Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
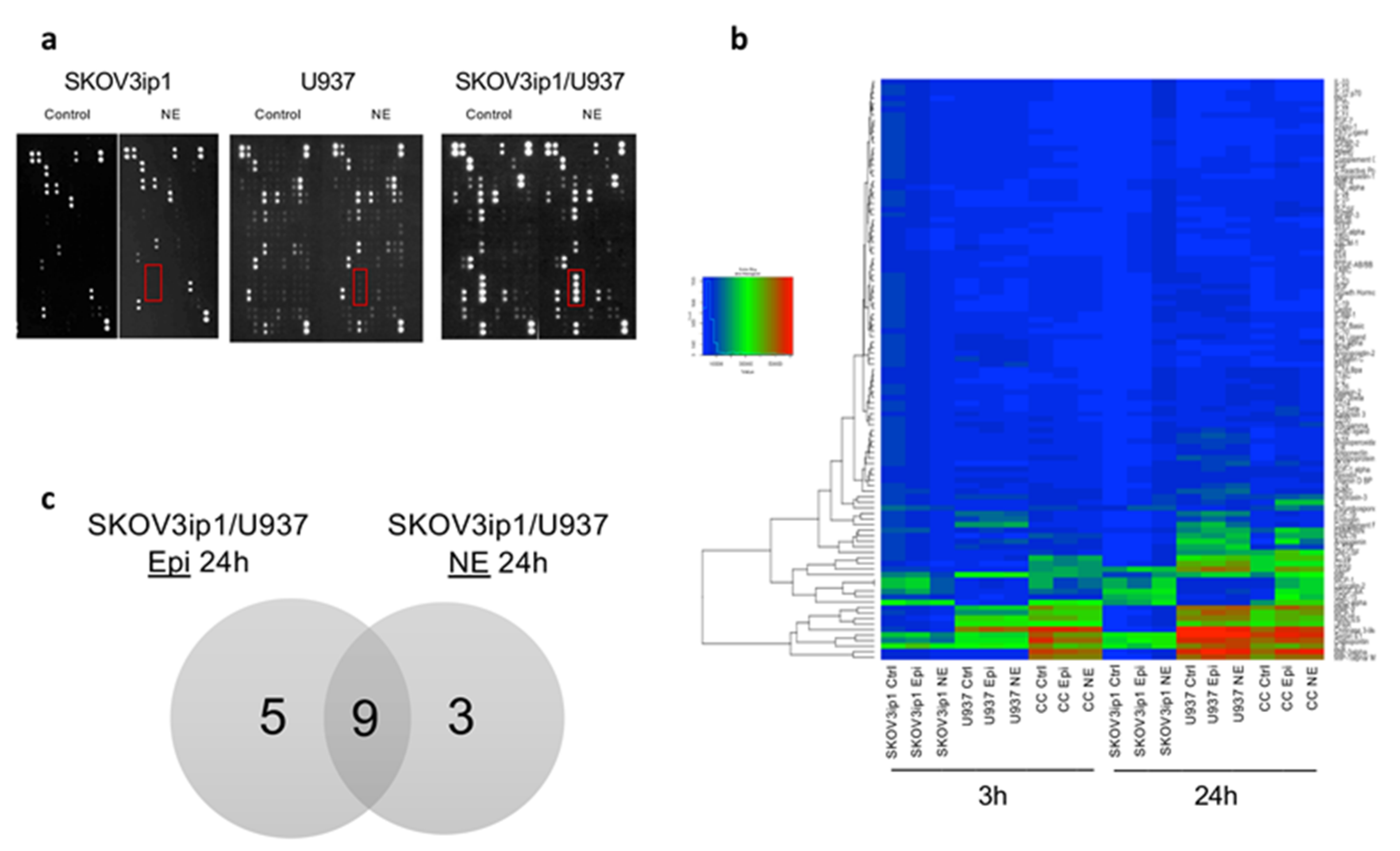
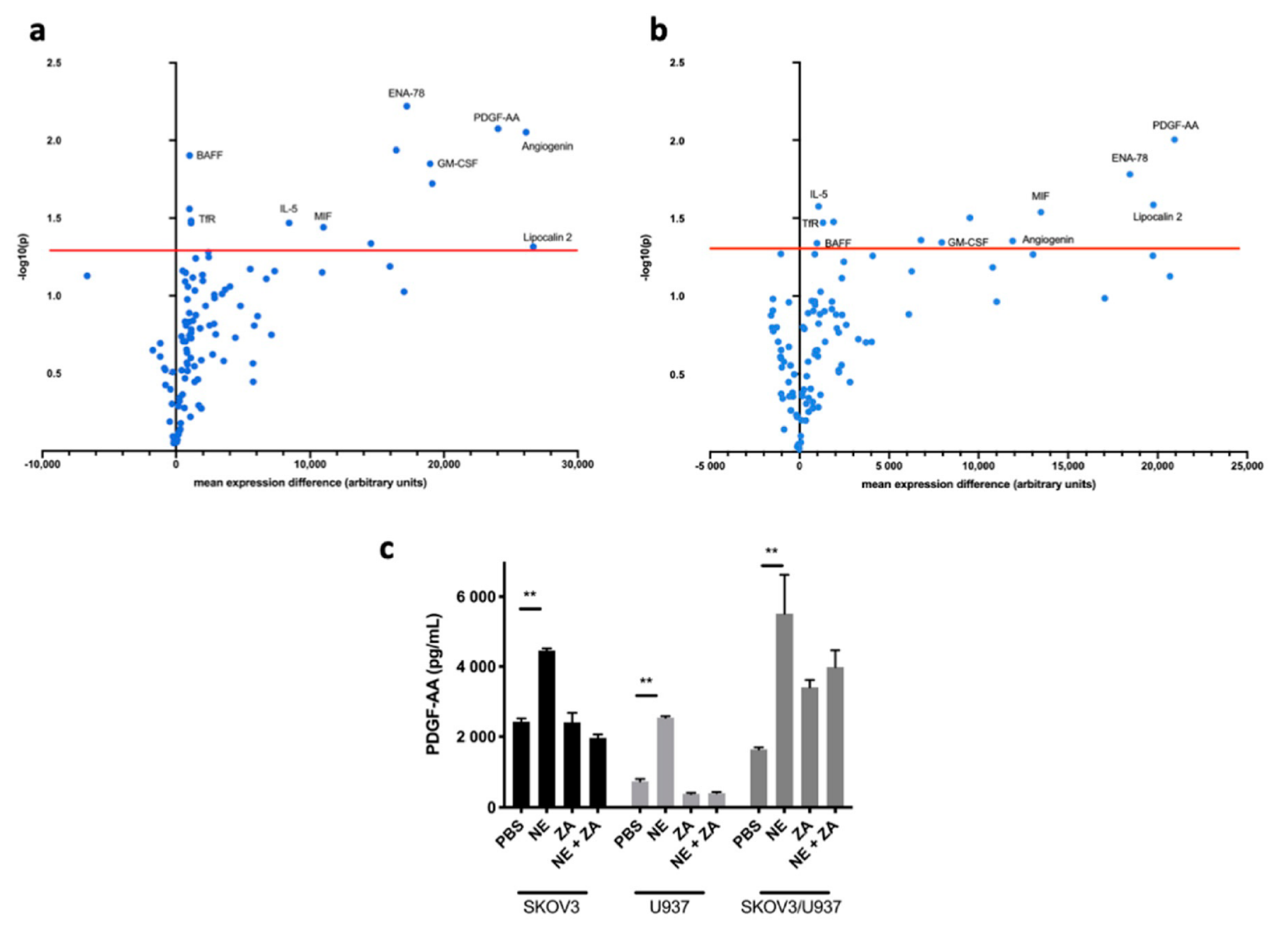
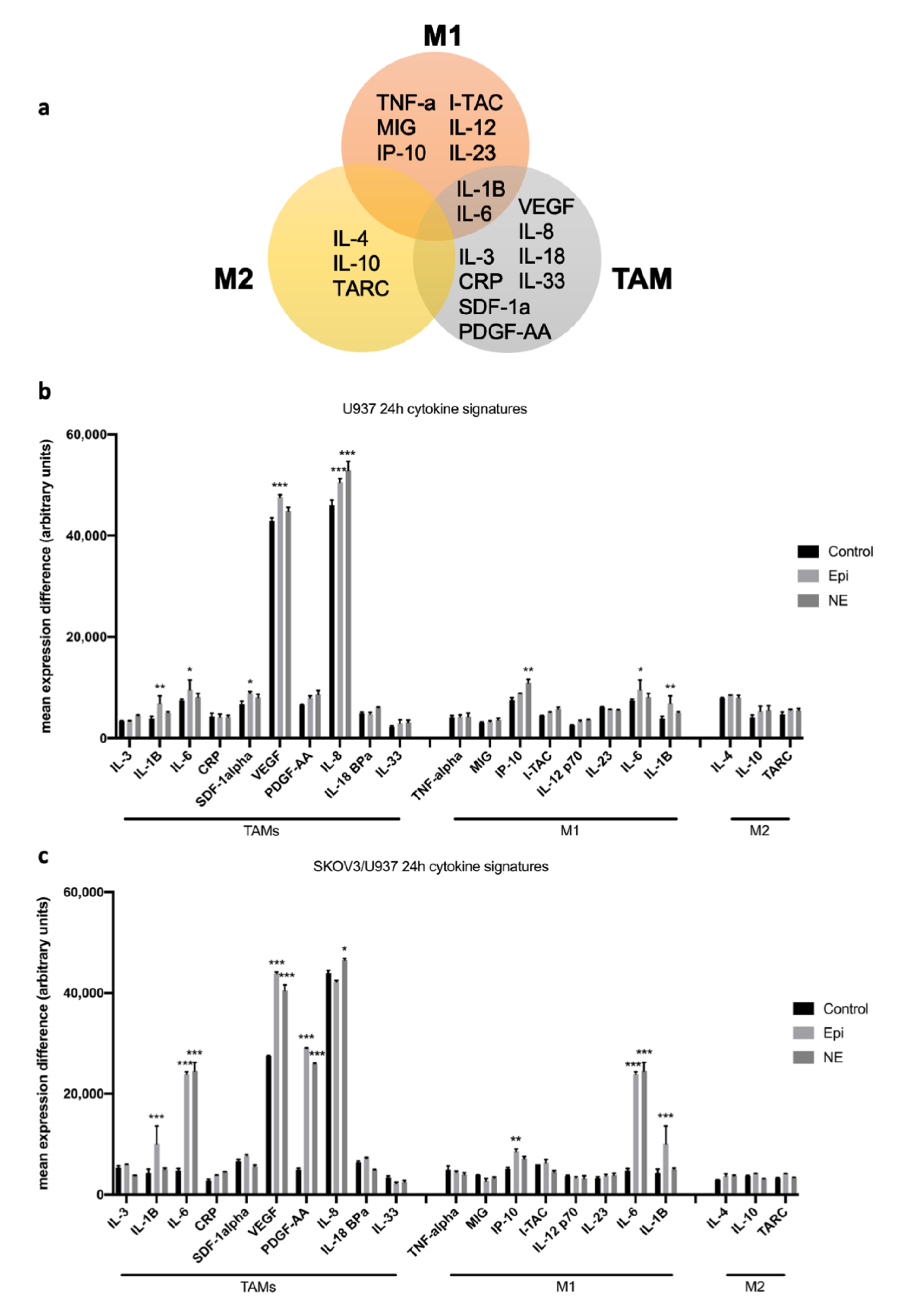
2.1. Adrenergic Stimuli Increase Pro-Inflammatory Cytokine Production
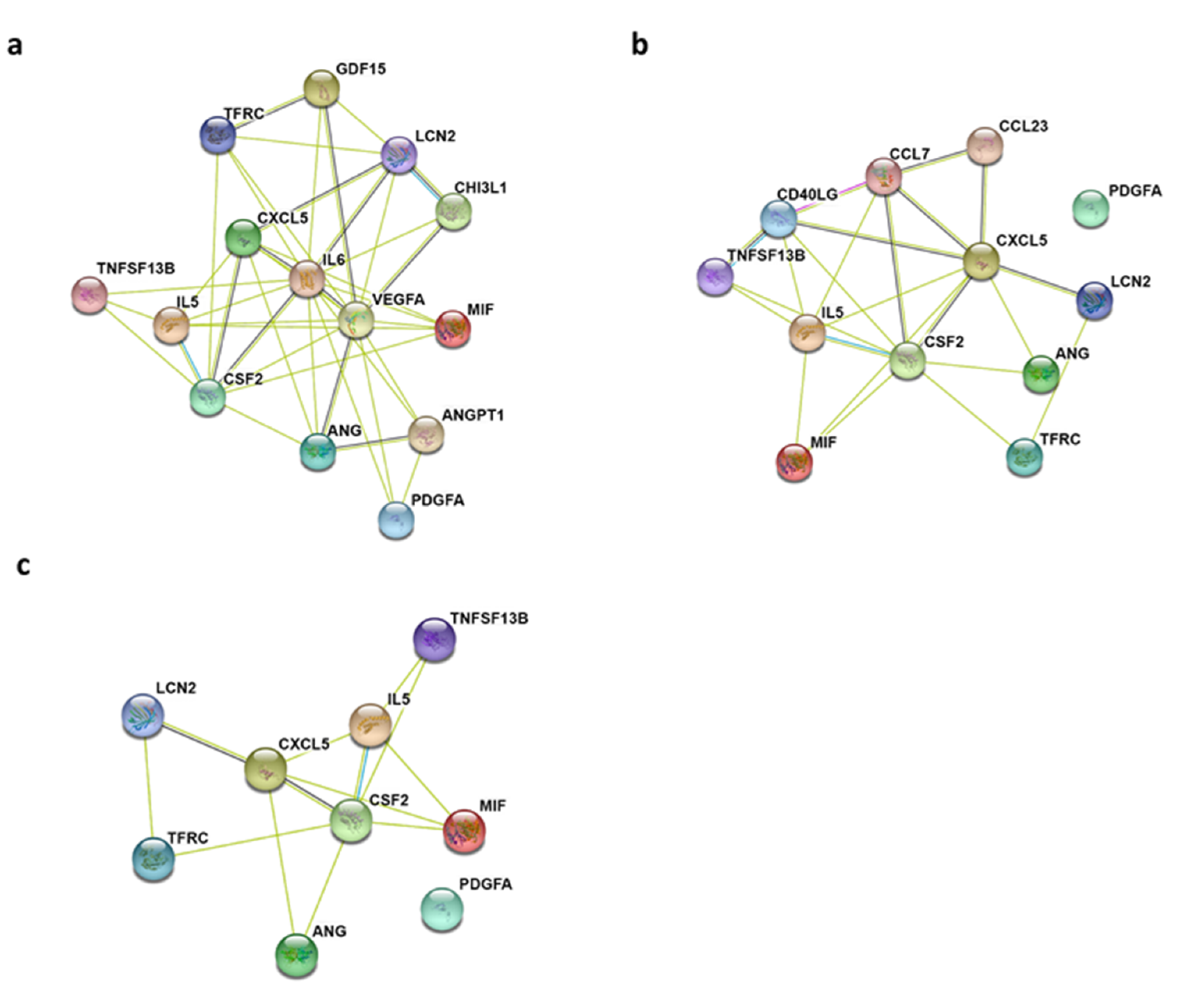
2.2. Gene Ontology Biological Process and KEGG Pathway Enrichment Analyses of Differentially Expressed Cytokines
