Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook
Abstract
:Simple Summary
Abstract
1. Glioblastoma: Occurrence, Mechanisms, Treatments, and Challenges
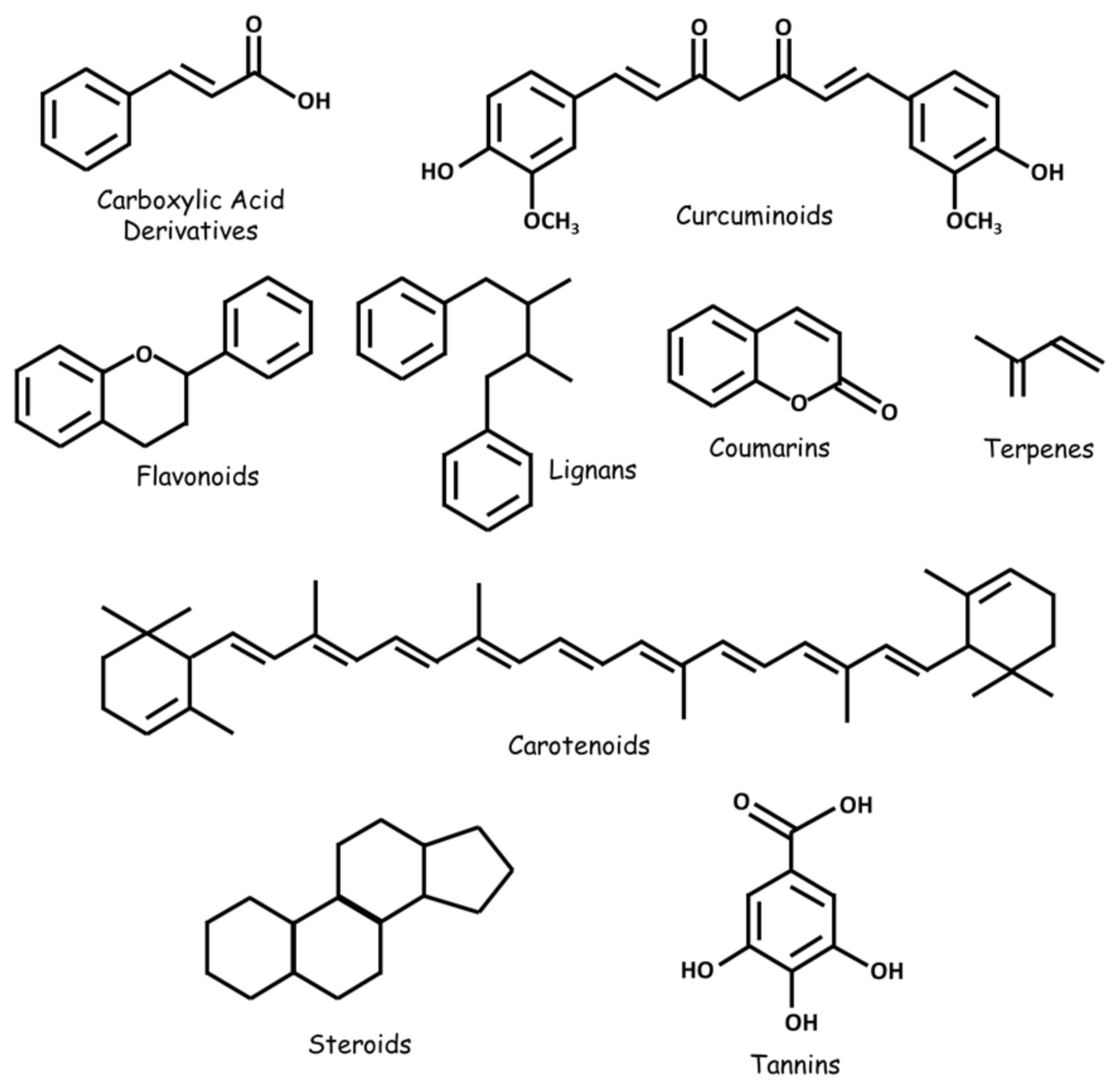
2. Natural Compounds Modulating Glioblastoma
2.1. Alkaloids
2.2. Carboxylic Acid Derivatives
2.3. Carotenoids
2.4. Flavonoids
2.5. Coumarins
2.6. Curcuminoids
2.7. Terpenes
2.8. Lignans
2.9. Natural Steroids
2.10. Tannins
2.11. Crude and Purified Plant Extracts
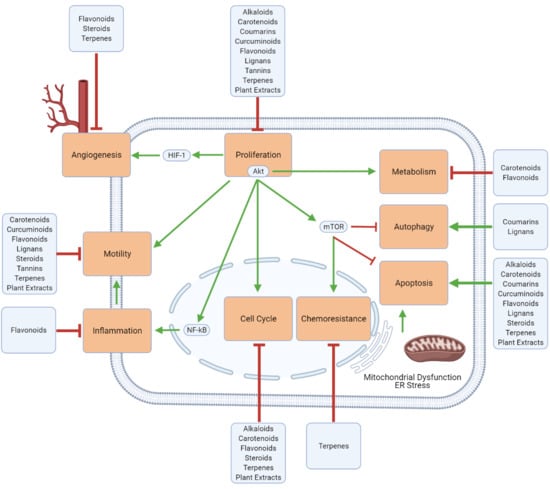
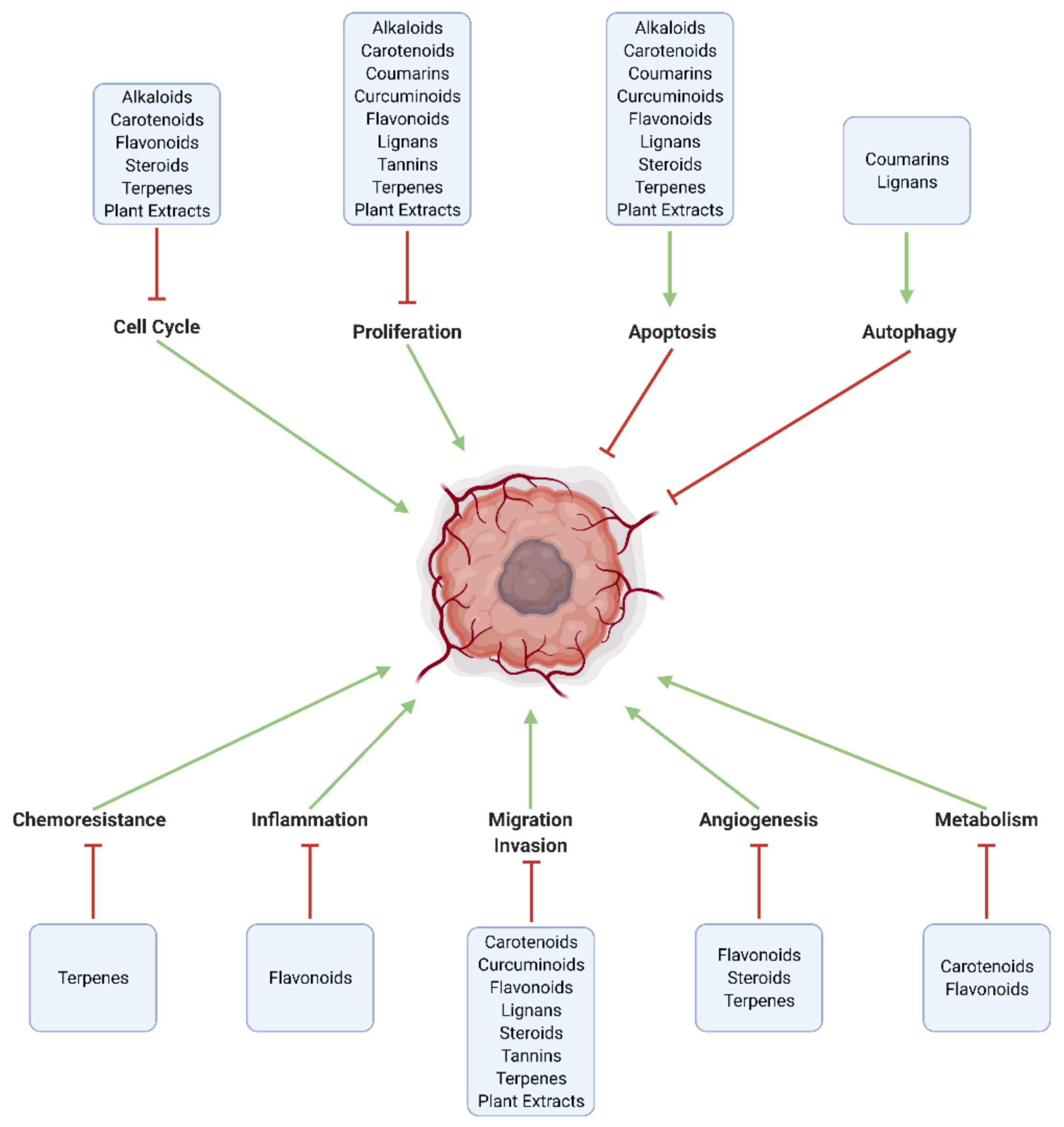
3. Mechanistic Effects of Natural Compounds on Glioblastoma
3.1. Generalized Anti-Cancer Markers
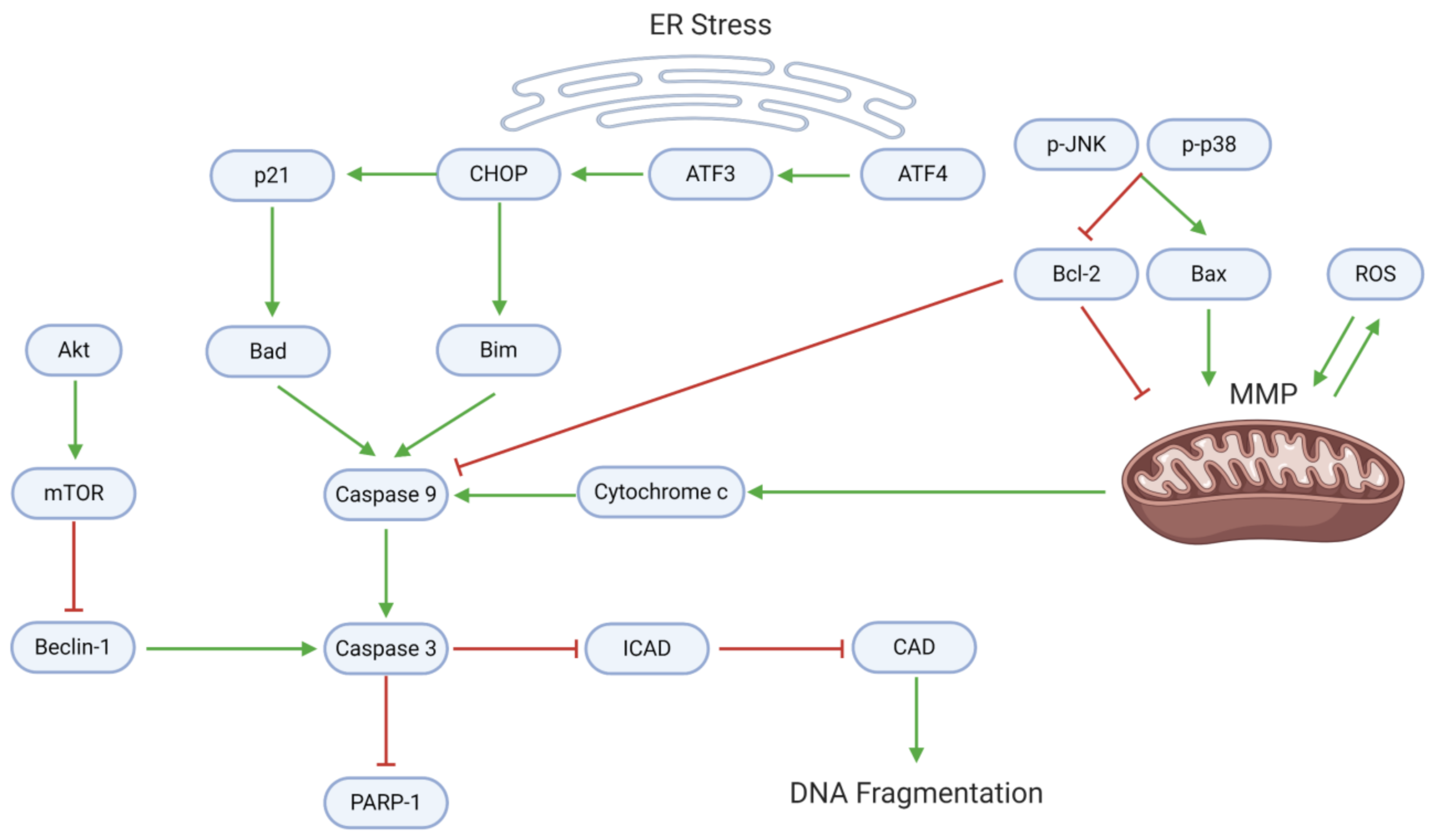
3.2. Proliferation, Apoptosis, and Autophagy
3.3. Cell Cycle
3.4. Inflammation and Immune Cell Modulation
3.5. Migration, Invasion, and Metastasis
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Reduces cell migration | Eucalyptal A | U87MG, LN229 | [32] |
| Astaxanthin | GL261, U251MG | [35] | |
| Adonixanthin | GL261, U251MG | [35] | |
| Arctigenin | U87MG, T98G | [61] | |
| Crocetin | U87, U251 | [34] | |
| CP | GAMG | [36] | |
| McC1 | U251, GAMG | [36] | |
| Tannic Acid | C6 | [39] | |
| TBMS1 | U87, LN229 | [41] | |
| Curcumin | U87 | [52] | |
| Paeoniflorin | U251, T98G | [53] | |
| Diosgenin | C6, T98G | [54] | |
| Rutin | C6 | [63] | |
| Magnolol | LN229, U87MG | [71] | |
| Gamabufotalin | U87 | [72] | |
| Quercetin | C6 | [63] | |
| Reduces cell invasion | Eucalyptal A | U87MG, LN229 | [32] |
| Arctigenin | U87MG, T98G | [61] | |
| McC1 | GAMG, U251 | [36] | |
| CrataBL | U87 | [49] | |
| Curcumin | U87 | [52] | |
| Paeoniflorin | U251, T98G | [53] | |
| Diosgenin | C6, T98G | [54] | |
| Downregulates MMP-2 (protein) | Astaxanthin | GL261 | [35] |
| Adonixanthin | GL261 | [35] | |
| Arctigenin | U87MG | [61] | |
| TBMS1 | U87, LN229 | [41] | |
| Diosgenin | T98G | [54] | |
| Downregulates MMP-7 (protein) | TBMS1 | U87, LN229 | [41] |
| Downregulates MMP-9 (protein) | Arctigenin | U87MG | [61] |
| Diosgenin | C6 | [54] | |
| Downregulates p-PKA 1/2/3 (prot.) | Eucalyptal A | U87MG, LN229 | [32] |
| Downregulates p-Cofilin (protein) | Eucalyptal A | U87MG, LN229 | [32] |
| Downregulates fibronectin (protein) | Adonixanthin | GL261 | [35] |
| Downregulates laminin (protein) | CrataBL | U87 | [49] |
| Downregulates Snail (protein) | TBMS1 | U87, LN229 | [41] |
| Galangin | U87, U251 | [70] | |
| Downregulates Snail (mRNA) | Galangin | U87, U251 | [70] |
| Downregulates Slug (protein) | TBMS1 | U87, LN229 | [41] |
| Downregulates Fascin (protein) | Curcumin | U87 | [52] |
| Reduces actin filament number | Paeoniflorin | T98G, U251 | [53] |
| Downregulates GTP-RhoA (protein) | Paeoniflorin | T98G, U251 | [53] |
| Downregulates ROCK (protein) | Paeoniflorin | T98G, U251 | [53] |
| Downregulates (p-)Limk1 (protein) | Paeoniflorin | T98G, U251 | [53] |
3.6. Angiogenesis
3.7. Metabolism
3.8. Chemoresistance
4. Synergistic and Combinatory Effects (Multiple Natural Compounds)
5. Challenges and Considerations in the Use of Natural Substances for GBM Treatment
5.1. Bioavailabilty and BBB Permeability
5.2. Selectivity of Natural Compounds for GBM
5.3. Delivery Mechanisms to Enhance Natural Compounds’ Anti-GBM Properties
6. Implications for Lifestyle Medicine
6.1. Lifestyle Approaches to (Brain) Cancer
6.2. Availability of Natural Substances as Supplements
6.3. Promising Natural Compounds and the Path to Clinical Trials
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS1 | A Disintegrin And Metalloproteinase with ThromboSpondin motifs 1 |
| Akt | serine-threonine kinase Akt |
| AM01 | 4β,5-dihydro-15-deoxy-eremantholide |
| AM02 | 4β,5-dihydro-2′,3′-epoxy-15-deoxy-goyazensolide |
| AM03 | 4β,5-dihydro-1′,2′-epoxy-15-deoxy-eremantholide |
| AM04 | goyazensolide |
| AM05 | lychnofolide |
| AM06 | 15-deoxy-goyazenolide |
| AMPK | 5’ Adenosine Monophosphate-activated Protein Kinase |
| APC | κN′,N’’-3-acetyloxy-BA-28-[2-(2-aminoethyl)aminoethyl]amide dichlorido platinum(II) |
