EVI1 in Leukemia and Solid Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
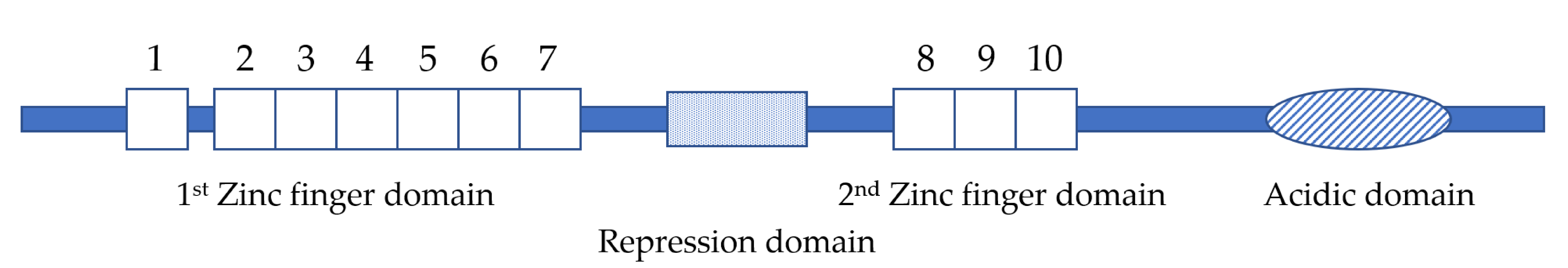
1.1. Structure of EVI1 and MDS1/EVI1
1.2. Expression and Function of EVI1 and MDS1/EVI1 in Development
1.3. EVI1 in Myeloid Leukemias
1.4. Chromosomal Rearrangements Activate EVI1 Transcription in Leukemia
2. EVI1, MDS1/EVI1 and AML1/MDS1/EVI1 in Leukemogenesis
2.1. EVI1 and MDS1/EVI1 in Leukemogenesis
2.1.1. Differentiation
2.1.2. Apoptosis
2.1.3. Cell Quiescence
2.2. AML1/MDS1/EVI1 in Leukemogenesis
2.2.1. Repression of TGF-β-Mediated Growth Inhibition
2.2.2. Stimulation of Proliferation
3. EVI1 and MDS1/EVI1 in Solid Tumors
4. Downstream Signaling Pathways
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fears, S.; Mathieu, C.; Zeleznik-Le, N.; Huang, S.; Rowley, J.D.; Nucifora, G. Intergenic splicing of MDS1 and EVI1 occurs in normal tissues as well as in myeloid leukemia and produces a new member of the PR domain family. Proc. Natl. Acad. Sci. USA 1996, 93, 1642–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucifora, G.; Begy, C.R.; Kobayashi, H.; Roulston, D.; Claxton, D.; Pedersen-Bjergaard, J.; Parganas, E.; Ihle, J.N.; Rowley, J.D. Consistent intergenic splicing and production of multiple transcripts between AML1 at 21q22 and unrelated genes at 3q26 in (3;21)(q26;q22) translocations. Proc. Natl. Acad. Sci. USA 1994, 91, 4004–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitani, K.; Ogawa, S.; Tanaka, T.; Miyoshi, H.; Kurokawa, M.; Mano, H.; Yazaki, Y.; Ohki, M.; Hirai, H. Generation of the AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994, 13, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, C.; Kilbey, A.; Clark, A.M.; Walker, M. The Evi-1 proto-oncogene encodes a transcriptional repressor activity associated with transformation. Oncogene 1997, 14, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soderholm, J.; Kobayashi, H.; Mathieu, C.; Rowley, J.D.; Nucifora, G. The leukemia-associated gene MDS1/EVI1 is a new type of GATA-binding transactivator. Leukemia 1997, 11, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Tanaka, T.; Kurokawa, M.; Imai, Y.; Ogawa, S.; Mitani, K.; Yazaki, Y.; Hirai, H. The AML1/ETO(MTG8) and AML1/Evi-1 Leukemia-Associated Chimeric Oncoproteins Accumulate PEBP2β(CBFβ) in the Nucleus More Efficiently Than Wild-Type AML1. Blood 1998, 91, 1688–1699. [Google Scholar] [CrossRef]
- Senyuk, V.; Chakraborty, S.; Mikhail, F.M.; Zhao, R.; Chi, Y.; Nucifora, G. The leukemia-associated transcription repressor aml1/mds1/evi1 requires ctbp to induce abnormal growth and differentiation of murine hematopoietic cells. Oncogene 2002, 21, 3232–3240. [Google Scholar] [CrossRef]
- Arai, S.; Yoshimi, A.; Shimabe, M.; Ichikawa, M.; Nakagawa, M.; Imai, Y.; Goyama, S.; Kurokawa, M. Evi-1 is a transcriptional target of mixed-lineage leukemia oncoproteins in hematopoietic stem cells. Blood 2011, 117, 6304–6314. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, K.; Sato, T.; Yoshimi, A.; Goyama, S.; Tsuruta, T.; Kobayashi, H.; Shimabe, M.; Arai, S.; Nakagawa, M.; Imai, Y.; et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. J. Exp. Med. 2011, 208, 2403–2416. [Google Scholar] [CrossRef] [Green Version]
- Konantz, M.; André, M.C.; Ebinger, M.; Grauer, M.; Wang, H.; Grzywna, S.; Rothfuss, O.C.; Lehle, S.; Kustikova, O.S.; Salih, H.R.; et al. EVI-1 modulates leukemogenic potential and apoptosis sensitivity in human acute lymphoblastic leukemia. Leukemia 2013, 27, 56–65. [Google Scholar] [CrossRef]