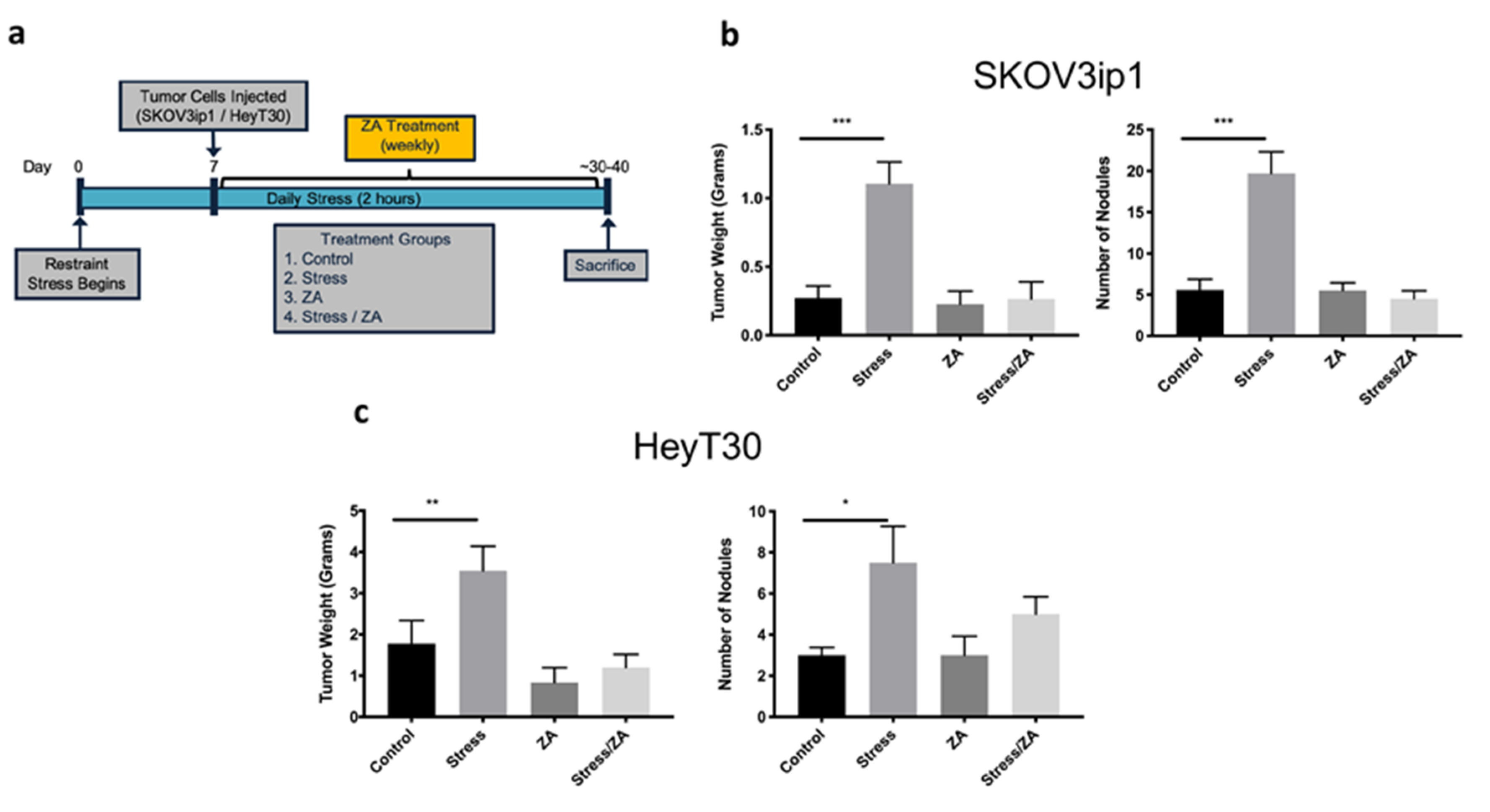
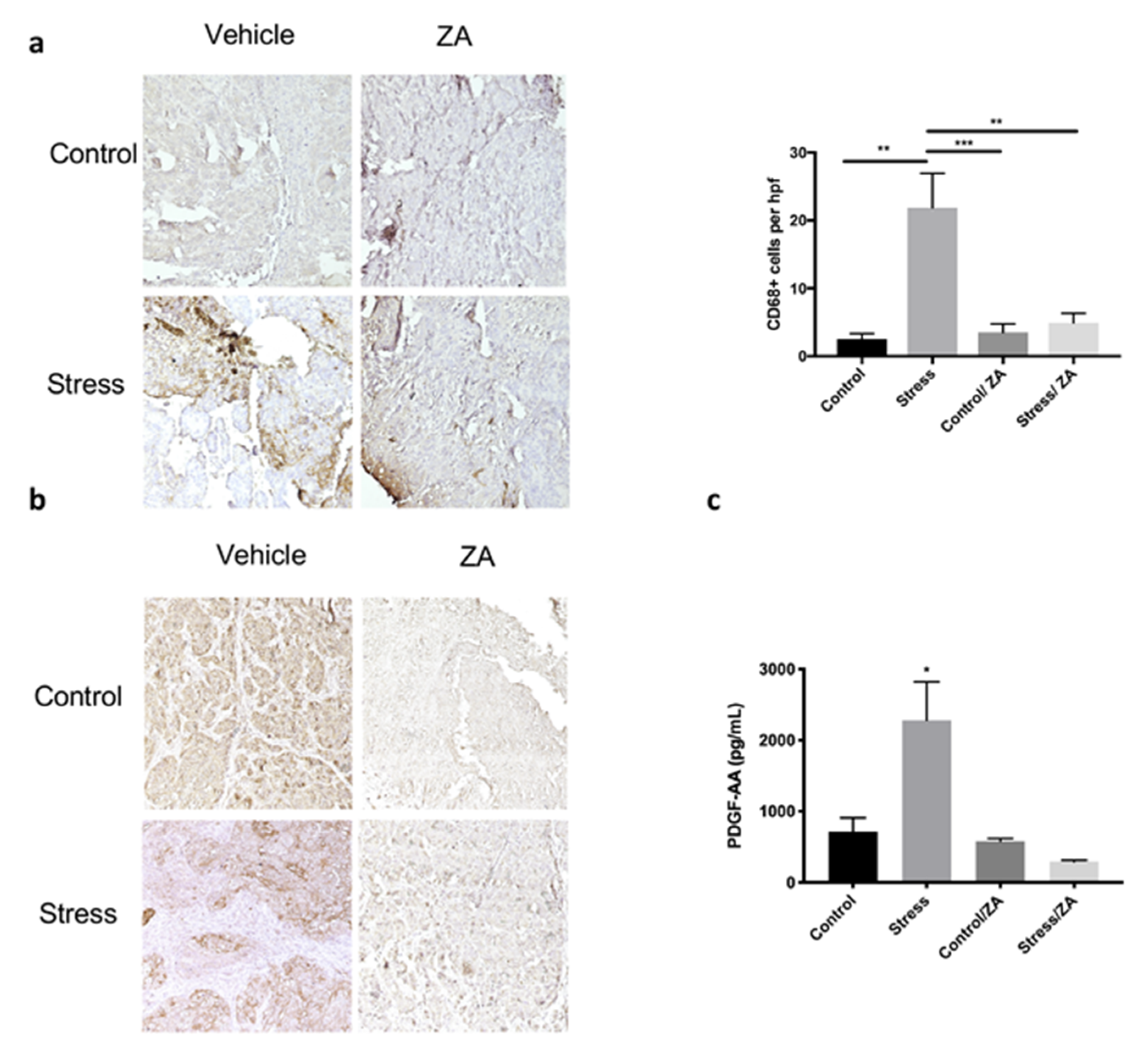
2.3. Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression and Tumor Growth in Orthotopic Mouse Models of Ovarian Cancer
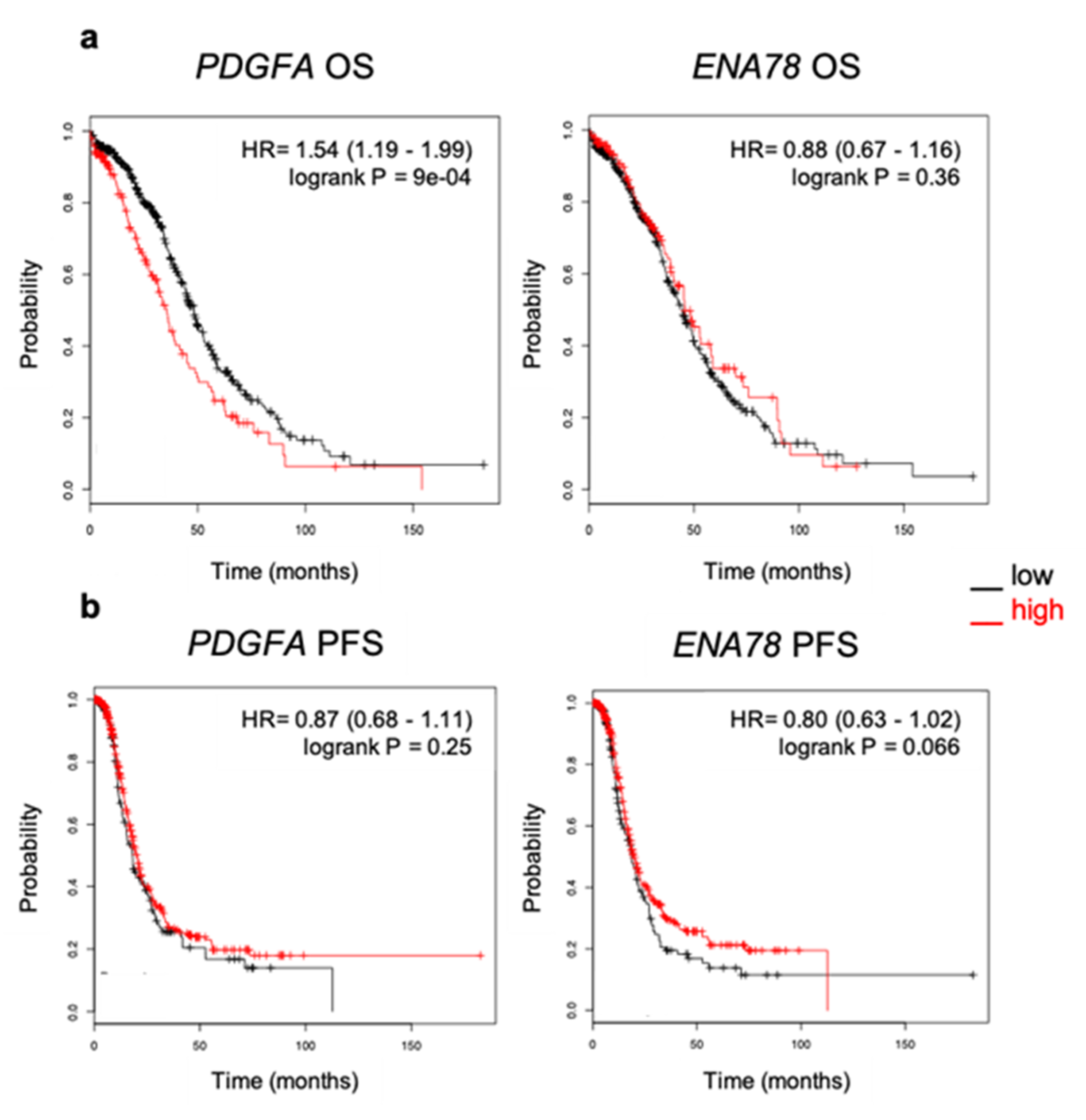
2.4. Elevated Expression of PDGFA Correlates with Poor Outcome in