| ATF4 | Activating Transcription Factor 4 |
| ATF6 | Activating Transcription Factor 6 |
| Bad | Bcl-2 associated death promoter |
| Bax | Bcl-2 associated x protein |
| BBR | BerBeRine |
| Bcl-2 | B-cell lymphoma 2 |
| c-Myc | MYC proto-oncogene |
| CA9 | Carbonic Anhydrase 9 |
| CAD | Caspase-Activated DNAse |
| CAT | CATalase |
| CCL2 | C-C motif chemokine Ligand 2 |
| CCL5 | C-C motif chemokine Ligand 5 |
| CD105 | Cluster of Differentiation 105 |
| CD31 | Cluster of Differentiation 31 |
| CDK2 | Cyclin-Dependent Kinase 2 |
| CDK4 | Cyclin-Dependent Kinase 4 |
| CHOP | C/EBP HOmologous Protein |
| CX3CL1 | chemokine (C-X3-C motif) Ligand 1 |
| DE9B | 3-acetyloxy-BA-28-[2-(2-aminoethyl)aminoethyl]amide |
| DSLD | Dietary Supplement Label Database |
| ECAR | ExtraCellular Acidification Rate |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-Mesenchymal Transition |
| ERK | Extracellular signal-Regulated Kinase |
| FASN | Fatty Acid SyNthase |
| FBW7 | F-Box and WD repeat domain-containing 7 |
| FPR1 | Formyl Peptide Receptor 1 |
| GBM | GlioBlastoMa |
| GDNF | Glial cell-Derived Neurotrophic Factor |
| GSC | Glioma Stem Cell |
| GSK3β | Glycogen Synthase Kinase 3 βeta |
| GTP-RhoA | Guanosine TriPhosphate-RhoA |
| H2AX | H2A histone family member X |
| HDAC1 | Histone DeACetylase 1 |
| HDAC3 | Histone DeACetylase 3 |
| HDGF | Hepatoma-Derived Growth Factor |
| HIF-1α | Hypoxia Inducible Factor-1 αlpha |
| HK2 | HexoKinase 2 |
| ICAD | Inhibitor of Caspase-Activated DNAse |
| IGF | Insulin-like Growth Factor |
| IL-1(β) | InterLeukin 1(βeta) |
| IL-18 | InterLeukin 18 |
| IL-4 | InterLeukin 4 |
| IL-6 | InterLeukin 6 |
| IL-10 | InterLeukin 10 |
| JNK | c-Jun N-terminal Kinase |
| LC3B-II | microtubule-associated proteins 1A/1B Light Chain 3B |
| MAPK | Mitogen Activated Protein Kinase |
| Mcl-1 | Myeloid cell leukemia 1 |
| MDR1 | MultiDrug Resistance protein 1 |
| MGMT | O6-MethlyGuanine-DNA-MethylTransferase |
| MMP-2 | Matrix MetalloProteinase-2 |
| MMP-9 | Matrix MetalloProteinase-9 |
| mTOR | mammalian Target Of Rapamycin |
| MYO1B | MYOsin 1B |
| NOS2 | Nitric Oxide Synthase 2 |
| Nox-4 | NADPH oxidase 4 |
| OCR | Oxygen Consumption Rate |
| OS | Overall Survival |
| OTC | Over-The-Counter |
| PAK 1/2/3 | p21/Cdc42/Rac1-Activated Kinase 1/2/3 |
| PARP | Poly (ADP-Ribose) Polymerase |
| PDK1 | Pyruvate Dehydrogenase Kinase 1 |
| PDK4 | Pyruvate Dehydrogenase Kinase 4 |
| PFS | Progression Free Survival |
| PI3K | PhosphoInositide 3-Kinase |
| PTGS2 | ProsTaGlandin-endoperoxide Synthase 2 |
| PTPN1 | Protein Tyrosine Phosphatase Non-receptor type 1 |
| Raf | Rapidly accelerated fibrosarcoma |
| ROCK | RhO-assoCiated protein Kinase |
| ROS | Reactive Oxygen Species |
| SLCP | Solid Lipid Curcumin Particles |
| SOD | SuperOxide Dismutase |
| SRSF1 | Serine/arginine-Rich Splicing Factor 1 |
| TBMS1 | TuBeiMoSide-1 |
| TGF(-β) | Tumor Growth Factor (βeta) |
| TIMP-3 | Tissue Inhibitor of MetalloProteinases 3 |
| TMZ | TeMoZolomide |
| TNF(-α) | Tumor Necrosis Factor (αlpha) |
| uPA | urokinase Plasminogen Activator |
| VEGF | Vascular Endothelial Growth Factor |
| XBP1 | X-box Binding Protein 1 |
References
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; de Vleeschouwer, S., Ed.; Exon Publications: Brisbane, QLD, Australia, 2017. [Google Scholar] [CrossRef]
- Darefsky, A.S.; King, J.T., Jr.; Dubrow, R. Adult glioblastoma multiforme survival in the temozolomide era: A population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 2012, 118, 2163–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, F.R.; Kahn, S.A.; Soletti, R.C.; Biasoli, D.; Alves, T.; da Fonseca, A.C.; Garcia, C.; Romao, L.; Brito, J.; Holanda-Afonso, R.; et al. Glioblastoma: Therapeutic challenges, what lies ahead. Biochim. Biophys. Acta 2012, 1826, 338–349. [Google Scholar] [CrossRef]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016, 18, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haar, C.P.; Hebbar, P.; Wallace, G.C.t.; Das, A.; Vandergrift, W.A., 3rd; Smith, J.A.; Giglio, P.; Patel, S.J.; Ray, S.K.; Banik, N.L. Drug resistance in glioblastoma: A mini review. Neurochem. Res. 2012, 37, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Kalokhe, G.; Grimm, S.A.; Chandler, J.P.; Helenowski, I.; Rademaker, A.; Raizer, J.J. Metastatic glioblastoma: Case presentations and a review of the literature. J. Neurooncol. 2012, 107, 21–27. [Google Scholar] [CrossRef]