- Sato, T.; Goyama, S.; Kataoka, K.; Nasu, R.; Tsuruta-Kishino, T.; Kagoya, Y.; Nukina, A.; Kumagai, K.; Kubota, N.; Nakagawa, M.; et al. Evi1 defines leukemia-initiating capacity and tyrosine kinase inhibitor resistance in chronic myeloid leukemia. Oncogene 2014, 33, 5028–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, G.; Rommer, A.; Steinleitner, K.; Etzler, J.; Hackl, H.; Heffeter, P.; Tomasich, E.; Filipits, M.; Steinmetz, B.; Topakian, T.; et al. EVI1 promotes tumor growth via transcriptional repression of MS4A3. J. Hematol. Oncol. 2015, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Wang, H.; Deng, J.; Zheng, X.; Ling, Y.; Gong, Y. Prognostic significance of the EVI1 gene expression in patients with acute myeloid leukemia: A meta-analysis. Ann. Hematol. 2019, 98, 2485–2496. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, S.E.; Rogers, J.R.; Harrison, G.; Wheatley, K.; Walker, H.; Bain, B.J.; Burnett, A.K.; Goldstone, A.H.; Linch, D.C.; Grimwade, D.; et al. EVI1 expression in acute myeloid leukaemia. Br. J. Haematol. 2001, 112, 208–211. [Google Scholar] [CrossRef]
- Van Waalwijk van Doorn-Khosrovani, S.B.; Erpelinck, C.; Van Putten, W.L.J.; Valk, P.J.M.; Van der Poel-van de Luytgaarde, S.; Hack, R.; Slater, R.; Smit, E.M.E.; Beverloo, H.B.; Verhoef, G.; et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: A study of 319 de novo AML patients. Blood 2002, 101, 837–845. [Google Scholar] [CrossRef]
- Lugthart, S.; Van Drunen, E.; Van Norden, Y.; Van Hoven, A.; Erpelinck, C.A.J.; Valk, P.J.M.; Beverloo, H.B.; Löwenberg, B.; Delwel, R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: Prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood 2008, 111, 4329–4337. [Google Scholar] [CrossRef] [Green Version]
- Gröschel, S.; Lugthart, S.; Schlenk, R.F.; Valk, P.J.M.; Eiwen, K.; Goudswaard, C.; Van Putten, W.J.L.; Kayser, S.; Verdonck, L.F.; Lübbert, M.; et al. High EVI1 Expression Predicts Outcome in Younger Adult Patients With Acute Myeloid Leukemia and Is Associated With Distinct Cytogenetic Abnormalities. J. Clin. Oncol. 2010, 28, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Ji, X. Overexpression of ecotropic viral integration site-1 is a prognostic factor of lung squamous cell cancer. OncoTargets Ther. 2017, 10, 2739–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Schäfer, T.; Konantz, M.; Braun, M.; Varga, Z.; Paczulla, A.M.; Reich, S.; Jacob, F.; Perner, S.; Moch, H.; et al. Prominent Oncogenic Roles of EVI1 in Breast Carcinoma. Cancer Res. 2017, 77, 2148–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Cao, Y.; Liu, Y.; Li, F.; Sambandam, K.; Rajaraman, S.; Perkins, A.S.; Fields, A.P.; Hellmich, M.R.; Townsend, C.M., Jr.; et al. Overexpression of Evi-1 oncoprotein represses TGF-β signaling in colorectal cancer. Mol. Carcinog. 