Ovarian Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Treatments, and Co-Culture System
4.2. Cytokine Arrays
4.3. Protein-Protein Interaction (PPI) Network Construction
4.4. Animal Experiments
4.5. Immunohistochemistry
4.6. ELISA
4.7. Kaplan–Meier Survival Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DAB | 3,3′-Diaminobenzidine |
| Epi | epinephrine |
| NE | norepinephrine |
| ZA | zoledronic acid |
| hrs | hours |
| BAFF | B-cell activating factor |
| ENA-78 | epithelial cell-derived neutrophil-activating peptide |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GO | gene ontology |
| HR | hazard ratio |
| HPF | high-power field |
| IL | interleukin |
| MIF | macrophage migration inhibitory factor |
| OS | overall survival |
| PDGF-AA | platelet-derived growth factor AA |
| PFS | progression-free survival |
| TAMs | tumor-associated macrophages |
| TfR | transferrin receptor |
| VEGF | vascular endothelial growth factor |
| PPI | protein-protein interaction |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| TNF | tumor necrosis factor |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| KM | Kaplan–Meier |
| MMP | matrix metalloprotein |
| PMA | phorbol 12-myristate 13-acetate |
| MeV | Multiexperiment viewer |
| IHC | immunohistochemistry |
| CI | confidence interval |
References
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2013; National Cancer Institute: Bethesda, MD, USA, 2016; p. 19.