- Balca-Silva, J.; Matias, D.; Carmo, A.D.; Sarmento-Ribeiro, A.B.; Lopes, M.C.; Moura-Neto, V. Cellular and molecular mechanisms of glioblastoma malignancy: Implications in resistance and therapeutic strategies. Semin. Cancer Biol. 2019, 58, 130–141. [Google Scholar] [CrossRef]
- Bodai, B.I.; Nakata, T.E.; Wong, W.T.; Clark, D.R.; Lawenda, S.; Tsou, C.; Liu, R.; Shiue, L.; Cooper, N.; Rehbein, M.; et al. Lifestyle Medicine: A Brief Review of Its Dramatic Impact on Health and Survival. Perm. J. 2018, 22, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Alternat. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Anticancer Alkaloids from Trees: Development into Drugs. Pharmacogn. Rev. 2016, 10, 90–99. [Google Scholar] [CrossRef] [Green Version]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents–A review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Chapter 1. Structures, Nomenclature and General Chemistry of Carotenoids and Their Esters. In Carotenoid Esters in Foods; Royal Society of Chemistry: London, UK, 2019; pp. 1–50. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Busselberg, D.; et al. Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.; et al. Carotenoids in Cancer Metastasis-Status Quo and Outlook. Biomolecules 2020, 10, 1653. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype-concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Mersakova, S.; Strnadel, J.; Kajo, K.; Pec, M.; Zhai, K.; Smejkal, K.; Mirzaei, S.; et al. Flavonoids Targeting HIF-1: Implications on Cancer Metabolism. Cancers 2021, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Sameri, S.; Liskova, A.; Zhai, K.; Varghese, E.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Shakibaei, M. Resveratrol’s Anti-Cancer Effects through the Modulation of Tumor Glucose Metabolism. Cancers 2021, 13, 188. [Google Scholar] [CrossRef]
- Riveiro, M.E.; de Kimpe, N.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Brockmuller, A.; Kubatka, P.; Shakibaei, M.; Busselberg, D. Curcumin’s Beneficial Effects on Neuroblastoma: Mechanisms, Challenges, and Potential Solutions. Biomolecules 2020, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [CrossRef]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Kuttan, G.; Pratheeshkumar, P.; Manu, K.A.; Kuttan, R. Inhibition of tumor progression by naturally occurring terpenoids. Pharm. Biol. 2011, 49, 995–1007. [Google Scholar] [CrossRef]
- Lee, K.-H.; Xiao, Z. Lignans in treatment of cancer and other diseases. Phytochem. Rev. 2003, 2, 341–362. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Toledo, E.; Delgado-Rodriguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef]
- Maiti, P.; Plemmons, A.; Dunbar, G.L. Combination treatment of berberine and solid lipid curcumin particles increased cell death and inhibited PI3K/Akt/mTOR pathway of human cultured glioblastoma cells more effectively than did individual treatments. PLoS ONE 2019, 14, e0225660. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, B.I.; Jeon, J.H.; Kim, D.K.; Kang, S.G.; Shim, J.K.; Kim, S.Y.; Kang, S.W.; Jang, H. Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres. Biomolecules 2019, 9, 595. [Google Scholar] [CrossRef] [Green Version]
- Oppermann, H.; Faust, H.; Yamanishi, U.; Meixensberger, J.; Gaunitz, F. Carnosine inhibits glioblastoma growth independent from PI3K/Akt/mTOR signaling. PLoS ONE 2019, 14, e0218972. [Google Scholar] [CrossRef] [Green Version]