2011, 52, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, L.; Ko, T.C.; Fields, A.P.; Thompson, K.J. Evi1 is a survival factor which conveys resistance to both TGFβ- and taxol-mediated cell death via PI3K/AKT. Oncogene 2006, 25, 3565–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, A.K.; Das Mohapatra, A.; Nayak, K.B.; Chakraborty, S. Acetylation of the Proto-Oncogene EVI1 Abrogates Bcl-xL Promoter Binding and Induces Apoptosis. PLoS ONE 2011, 6, e25370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, K.B.; Kuila, N.; Das Mohapatra, A.; Panda, A.K.; Chakraborty, S. EVI1 targets ΔNp63 and upregulates the cyclin dependent kinase inhibitor p21 independent of p53 to delay cell cycle progression and cell proliferation in colon cancer cells. Int. J. Biochem. Cell Biol. 2013, 45, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.B.; Sajitha, I.S.; Kumar, T.R.S.; Chakraborty, S. Ecotropic viral integration site 1 promotes metastasis independent of epithelial mesenchymal transition in colon cancer cells. Cell Death Dis. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, D.; Woodward, S.; Thompson, F.; Dos Santos, B.; Russell, M.; Yang, J.-M.; Guan, X.-Y.; Trent, J.; Alberts, D.; Taetle, R. Expression of the zinc finger gene EVI-1 in ovarian and other cancers. Br. J. Cancer 1996, 74, 1518–1525. [Google Scholar] [CrossRef]
- Queisser, A.; Hagedorn, S.; Wang, H.; Schäfer, T.; Konantz, M.; Alavi, S.; Deng, M.; Vogel, W.; Von Mässenhausen, A.; Kristiansen, G.; et al. Ecotropic viral integration site 1, a novel oncogene in prostate cancer. Oncogene 2016, 36, 1573–1584. [Google Scholar] [CrossRef]
- Kurokawa, M.; Mitani, K.; Irie, K.; Matsuyama, T.; Takahashi, T.; Chiba, S.; Yazaki, Y.; Matsumoto, K.; Hirai, H. The oncoprotein Evi-1 represses TGF-β signalling by inhibiting Smad3. Nature 1998, 394, 92–96. [Google Scholar] [CrossRef]
- Izutsu, K.; Kurokawa, M.; Imai, Y.; Maki, K.; Mitani, K.; Hirai, H. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor β signaling. Blood 2001, 97, 2815–2822. [Google Scholar] [CrossRef] [Green Version]
- Alliston, T.; Ko, T.C.; Cao, Y.; Liang, Y.-Y.; Feng, X.-H.; Chang, C.; Derynck, R. Repression of Bone Morphogenetic Protein and Activin-inducible Transcription by Evi-1. J. Biol. Chem. 2005, 280, 24227–24237. [Google Scholar] [CrossRef] [Green Version]
- Yoshimi, A.; Goyama, S.; Watanabe-Okochi, N.; Yoshiki, Y.; Nannya, Y.; Nitta, E.; Arai, S.; Sato, T.; Shimabe, M.; Nakagawa, M.; et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood 2011, 117, 3617–3628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-M.; Liu, Z.-L.; Qiu, B.; Xu, Y.-F.; Pan, C.; Zhang, Z.-L. Downregulation of EVI1 Expression Inhibits Cell Proliferation and Induces Apoptosis in Hilar Cholangiocarcinoma via the PTEN/AKT Signalling Pathway. J. Cancer 2020, 11, 1412–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Nishida, J.; Mitani, K.; Ogawa, S.; Yazaki, Y.; Hirai, H. Evi-1 raises AP-1 activity and stimulates c-fos promoter transactivation with dependence on the second zinc finger domain. J. Biol. Chem. 1994, 269, 24020–24026. [Google Scholar] [PubMed]
- Kurokawa, M.; Mitani, K.; Yamagata, T.; Takahashi, T.; Izutsu, K.; Ogawa, S.; Moriguchi, T.; Nishida, E.; Yazaki, Y.; Hirai, H. The Evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents stress-induced cell death. EMBO J. 2000, 19, 2958–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonamici, S.; Li, D.; Mikhail, F.M.; Sassano, A.; Platanias, L.C.; Colamonici, O.; Anastasi, J.; Nucifora, G. EVI1 Abrogates Interferon-alpha Response by Selectively Blocking PML Induction. J. Biol. Chem. 2004, 280, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Woo, C.-H.; Steere, R.R.; Lee, B.C.; Huang, Y.; Wu, J.; Pang, J.; Lim, J.H.; Xu, H.; Zhang, W.; et al. EVI1 acts as an inducible negative-feedback regulator of NF-κB by inhibiting p65 acetylation. J. Immunol. 2012, 188, 6371–6380. [Google Scholar] [CrossRef] [Green Version]