- Tortolero-Luna, G.; Zavala-Zegarra, D.; Pérez-Ríos, N.; Torres-Cintrón, C.; Ortiz-Ortiz, K.; Traverso-Ortiz, M.; Román-Ruiz, Y.; Veguilla-Rosario, I.; Vázquez-Cubano, N.; Merced-Vélez, M.; et al. Cancer in Puerto Rico, 2006–2010; Puerto Rico Central Cancer Registry: San Juan, PR, USA, 2013; p. 107. [Google Scholar]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Bodurka-Bevers, D.; Basen-Engquist, K.; Carmack, C.L.; Fitzgerald, M.A.; Wolf, J.K.; de Moor, C.; Gershenson, D.M. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol. Oncol. 2000, 78, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.L.; Thaker, P.H.; Nick, A.M.; Ramondetta, L.M.; Kumar, S.; Urbauer, D.L.; Matsuo, K.; Squires, K.C.; Coleman, R.L.; Lutgendorf, S.K.; et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 2015, 121, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M. Quality of life and mental health among women with ovarian cancer: Examining the role of emotional and instrumental social support seeking. Psychol. Health Med. 2016, 21, 551–561. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Andersen, B.L. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am. Psychol. 2015, 70, 186–197. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Nagaraja, A.S.; Dorniak, P.L.; Sadaoui, N.C.; Kang, Y.; Lin, T.; Armaiz-Pena, G.; Wu, S.Y.; Rupaimoole, R.; Allen, J.K.; Gharpure, K.M.; et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 2016, 35, 2390–2397. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Nagaraja, A.S.; Armaiz-Pena, G.N.; Dorniak, P.L.; Hu, W.; Rupaimoole, R.; Liu, T.; Gharpure, K.M.; Previs, R.A.; Hansen, J.M.; et al. Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 1713–1724. [Google Scholar] [CrossRef] [Green Version]
- Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Nagaraja, A.S.; Rodriguez-Aguayo, C.; Sadaoui, N.C.; Stone, R.L.; Matsuo, K.; Dalton, H.J.; Previs, R.A.; Jennings, N.B.; et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015, 6, 4266–4273. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.H.; Du, Y.; Mao, D.; Wang, Z.L.; He, Z.Q.; Qiu, J.D.; Ma, X.B.; Shang, W.T.; Ding, D.; Tian, J. Zoledronic acid prevents the tumor-promoting effects of mesenchymal stem cells via MCP-1 dependent recruitment of macrophages. Oncotarget 2015, 6, 26018–26028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Villasana, V.; Rodriguez-Aguayo, C.; Arumugam, T.; Cruz-Monserrate, Z.; Fuentes-Mattei, E.; Deng, D.; Hwang, R.F.; Wang, H.; Ivan, C.; Garza, R.J.; et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound paclitaxel in pancreatic ductal adenocarcinoma. Mol. Cancer 2014, 13, 2583–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulbe, H.; Chakravarty, P.; Leinster, D.A.; Charles, K.A.; Kwong, J.; Thompson, R.G.; Coward, J.I.; Schioppa, T.; Robinson, S.C.; Gallagher, W.M.; et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012, 72, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinartz, S.; Finkernagel, F.; Adhikary, T.; Rohnalter, V.; Schumann, T.; Schober, Y.; Nockher, W.A.; Nist, A.; Stiewe, T.; Jansen, J.M.; et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Bax, H.J.; Scotto, D.; Souri, E.A.; Sollie, S.; Harris, R.J.; Hammar, N.; Walldius, G.; Winship, A.; Ghosh, S.; et al. Immune mediator expression signatures are associated with improved outcome in ovarian carcinoma. Oncoimmunology 2019, 8, e1593811. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Gyorffy, B.; Lanczky, A.; Szallasi, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Costantini, S.; Capone, F.; Guerriero, E.; Castello, G. An approach for understanding the inflammation and cancer relationship. Immunol Lett 2009, 126, 91–92. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Savant, S.S.; Sriramkumar, S.; O’Hagan, H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaishi, K.; Komohara, Y.; Tashiro, H.; Ohtake, H.; Nakagawa, T.; Katabuchi, H.; Takeya, M. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010, 101, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A.; Medina, L.; Piura, B.; Segal, S.; Huleihel, M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res. 2007, 27, 267–272. [Google Scholar] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Gorouhi, F.; Ramirez, S.; Granick, J.L.; Byrne, B.A.; Soulika, A.M.; Simon, S.I.; Rivkah Isseroff, R. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J. Investig. Dermatol. 2014, 134, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Costantini, S.; Sharma, A.; Colonna, G. The Value of the Cytokinome Profile. In Inflammatory Diseases—A Modern Perspective; Nagal, A., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Shikada, Y.; Yonemitsu, Y.; Koga, T.; Onimaru, M.; Nakano, T.; Okano, S.; Sata, S.; Nakagawa, K.; Yoshino, I.; Maehara, Y.; et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005, 65, 7241–7248. [Google Scholar] [CrossRef] [Green Version]