- Hua, D.; Zhao, Q.; Yu, Y.; Yu, H.; Yu, L.; Zhou, X.; Wang, Q.; Sun, C.; Shi, C.; Luo, W.; et al. Eucalyptal A inhibits glioma by rectifying oncogenic splicing of MYO1B mRNA via suppressing SRSF1 expression. Eur. J. Pharmacol. 2020, 173669. [Google Scholar] [CrossRef]
- Chang, K.F.; Huang, X.F.; Chang, J.T.; Huang, Y.C.; Lo, W.S.; Hsiao, C.Y.; Tsai, N.M. Cedrol, a Sesquiterpene Alcohol, Enhances the Anticancer Efficacy of Temozolomide in Attenuating Drug Resistance via Regulation of the DNA Damage Response and MGMT Expression. J. Nat. Prod. 2020, 83, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Angelucci, A.; Llorens, S.; Mattei, V.; Gravina, G.L.; Alonso, G.L.; Festuccia, C. Crocetin Extracted from Saffron Shows Antitumor Effects in Models of Human Glioblastoma. Int. J. Mol. Sci. 2020, 21, 423. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, S.; Nakamura, S.; Maoka, T.; Yamada, T.; Imai, T.; Ohba, T.; Yako, T.; Hayashi, M.; Endo, K.; Saio, M.; et al. Antitumour Effects of Astaxanthin and Adonixanthin on Glioblastoma. Mar. Drugs 2020, 18, 474. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Silva, V.A.O.; Oliveira, R.J.S.; de Rezende, A.R.; Chagas, R.C.R.; Pimenta, L.P.S.; Romao, W.; Santos, H.B.; Thome, R.G.; Reis, R.M.; et al. Matteucinol, isolated from Miconia chamissois, induces apoptosis in human glioblastoma lines via the intrinsic pathway and inhibits angiogenesis and tumor growth in vivo. Investig. New Drugs 2020, 38, 1044–1055. [Google Scholar] [CrossRef]
- Aroui, S.; Fetoui, H.; Kenani, A. Natural dietary compound naringin inhibits glioblastoma cancer neoangiogenesis. BMC Pharmacol. Toxicol. 2020, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Peng, G.; Xiao, G.; Yang, Z.; Huang, J.; Liu, Q.; Yang, Z.; Liu, D. Xanthohumol suppresses glioblastoma via modulation of Hexokinase 2 -mediated glycolysis. J. Cancer 2020, 11, 4047–4058. [Google Scholar] [CrossRef]
- Bona, N.P.; Pedra, N.S.; Azambuja, J.H.; Soares, M.S.P.; Spohr, L.; Gelsleichter, N.E.; de, M.M.B.; Sekine, F.G.; Mendonca, L.T.; de Oliveira, F.H.; et al. Tannic acid elicits selective antitumoral activity in vitro and inhibits cancer cell growth in a preclinical model of glioblastoma multiforme. Metab. Brain Dis. 2020, 35, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ren, L.; Liu, J.; Li, W.; Zheng, X.; Wang, J.; Du, G. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis. Cell Prolif. 2020, 53, e12706. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhao, E.; Zhu, Q.; Ji, J.; Wei, Z.; Xu, B.; Cui, H. Tubeimoside-1 Inhibits Glioblastoma Growth, Migration, and Invasion via Inducing Ubiquitylation of MET. Cells 2019, 8, 774. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Lamb, H.K.; Brady, C.; Lefkove, B.; Bonner, M.Y.; Thompson, P.; Lovat, P.E.; Arbiser, J.L.; Hawkins, A.R.; Redfern, C.P. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br. J. Cancer 2013, 109, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Naumowicz, M.; Kusaczuk, M.; Zajac, M.; Gal, M.; Kotynska, J. Monitoring of the Surface Charge Density Changes of Human Glioblastoma Cell Membranes upon Cinnamic and Ferulic Acids Treatment. Int. J. Mol. Sci. 2020, 21, 6972. [Google Scholar] [CrossRef]
- Izumi, C.; Laure, H.J.; Barbosa, N.G.; Thome, C.H.; Ferreira, G.A.; Sousa, J.P.B.; Lopes, N.P.; Rosa, J.C. Sequesterpene Lactones Isolated from a Brazilian Cerrado Plant (Eremanthus spp.) as Anti-Proliferative Compounds, Characterized by Functional and Proteomic Analysis, are Candidates for New Therapeutics in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 4713. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.G.; Quan, L.Q.; Cui, X.Y.; Li, H.M.; Zhao, X.D.; Li, R.T. A natural compound obtained from Valeriana jatamansi selectively inhibits glioma stem cells. Oncol. Lett. 2020, 19, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.M.; Faria, B.M.; Ascari, L.M.; Souza, J.M.; Soares, A.G.; Cordeiro, Y.; Romao, L.F. Diosmin induces caspase-dependent apoptosis in human glioblastoma cells. An. Acad. Bras. Cienc. 2019, 91, e20191031. [Google Scholar] [CrossRef] [PubMed]