- Mucenski, M.L.; Taylor, B.A.; Ihle, J.N.; Hartley, J.W.; Morse, H.C.; Jenkins, N.A.; Copeland, N.G. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol. Cell. Biol. 1988, 8, 301–308. [Google Scholar] [CrossRef]
- Perkins, A.S.; Fishel, R.; Jenkins, N.A.; Copeland, N.G. Evi-1, a murine zinc finger proto-oncogene, encodes a sequence-specific DNA-binding protein. Mol. Cell. Biol. 1991, 11, 2665–2674. [Google Scholar] [CrossRef] [Green Version]
- Delwel, R.; Funabiki, T.; Kreider, B.L.; Morishita, K.; Ihle, J.N. Four of the seven zinc fingers of the Evi-1 myeloid-transforming gene are required for sequence-specific binding to GA(C/T)AAGA(T/C)AAGATAA. Mol. Cell. Biol. 1993, 13, 4291–4300. [Google Scholar] [CrossRef] [Green Version]
- Funabiki, T.; Kreider, B.L.; Ihle, J.N. The carboxyl domain of zinc fingers of the Evi-1 myeloid transforming gene binds a consensus sequence of GAAGATGAG. Oncogene 1994, 9, 1575–1581. [Google Scholar]
- Morishita, K.; Parganas, E.; Douglass, E.C.; Ihle, J.N. Unique expression of the human Evi-1 gene in an endometrial carcinoma cell line: Sequence of cDNAs and structure of alternatively spliced transcripts. Oncogene 1990, 5, 963–971. [Google Scholar]
- Nucifora, G.; Begy, C.R.; Erickson, P.; Drabkin, H.A.; Rowley, J.D. The 3;21 translocation in myelodysplasia results in a fusion transcript between the AML1 gene and the gene for EAP, a highly conserved protein associated with the Epstein-Barr virus small RNA EBER 1. Proc. Natl. Acad. Sci. USA 1993, 90, 7784–7788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchi, N.; Nisson, P.E.; Watkins, P.C.; Faustinella, F.; Wijsman, J.; Hagemeijer, A. AMLI fusion transcripts in t(3;21) positive leukemia: Evidence of molecular heterogeneity and usage of splicing sites frequently involved in the generation of normalAMLI transcripts. Genes Chromosom. Cancer 1994, 11, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Garriga, G.; Guenther, C.; Horvitz, H.R. Migrations of the Caenorhabditis elegans HSNs are regulated by egl-43, a gene encoding two zinc finger proteins. Genes Dev. 1993, 7, 2097–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S. Blimp-1 is the murine homolog of the human transcriptional repressor PRDI-BF1. Cell 1994, 78, 9. [Google Scholar] [CrossRef]
- Lin, Y.; Wong, K.-K.; Calame, K. Repression of c-myc Transcription by Blimp-1, an Inducer of Terminal B Cell Differentiation. Science 1997, 276, 596–599. [Google Scholar] [CrossRef]
- Turner, C.; Mack, D.H.; Davis, M.M. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 1994, 77, 297–306. [Google Scholar] [CrossRef]
- Buyse, I.M.; Shao, G.; Huang, S. The retinoblastoma protein binds to RIZ, a zinc-finger protein that shares an epitope with the adenovirus E1A protein. Proc. Natl. Acad. Sci. USA 1995, 92, 4467–4471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Shao, G.; Steele-Perkins, G.; Huang, S. The Retinoblastoma Interacting Zinc Finger GeneRIZProduces a PR Domain-lacking Product through an Internal Promoter. J. Biol. Chem. 1997, 272, 2984–2991. [Google Scholar] [CrossRef] [Green Version]
- Hoyt, P.R.; Bartholomew, C.; Davis, A.J.; Yutzey, K.E.; Gamer, L.W.; Potter, S.; Ihle, J.N.; Mucenski, M.L. The Evil proto-oncogene is required at midgestation for neural, heart, and paraxial mesenchyme development. Mech. Dev. 1997, 65, 55–70. [Google Scholar] [CrossRef]