- Zaynagetdinov, R.; Sherrill, T.P.; Gleaves, L.A.; McLoed, A.G.; Saxon, J.A.; Habermann, A.C.; Connelly, L.; Dulek, D.; Peebles, R.S., Jr.; Fingleton, B.; et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015, 75, 1624–1634. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; McNeish, B.; Butterfield, C.; Moses, M.A. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013, 27, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Simpson, K.D.; Templeton, D.J.; Cross, J.V. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J. Immunol. 2012, 189, 5533–5540. [Google Scholar] [CrossRef] [Green Version]
- Jansson, S.; Aaltonen, K.; Bendahl, P.O.; Falck, A.K.; Karlsson, M.; Pietras, K.; Ryden, L. The PDGF pathway in breast cancer is linked to tumour aggressiveness, triple-negative subtype and early recurrence. Breast Cancer Res. Treat. 2018, 169, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Andersson, P.; Hosaka, K.; Zhang, Y.; Cao, R.; Iwamoto, H.; Yang, X.; Nakamura, M.; Wang, J.; Zhuang, R.; et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat. Commun. 2016, 7, 11385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoschek, M.; Pietras, K. PDGF family function and prognostic value in tumor biology. Biochem. Biophys. Res. Commun. 2018, 503, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Milanezi, F.; Martins, A.; Reis, R.M.; Schmitt, F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005, 7, R788–R795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Nishimura, M.; Komurov, K.; Shahzad, M.M.; Ali-Fehmi, R.; Roh, J.W.; Lu, C.; Cody, D.D.; Ram, P.T.; Loizos, N.; et al. Platelet-derived growth factor receptor alpha (PDGFRalpha) targeting and relevant biomarkers in ovarian carcinoma. Gynecol. Oncol. 2014, 132, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E.; Collinson, M.; Gregory, W.; Marshall, H.; Bell, R.; Dodwell, D.; Keane, M.; Gil, M.; Barrett-Lee, P.; Ritchie, D.; et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J. Bone Oncol. 2018, 13, 123–135. [Google Scholar] [CrossRef]
- Patntirapong, S.; Poolgesorn, M. Alteration of macrophage viability, differentiation, and function by bisphosphonates. Oral Dis. 2018, 24, 1294–1302. [Google Scholar] [CrossRef]
- Huang, G.S.; Brouwer-Visser, J.; Ramirez, M.J.; Kim, C.H.; Hebert, T.M.; Lin, J.; Arias-Pulido, H.; Qualls, C.R.; Prossnitz, E.R.; Goldberg, G.L.; et al. Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin. Cancer Res. 2010, 16, 2999–3010. [Google Scholar] [CrossRef] [Green Version]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef]
- Barbieri, A.; Bimonte, S.; Palma, G.; Luciano, A.; Rea, D.; Giudice, A.; Scognamiglio, G.; La Mantia, E.; Franco, R.; Perdona, S.; et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int. J. Oncol. 2015, 47, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Lamboy-Caraballo, R.; Ortiz-Sanchez, C.; Acevedo-Santiago, A.; Matta, J.; NA Monteiro, A.; Armaiz-Pena, G.N. Norepinephrine-Induced DNA Damage in Ovarian Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2250. [Google Scholar] [CrossRef] [Green Version]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Various R Programming Tools for Plotting Data; Version 3.0.4, CRAN Repository. 2020. Available online: https://cran.r-project.org/web/packages/gplots/index.html (accessed on 9 September 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Morales, J.; Mejías-Morales, D.; Rivera-Rivera, S.; González-Flores, J.; González-Loperena, M.; Cordero-Báez, F.Y.; Pedreira-García, W.M.; Chardón-Colón, C.; Cabán-Rivera, J.; Cress, W.D.; et al. Hyper-phosphorylation of Rb S249 together with CDK5R2/p39 overexpression are associated with impaired cell adhesion and epithelial-to-mesenchymal transition: Implications as a potential lung cancer grading and staging biomarker. PLoS ONE 2018, 13, e0207483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colon-Echevarria, C.B.; Ortiz, T.; Maldonado, L.; Hidalgo-Vargas, M.J.; Pérez-Morales, J.; Aquino-Acevedo, A.N.; Herrera-Noriega, R.; Bonilla-Claudio, M.; Castro, E.M.; Armaiz-Pena, G.N. Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth. Cancers 2020, 12, 2671. https://doi.org/10.3390/cancers12092671
Colon-Echevarria CB, Ortiz T, Maldonado L, Hidalgo-Vargas MJ, Pérez-Morales J, Aquino-Acevedo AN, Herrera-Noriega R, Bonilla-Claudio M, Castro EM, Armaiz-Pena GN. Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth. Cancers. 2020; 12(9):2671. https://doi.org/10.3390/cancers12092671
Chicago/Turabian StyleColon-Echevarria, Claudia B., Tatiana Ortiz, Lizette Maldonado, Melanie J. Hidalgo-Vargas, Jaileene Pérez-Morales, Alexandra N. Aquino-Acevedo, Roberto Herrera-Noriega, Margarita Bonilla-Claudio, Eida M. Castro, and Guillermo N. Armaiz-Pena. 2020. "Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth" Cancers 12, no. 9: 2671. https://doi.org/10.3390/cancers12092671