- Franco, Y.E.M.; Okubo, M.Y.; Torre, A.D.; Paiva, P.P.; Rosa, M.N.; Silva, V.A.O.; Reis, R.M.; Ruiz, A.L.T.G.; Imamura, P.M.; de Carvalho, J.E.; et al. Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line. Molecules 2019, 24, 4498. [Google Scholar] [CrossRef] [Green Version]
- Bache, M.; Hein, A.; Petrenko, M.; Guttler, A.; Kessler, J.; Wichmann, H.; Kappler, M.; Emmerich, D.; Paschke, R.; Vordermark, D. Evaluation of the Betulinic Acid-Cisplatin conjugate APC and its precursor DE9B for the treatment of human malignant glioma. Chem. Biol. Interact. 2019, 314, 108841. [Google Scholar] [CrossRef] [PubMed]
- Bonturi, C.R.; Silva, M.C.C.; Motaln, H.; Salu, B.R.; Ferreira, R.D.S.; Batista, F.P.; Correia, M.; Paiva, P.M.G.; Turnsek, T.L.; Oliva, M.L.V. A Bifunctional Molecule with Lectin and Protease Inhibitor Activities Isolated from Crataeva tapia Bark Significantly Affects Cocultures of Mesenchymal Stem Cells and Glioblastoma Cells. Molecules 2019, 24, 2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, L.T.; Hsu, L.S.; Chung, Y.H.; Chen, C.J. Tectorigenin Inhibits Glioblastoma Proliferation by G0/G1 Cell Cycle Arrest. Medicina 2020, 56, 681. [Google Scholar] [CrossRef]
- Onay Ucar, E.; Sengelen, A. Resveratrol and siRNA in combination reduces Hsp27 expression and induces caspase-3 activity in human glioblastoma cells. Cell Stress Chaperones 2019, 24, 763–775. [Google Scholar] [CrossRef]
- Park, K.S.; Yoon, S.Y.; Park, S.H.; Hwang, J.H. Anti-Migration and Anti-Invasion Effects of Curcumin via Suppression of Fascin Expression in Glioblastoma Cells. Brain Tumor Res. Treat. 2019, 7, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, Z.; Zeng, S.; Liu, S.; Zhu, C.; Xu, R.; Liu, R.E. Paeoniflorin Inhibits Hepatocyte Growth Factor- (HGF-) Induced Migration and Invasion and Actin Rearrangement via Suppression of c-Met-Mediated RhoA/ROCK Signaling in Glioblastoma. Biomed. Res. Int. 2019, 2019, 9053295. [Google Scholar] [CrossRef] [Green Version]
- Khathayer, F.; Ray, S.K. Diosgenin as a Novel Alternative Therapy for Inhibition of Growth, Invasion, and Angiogenesis Abilities of Different Glioblastoma Cell Lines. Neurochem. Res. 2020, 45, 2336–2351. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.; Naliwajko, S.K.; Moskwa, J.; Bielecka, J.; Grabia, M.; Borawska, M.; Socha, K. Mushrooms as potential therapeutic agents in the treatment of cancer: Evaluation of anti-glioma effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus extracts. Biomed. Pharmacother. 2021, 133. [Google Scholar] [CrossRef]
- Czarnik-Kwasniak, J.; Kwasniak, K.; Kwasek, P.; Swierzowska, E.; Strojewska, A.; Tabarkiewicz, J. The Influence of Lycopene, [6]-Gingerol, and Silymarin on the Apoptosis on U-118MG Glioblastoma Cells In Vitro Model. Nutrients 2019, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Moskwa, J.; Naliwajko, S.K.; Markiewicz-Zukowska, R.; Gromkowska-Kepka, K.J.; Nowakowski, P.; Strawa, J.W.; Borawska, M.H.; Tomczyk, M.; Socha, K. Chemical composition of Polish propolis and its antiproliferative effect in combination with Bacopa monnieri on glioblastoma cell lines. Sci. Rep. 2020, 10, 21127. [Google Scholar] [CrossRef] [PubMed]
- Palma, T.V.; Lenz, L.S.; Bottari, N.B.; Pereira, A.; Schetinger, M.R.C.; Morsch, V.M.; Ulrich, H.; Pillat, M.M.; de Andrade, C.M. Berberine induces apoptosis in glioblastoma multiforme U87MG cells via oxidative stress and independent of AMPK activity. Mol. Biol. Rep. 2020, 47, 4393–4400. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, D.; Chen, Q.; Lin, S.; Shi, S.; Chen, C. Polysaccharide peptide isolated from grass-cultured Ganoderma lucidum induces anti-proliferative and pro-apoptotic effects in the human U251 glioma cell line. Oncol. Lett. 2018, 15, 4330–4336. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, W.A.S.; Burbano, R.R.; Pessoa, C.D.O.; Harada, M.L.; Borges, B.D.N.; de Oliveira, E.H.C. Pisosterol Induces G2/M Cell Cycle Arrest and Apoptosis via the ATM/ATR Signaling Pathway in Human Glioma Cells. Anticancer Agents Med. Chem. 2020, 20, 734–750. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Hong, W.; Fei, X.; Liu, R. Arctigenin Inhibits Glioblastoma Proliferation through the AKT/mTOR Pathway and Induces Autophagy. Biomed. Res. Int 2020, 2020, 3542613. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Liskova, A.; Kubatka, P.; Busselberg, D. Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells. Int. J. Mol. Sci. 2020, 21, 4177. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.B.; Coelho, P.L.C.; Oliveira, M.D.N.; Oliveira, J.L.; Amparo, J.A.O.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; Souza, C.D.S.; de Faria Lopes, G.P.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zajac, A.; Langner, E.; Skalicka-Wozniak, K.; Maciejczyk, A.; Rzeski, W.; Jakubowicz-Gil, J. Antiglioma Potential of Coumarins Combined with Sorafenib. Molecules 2020, 25, 5192. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Lotfi, M.; Soukhtanloo, M.; Ghayour Mobarhan, M.; Jaliani, H.Z.; Sadeghnia, H.R.; Ghorbani, A. Effects of Galbanic Acid on Proliferation, Migration, and Apoptosis of Glioblastoma Cells Through the PI3K/Akt/MTOR Signaling Pathway. Curr. Mol. Pharmacol. 2021, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, Y.; Li, H.; Ji, Y.; Fang, F.; Tang, H.; Qiu, P. A steroidal saponin form Paris vietnamensis (Takht.) reverses temozolomide resistance in glioblastoma cells via inducing apoptosis through ROS/PI3K/Akt pathway. Biosci. Trends 2020, 14, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Lim, J.H.; Hong, Y.K.; Yang, S.H. High-Dose Metformin Plus Temozolomide Shows Increased Anti-tumor Effects in Glioblastoma In Vitro and In Vivo Compared with Monotherapy. Cancer Res. Treat. 2018, 50, 1331–1342. [Google Scholar] [CrossRef]
- Lo Dico, A.; Valtorta, S.; Ottobrini, L.; Moresco, R.M. Role of Metformin and AKT Axis Modulation in the Reversion of Hypoxia Induced TMZ-Resistance in Glioma Cells. Front. Oncol. 2019, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Liu, Y.; Yang, Q.; Zhou, X.; Li, H.; Liu, Y.; Li, J.; Lu, Y.; Tang, H. Paris saponin H inhibits the proliferation of glioma cells through the A1 and A3 adenosine receptormediated pathway. Int. J. Mol. Med. 2021, 47. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Xu, X.B.; Qiu, S.; Luo, S.; Qiu, E.; Rong, Z.; Zhang, J.; Zheng, D. Galangin inhibits epithelial-mesenchymal transition and angiogenesis by downregulating CD44 in glioma. J. Cancer 2019, 10, 4499–4508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.C.; Tsao, M.J.; Chiu, C.Y.; Kan, P.C.; Chen, Y. Magnolol Inhibits Human Glioblastoma Cell Migration by Regulating N-Cadherin. J. Neuropathol. Exp. Neurol. 2018, 77, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Shimada, R.; Xu, K.; Han, L.; Si, N.; Zhao, H.; Bian, B.; Hayashi, H.; Okazaki, M.; Takagi, N. Multiple cytotoxic effects of gamabufotalin against human glioblastoma cell line U-87. Chem. Biol. Interact. 2019, 314, 108849. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkaria, J.N.; Kitange, G.J.; James, C.D.; Plummer, R.; Calvert, H.; Weller, M.; Wick, W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin. Cancer Res. 2008, 14, 2900–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.L.; Chen, C.M.; Chang, Y.Z.; Liu, G.Y.; Hung, H.C.; Hsieh, T.Y.; Lin, C.L. Pine (Pinus morrisonicola Hayata) needle extracts sensitize GBM8901 human glioblastoma cells to temozolomide by downregulating autophagy and O(6)-methylguanine-DNA methyltransferase expression. J. Agric. Food Chem. 2014, 62, 10458–10467. [Google Scholar] [CrossRef]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- You, L.; Feng, S.; An, R.; Wang, X. Osthole: A Promising Lead Compound for Drug Discovery from a Traditional Chinese Medicine (TCM). Nat. Prod. Commun. 2009, 4. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Kim, I.S.; Kang, K.S.; Yoo, H.H. HPLC Determination of Esculin and Esculetin in Rat Plasma for Pharmacokinetic Studies. J. Chromatogr. Sci. 2015, 53, 1322–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Xing, D.; Su, H.; Ma, C.; Ding, Y.; Du, L. Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci. 2005, 77, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Xu, W.; Song, W.; Ye, M.; Yang, X.W. Transport of Twelve Coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR Cell Monolayer-An in Vitro Model for Blood-Brain Barrier Permeability. Molecules 2015, 20, 11719–11732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can Xanthophyll-Membrane Interactions Explain Their Selective Presence in the Retina and Brain? Foods 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Ren, Y.S.; Lv, Y.Y.; Zhang, J.S.; Xu, X.L.; Wang, X.Z.; Yao, J.C.; Zhang, G.M.; Liu, Z. Elucidation of Arctigenin Pharmacokinetics and Tissue Distribution after Intravenous, Oral, Hypodermic and Sublingual Administration in Rats and Beagle Dogs: Integration of In Vitro and In Vivo Findings. Front. Pharmacol. 2017, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Nomoto, K.; Tohda, C. Diosgenin content is a novel criterion to assess memory enhancement effect of yam extracts. J. Nat. Med. 2021, 75, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.B.; Zhao, Z.X.; Peng, R.; Pan, L.B.; Fu, J.; Ma, S.R.; Han, P.; Cong, L.; Zhang, Z.W.; Sun, L.X.; et al. Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcumin-loaded zein nanoparticles for transport across the blood-brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Ghosh, S.; Dungdung, S.R.; Chowdhury, S.T.; Mandal, A.K.; Sarkar, S.; Ghosh, D.; Das, N. Encapsulation of the flavonoid quercetin with an arsenic chelator into nanocapsules enables the simultaneous delivery of hydrophobic and hydrophilic drugs with a synergistic effect against chronic arsenic accumulation and oxidative stress. Free Radic. Biol. Med. 2011, 51, 1893–1902. [Google Scholar] [CrossRef]
- Kong, L.; Li, X.T.; Ni, Y.N.; Xiao, H.H.; Yao, Y.J.; Wang, Y.Y.; Ju, R.J.; Li, H.Y.; Liu, J.J.; Fu, M.; et al. Transferrin-Modified Osthole PEGylated Liposomes Travel the Blood-Brain Barrier and Mitigate Alzheimer’s Disease-Related Pathology in APP/PS-1 Mice. Int. J. Nanomed. 2020, 15, 2841–2858. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, Z.; Mohammadinejad, R.; Ashrafizadeh, M. Drug delivery systems for resveratrol, a non-flavonoid polyphenol: Emerging evidence in last decades. J. Drug Deliv. Sci. Technol. 2019, 51, 591–604. [Google Scholar] [CrossRef]
- Singh, A.; Kim, W.; Kim, Y.; Jeong, K.; Kang, C.S.; Kim, Y.; Koh, J.; Mahajan, S.D.; Prasad, P.N.; Kim, S. Multifunctional Photonics Nanoparticles for Crossing the Blood-Brain Barrier and Effecting Optically Trackable Brain Theranostics. Adv. Funct. Mater. 2016, 26, 7057–7066. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active Targeting of Orthotopic Glioma Using Biomimetic Proteolipid Nanoparticles. ACS Nano 2019, 13, 386–398. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; La Vecchia, C.; Negri, E.; Chatenoud, L.; Bosetti, C.; Jia, X.; Liu, R.; Huang, G.; Bi, D.; Wang, C. Diet and brain cancer in adults: A case-control study in Northeast China. Int. J. Cancer 1999, 81, 20–23. [Google Scholar] [CrossRef]
- Hu, J.; Johnson, K.C.; Mao, Y.; Guo, L.; Zhao, X.; Jia, X.; Bi, D.; Huang, G.; Liu, R. Risk factors for glioma in adults: A case-control study in northeast China. Cancer Detect. Prev. 1998, 22, 100–108. [Google Scholar] [CrossRef]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Artzi, M.; Liberman, G.; Vaisman, N.; Bokstein, F.; Vitinshtein, F.; Aizenstein, O.; Ben Bashat, D. Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: (1)H-MRS study. J. Neurooncol. 2017, 132, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef] [Green Version]