- Kitabayashi, I.; Yokoyama, A.; Shimizu, K.; Ohki, M. Interaction and functional cooperation of the leukemia-associated factors AML1 and p300 in myeloid cell differentiation. EMBO J. 1998, 17, 2994–3004. [Google Scholar] [CrossRef] [Green Version]
- Perkins, A.S.; Mercer, J.A.; Jenkins, N.A.; Copeland, N.G. Patterns of Evi-1 expression in embryonic and adult tissues suggest that Evi-1 plays an important regulatory role in mouse development. Development 1991, 111, 479–487. [Google Scholar] [PubMed]
- Sitailo, S.; Sood, R.; Barton, K.; Nucifora, G. Forced expression of the leukemia-associated gene EVI1 in ES cells: A model for myeloid leukemia with 3q26 rearrangements. Leukemia 1999, 13, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, B.L.; Orkin, S.H.; Ihle, J.N. Loss of erythropoietin responsiveness in erythroid progenitors due to expression of the Evi-1 myeloid-transforming gene. Proc. Natl. Acad. Sci. USA 1993, 90, 6454–6458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Stehling-Sun, S.; Lezon-Geyda, K.; Juneja, S.C.; Coillard, L.; Chatterjee, G.; Wuertzer, C.A.; Camargo, F.; Perkins, A.S. PR-domain–containing Mds1-Evi1 is critical for long-term hematopoietic stem cell function. Blood 2011, 118, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Kurokawa, M. Ecotropic viral integration site 1, stem cell self-renewal and leukemogenesis. Cancer Sci. 2012, 103, 1371–1377. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [Green Version]
- Haas, K.; Kundi, M.; Sperr, W.R.; Esterbauer, H.; Ludwig, W.-D.; Ratei, R.; Koller, E.; Gruener, H.; Sauerland, C.; Fonatsch, C.; et al. Expression and prognostic significance of different mRNA 5′-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosom. Cancer 2008, 47, 288–298. [Google Scholar] [CrossRef]
- Fontenay-Roupie, M.; Bouscary, D.; Melle, J.; Viguie, F.; Picard, F.; Guesnu, M.; Dreyfus, F. Expression of the transcription factor Evi-1 in human erythroleukemia cell lines and in leukemias. Hematol. Cell Ther. 1997, 39, 5–10. [Google Scholar] [CrossRef]
- Fichelson, S.; Dreyfus, F.; Berger, R.; Melle, J.; Bastard, C.; Miclea, J.M.; Gisselbrecht, S. Evi-1 expression in leukemic patients with rearrangements of the 3q25-q28 chromosomal region. Leukemia 1992, 6, 93–99. [Google Scholar]
- Jenkins, R.B.; Tefferi, A.; Solberg, L.A.; Dewald, G.W. Acute leukemia with abnormal thrombopoiesis and inversions of chromosome 3. Cancer Genet. Cytogenet. 1989, 39, 167–179. [Google Scholar] [CrossRef]
- Morishita, K.; Parganas, E.; William, C.L.; Whittaker, M.H.; Drabkin, H.; Oval, J.; Taetle, R.; Valentine, M.B.; Ihle, J.N. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc. Natl. Acad. Sci. USA 1992, 89, 3937–3941. [Google Scholar] [CrossRef] [Green Version]
- Suzukawa, K.; Parganas, E.; Gajjar, A.; Abe, T.; Takahashi, S.; Tani, K.; Asano, S.; Asou, H.; Kamada, N.; Yokota, J.; et al. Identification of a breakpoint cluster region 3′ of the ribophorin I gene at 3q21 associated with the transcriptional activation of the EVI1 gene in acute myelogenous leukemias with inv(3)(q21q26). Blood 1994, 84, 2681–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröschel, S.; Sanders, M.A.; Hoogenboezem, R.; De Wit, E.; Bouwman, B.A.M.; Erpelinck, C.; Van Der Velden, V.H.J.; Havermans, M.; Avellino, R.; Van Lom, K.; et al. A Single