Google Scholar] [PubMed]
- Yang, M.; Jiang, P.; Hoffman, R.M. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 2007, 67, 5195–5200. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, K.; Yang, M.; Jiang, P.; Xu, M.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Development of real-time subcellular dynamic multicolor imaging of cancer cell- trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006, 66, 4208–4214. [Google Scholar] [CrossRef] [Green Version]
- Sparks, H.; Kondo, H.; Hooper, S.; Munro, I.; Kennedy, G.; Dunsby, C.; French, P.; Sahai, E. Heterogeneity in tumor chromatin-doxorubicin binding revealed by in vivo fluorescence lifetime imaging confocal endomicroscopy. Nat. Commun. 2018, 9, 2662. [Google Scholar] [CrossRef] [PubMed]
- Batters, C.; Zhu, H.; Sale, J. Visualization of PCNA monoubiquitination in vivo by single pass spectral imaging FRET microscopy. PLoS ONE 2010, 5, e9008. [Google Scholar] [CrossRef]
- Ersoy, I.; Bunyak, F.; Chagin, V.; Cardoso, M.C.; Palaniappan, K. Segmentation and Classification of Cell Cycle Phases in Fluorescence Imaging. Lect. Notes Comput. Sci. 2009, 5762, 617–624. [Google Scholar]
- Jares-Erijman, E.A. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef]
- Algar, W.R.; Hildebrandt, N.; Vogel., S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef]
- Janssen, A.; Beerling, E.; Medema, R.; van Rheene, J. Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS ONE 2013, 8, e64029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavet, O.; Pines, J. Progression activation of cyclin B1-adk1 coordinates entry to mitosis. Dev. Cell. 2010, 18, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielke, N.; Edgar, B.A. FUCCI sensors: Powerful new tools for analysis of cell proliferation. WIREs Dev. Biol. 2015, 4, 469–487. [Google Scholar] [CrossRef]
- Vittadello, S.T.; McCue, S.W.; Gunasingh, G.; Haass, N.K.; Simpson, M.J. Mathematical models for cell migration with real-time cell cycle dynamics. Biophys. J. 2018, 114, 1241–1253. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, S.; Tazawa, H.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells. Cancers 2020, 12, 2655. https://doi.org/10.3390/cancers12092655
Yano S, Tazawa H, Kagawa S, Fujiwara T, Hoffman RM. FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells. Cancers. 2020; 12(9):2655. https://doi.org/10.3390/cancers12092655
Chicago/Turabian StyleYano, Shuya, Hiroshi Tazawa, Shunsuke Kagawa, Toshiyoshi Fujiwara, and Robert M. Hoffman. 2020. "FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells" Cancers 12, no. 9: 2655. https://doi.org/10.3390/cancers12092655
APA StyleYano, S., Tazawa, H., Kagawa, S., Fujiwara, T., & Hoffman, R. M. (2020). FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells. Cancers, 12(9), 2655. https://doi.org/10.3390/cancers12092655