Oncogenic Enhancer Rearrangement Causes Concomitant EVI1 and GATA2 Deregulation in Leukemia. Cell 2014, 157, 369–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottema, S.; Mulet-Lazaro, R.; Beverloo, H.B.; Erpelinck, C.A.; Van Herk, S.; Van Der Helm, R.; Havermans, M.; Grob, T.; Valk, P.; Bindels, E.; et al. Atypical 3q26/MECOM rearrangements genocopy inv(3)/t(3;3) in acute myeloid leukemia. Blood 2020, 136, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, S.; Baens, M.; Grosgeorge, J.; Rodgers, K.; Reid, C.; Dainton, M.; Dyer, M.; Fuzibet, J.; Gratecos, N.; Taillan, B.; et al. Fluorescence in situ hybridization analysis of t(3; 12)(q26; p13): A recurring chromosomal abnormality involving the TEL gene (ETV6) in myelodysplastic syndromes. Blood 1996, 88, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Rubin, C.; Larson, R.; Bitter, M.; Carrino, J.; Le Beau, M.; Diaz, M.; Rowley, J. Association of a chromosomal 3;21 translocation with the blast phase of chronic myelogenous leukemia. Blood 1987, 70, 1338–1342. [Google Scholar] [CrossRef] [Green Version]
- Rubin, C.; Larson, R.; Anastasi, J.; Winter, J.; Thangavelu, M.; Vardiman, J.; Rowley, J.; Le Beau, M. t(3;21)(q26;q22): A recurring chromosomal abnormality in therapy- related myelodysplastic syndrome and acute myeloid leukemia. Blood 1990, 76, 2594–2598. [Google Scholar] [CrossRef] [Green Version]
- Schneider, N.R.; Bowman, W.P.; Frenkel, E.P. Translocation (3;21)(q26;q22) in secondary leukemia. Report of two cases and literature review. Ann. Génét. 1991, 34, 256–263. [Google Scholar]
- Coyle, T.; Najfeld, V. Translocation (3;21) in Philadelphia chromosome—Positive chronic myelogenous leukemia prior to the onset of blast crisis. Am. J. Hematol. 1988, 27, 56–59. [Google Scholar] [CrossRef]
- Poirel, H.; Oury, C.; Carron, C.; Duprez, E.; Laâbi, Y.; Tsapis, A.; Romana, S.P.; Mauchauffe, M.; Le Coniat, M.; Berger, R.; et al. The TEL gene products: Nuclear phosphoproteins with DNA binding properties. Oncogene 1997, 14, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Jousset, C.; Carron, C.; Boureux, A.; Quang, C.T.; Oury, C.; Dusanter-Fourt, I.; Charon, M.; Levin, J.; Bernard, O.A.; Ghysdael, J. A domain of TEL conserved in a subset of ETS proteins defines a specific oligomerization interface essential to the mitogenic properties of the TEL-PDGFR beta oncoprotein. EMBO J. 1997, 16, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, P.; Wlodarska, I.; Baens, M.; Criel, A.; Selleslag, D.; Hagemeijer, A.; Van den Berghe, H.; Marynen, P. Fusion of ETV6 to MDS1/EVI1 as a result of t(3;12)(q26;p13) in myeloproliferative disorders. Cancer Res. 1997, 57, 564–569. [Google Scholar] [PubMed]
- Buijs, A.; Sherr, S.; Van Baal, S.; Van Bezouw, S.; Van Der Plas, D.; Van Kessel, A.G.; Riegman, P.; Deprez, R.L.; Zwarthoff, E.; Hagemeijer, A.; et al. Translocation (12;22) (p13;q11) in myeloproliferative disorders results in fusion of the ETS-like TEL gene on 12p13 to the MN1 gene on 22q11. Oncogene 1995, 10, 1511–1519. [Google Scholar] [PubMed]
- Romana, S.P.; Mauchauffe, M.; Le Coniat, M.; Chumakov, I.; Le Paslier, D.; Berger, R.; Bernard, O.A. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion. Blood 1995, 85, 3662–3670. [Google Scholar] [CrossRef] [Green Version]
- Golub, T.R.; Barker, G.F.; Lovett, M.; Gilliland, D. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994, 77, 307–316. [Google Scholar] [CrossRef]
- Khanna-Gupta, A.; Lopingco, M.C.; Savinelli, T.; Zibello, T.; Berliner, N.; Perkins, A.S. Retroviral insertional activation of the EVI1 oncogene does not prevent G-CSF-induced maturation of the murine pluripotent myeloid cell line 32Dcl3. Oncogene 1996, 12, 563–569. [Google Scholar]
- Louz, D.; Van Den Broek, M.; Verbakel, S.; Vankan, Y.; Van Lom, K.; Joosten, M.; Meijer, D.; Löwenberg, B.; Delwel, R. Erythroid defects and increased retrovirally-induced tumor formation in Evi1 transgenic mice. Leukemia 2000, 14, 1876–1884. [Google Scholar] [CrossRef] [Green Version]
- Glass, C.; Wuertzer, C.; Cui, X.; Bi, Y.; Davuluri, R.; Xiao, Y.-Y.; Wilson, M.; Owens, K.; Zhang, Y.; Perkins, A.S. Global Identification of EVI1 Target Genes in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e67134. [Google Scholar] [CrossRef] [Green Version]
- Akagi, T.; Thoennissen, N.H.; George, A.; Crooks, G.; Song, J.H.; Okamoto, R.; Nowak, D.; Gombart, A.F.; Phillip Koeffler, H. In Vivo Deficiency of Both C/EBPβ and C/EBPε Results in Highly Defective Myeloid Differentiation and Lack of Cytokine Response. PLoS ONE 2010, 5, e15419. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Watanabe, N.; Shibata, F.; Kitamura, T.; Ikeda, Y.; Handa, M. N-terminal Region of CCAAT/Enhancer-binding Protein epsilon Is Critical for Cell Cycle Arrest, Apoptosis, and Functional Maturation during Myeloid Differentiation. J. Biol. Chem. 2006, 281, 14494–14502. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, R.; Kim, G.-D.; Radomska, H.S.; Lekstrom-Himes, J.; Smith, L.T.; Antonson, P.; Tenen, D.G.; Xanthopoulos, K.G. CCAAT/enhancer binding protein ɛ is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc. Natl. Acad. Sci. USA 1997, 94, 6462–6467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Stunff, H.; Auger, R.; Kanellopoulos, J.; Raymond, M.-N. The Pro-451 to Leu Polymorphism within the C-terminal Tail of P2X7 Receptor Impairs Cell Death but Not Phospholipase D Activation in Murine Thymocytes. J. Biol. Chem. 2004, 279, 16918–16926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.J.; Zhang, W.; Worthington, R.A.; Sluyter, R.; Dao-Ung, P.; Petrou, S.; Barden, J.A.; Wiley, J.S. A Glu-496 to Ala Polymorphism Leads to Loss of Function of the Human P2X7 Receptor. J. Biol. Chem. 2001, 276, 11135–11142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placido, R.; Auricchio, G.; Falzoni, S.; Battistini, L.; Colizzi, V.; Brunetti, E.; Di Virgilio, F.; Mancino, R. P2X7 purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell. Immunol. 2006, 244, 10–18. [Google Scholar] [CrossRef]
- Suh, B.-C.; Kim, J.-S.; Namgung, U.; Ha, H.; Kim, K.-T. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J. Immunol. 2001, 166, 6754–6763. [Google Scholar] [CrossRef] [Green Version]
- Kahlenberg, J.M.; Lundberg, K.C.; Kertesy, S.B.; Qu, Y.; Dubyak, G.R. Potentiation of Caspase-1 Activation by the P2X7 Receptor Is Dependent on TLR Signals and Requires NF-κB-Driven Protein Synthesis. J. Immunol. 2005, 175, 7611–7622. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, B.D.; Rice, J.; Kertesy, S.B.; Dubyak, G.R. SAPK/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J. Biol. Chem. 2000, 275, 26792–26798. [Google Scholar] [CrossRef] [Green Version]
- Perkins, A.S.; Del Campo, J.J.; Xiao, Y.-Y.; Zhang, Y.; Lin, S.J.; Dudley, J.; Tuck, D.; Yatsula, B. EVI1 Blocks Apoptosis in DA-1 Myeloid Leukemia Cells Via Enhanced Transcription of the Prosurvival Gene Bcl2a1 (A1). Blood 2008, 112, 3805. [Google Scholar] [CrossRef]
- Glass, C.; Wilson, M.; González, R.; Zhang, Y.; Perkins, A.S. The role of EVI1 in myeloid malignancies. Blood Cells Mol. Dis. 2014, 53, 67–76. [Google Scholar] [CrossRef]
- Kustikova, O.S.; Schwarzer, A.; Stahlhut, M.; Brugman, M.H.; Neumann, T.; Yang, M.; Li, Z.; Schambach, A.; Heinz, N.; Gerdes, S.; et al. Activation of Evi1 inhibits cell cycle progression and differentiation of hematopoietic progenitor cells. Leukemia 2012, 27, 1127–1138. [Google Scholar] [CrossRef]
- Yamakawa, N.; Kaneda, K.; Saito, Y.; Ichihara, E.; Morishita, K. The increased expression of integrin α6 (itga6) enhances drug resistance in evi1 high leukemia. PLoS ONE 2012, 7, e30706. [Google Scholar] [CrossRef] [PubMed]
- Konrad, T.A.; Karger, A.; Hackl, H.; Schwarzinger, I.; Herbacek, I.; Wieser, R. Inducible expression of EVI1 in human myeloid cells causes phenotypes consistent with its role in myelodysplastic syndromes. J. Leukoc. Biol. 2009, 86, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, K.; Herbst, F.; Ball, C.; Glimm, H.; Krämer, A.; Löffler, H. Overexpression of EVI1 interferes with cytokinesis and leads to accumulation of cells with supernumerary centrosomes in G0/1 phase. Cell Cycle 2012, 11, 3492–3503. [Google Scholar] [CrossRef] [PubMed]
- Wieser, R. New functions for ecotropic viral integration site 1 (EVI1), an oncogene causing aggressive malignant disease. Cell Cycle 2012, 11, 3915. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Ogawa, S.; Tanaka, T.; Mitani, K.; Yazaki, Y.; Witte, O.N.; Hirai, H. The AML1/Evi-1 fusion protein in the t(3;21) translocation exhibits transforming activity on Rat1 fibroblasts with dependence on the Evi-1 sequence. Oncogene 1995, 11, 833–840. [Google Scholar]
- Tanaka, T.; Mitani, K.; Kurokawa, M.; Ogawa, S.; Tanaka, K.; Nishida, J.; Yazaki, Y.; Shibata, Y.; Hirai, H. Dual functions of the AML1/Evi-1 chimeric protein in the mechanism of leukemogenesis in t(3;21) leukemias. Mol. Cell. Biol. 1995, 15, 2383–2392. [Google Scholar] [CrossRef] [Green Version]
- Hou, A.; Zhao, L.; Zhao, F.; Wang, W.; Niu, J.; Li, B.; Zhou, Z.; Zhu, D. Expression of MECOM is associated with unfavorable prognosis in glioblastoma multiforme. OncoTargets Ther. 2016, 9, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Idel, C.; Ribbat-Idel, J.; Kuppler, P.; Krupar, R.; Offermann, A.; Vogel, W.; Rades, D.; Kirfel, J.; Wollenberg, B.; Perner, S. EVI1 as a marker for lymph node metastasis in HNSCC. Int. J. Mol. Sci. 2020, 21, 854. [Google Scholar] [CrossRef] [Green Version]
- Nucifora, G. The EVI1 gene in myeloid leukemia. Leukemia 1997, 11, 2022–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, M.; Thompson, F.; Spier, C.; Taetle, R. Expression of the EVI1 gene in chronic myelogenous leukemia in blast crisis. Leukemia 1993, 7, 1654–1657. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Wang, J. EVI1 in Leukemia and Solid Tumors. Cancers 2020, 12, 2667. https://doi.org/10.3390/cancers12092667
Liang B, Wang J. EVI1 in Leukemia and Solid Tumors. Cancers. 2020; 12(9):2667. https://doi.org/10.3390/cancers12092667
Chicago/Turabian StyleLiang, Beiyuan, and Jing Wang. 2020. "EVI1 in Leukemia and Solid Tumors" Cancers 12, no. 9: 2667. https://doi.org/10.3390/cancers12092667