- D’Addato, S.; Scandiani, L.; Mombelli, G.; Focanti, F.; Pelacchi, F.; Salvatori, E.; di Loreto, G.; Comandini, A.; Maffioli, P.; Derosa, G. Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q10 on lipid levels: A randomized, double-blind, placebo controlled study. Drug Des. Dev. Ther. 2017, 11, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Zanotta, D.; Puricelli, S.; Bonoldi, G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: A noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 2014, 10, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Hitoe, S.; Shimoda, H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct. Foods Health Dis. 2017, 7. [Google Scholar] [CrossRef]
- Ilic, D.; Forbes, K.M.; Hassed, C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst. Rev. 2011, CD008007. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Tabira, T.; Kawamura, N. A Study of a Supplement Containing Huperzine A and Curcumin in Dementia Patients and Individuals with Mild Cognitive Impairment. J. Alzheimers Dis. 2018, 63, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Duranti, G.; Ceci, R.; Patrizio, F.; Sgro, P.; di Luigi, L.; Sabatini, S.; Felici, F.; Bazzucchi, I. Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr. Res. 2018, 50, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimaraes, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Kumar, R.M.; van Gompel, J.J.; Bower, R.; Rabinstein, A.A. Spontaneous intraventricular hemorrhage associated with prolonged diosmin therapy. Neurocrit. Care 2011, 14, 438–440. [Google Scholar] [CrossRef]
- Nouri, K.; Walch, K.; Weghofer, A.; Imhof, M.; Egarter, C.; Ott, J. The Impact of a Standardized Oral Multinutrient Supplementation on Embryo Quality in in vitro Fertilization/Intracytoplasmic Sperm Injection: A Prospective Randomized Trial. Gynecol. Obstet. Investig. 2017, 82, 8–14. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Hosseinzadeh, H. A comprehensive review on biological activities and toxicology of crocetin. Food Chem. Toxicol. 2019, 130, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Choi, J.S. Effects of quercetin on the pharmacokinetics of Etoposide after oral or intravenous administration of etoposide in rats. Anticancer Res. 2009, 29, 1411–1415. [Google Scholar]
- Samuel, T.; Fadlalla, K.; Mosley, L.; Katkoori, V.; Turner, T.; Manne, U. Dual-mode Interaction Between Quercetin and DNA-damaging Drugs in Cancer Cells. Anticancer Res. 2012, 32, 61–71. [Google Scholar] [PubMed]
- Pal, D.; Mitra, A.K. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006, 78, 2131–2145. [Google Scholar] [CrossRef]
- Mitsunaga, Y.; Takanaga, H.; Matsuo, H.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. Effect of bioflavonoids on vincristine transport across blood–brain barrier. Eur. J. Pharmacol. 2000, 395, 193–201. [Google Scholar] [CrossRef]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-kappaB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef]
- Tripathi, D.N.; Jena, G.B. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chem. Biol. Interact. 2009, 180, 398–406. [Google Scholar] [CrossRef] [PubMed]




| Substance | Class/Type | Primary Source(s) |
|---|---|---|
| Alkaloids | ||
| Berberine | Quaternary Ammonium Salt | Barberry (Berberis) |
| Carboxylic Acid Derivatives | ||
| Cinnamic Acid | Monocarboxylic Acid | Cinnamon (Cinnamomum) |
| Ferulic Acid | Hydroxycinnamic Acid | Giant fennel (Ferula communis) |
| Carotenoids | ||
| Adonixanthin | Carotenone | Derivative of astaxanthin |
| Astaxanthin | Xanthophyll | Chlorophyte (Haematococcus pluvialis) |
| Crocetin | Apocarotenoid | Saffron (Crocus sativus) |
| Coumarins | ||
| Galbanic Acid | Sesquiterpene Coumarin | Celery/carrot/parsley family (Umbelliferae) |
| Osthole | Coumarin | Monnier’s snowparsley (Cnidium monnieri) |
| Curcuminoids | ||
| Curcumin | Curcumin | Turmeric (Curcuma longa) |
| Flavonoids | ||
| Chrysin | Dihydroxyflavone | Blue passion flower (Passiflora caerulea) |
| Diosmin | Flavone Glycoside | Germander (Teucrium gnaphalodes) |
| EGCG | Catechin | Green tea (Camellia sinensis) |
| Galangin | Trihydroxyflavone | Galangal (Alpinia officinarum) |
| Matteucinol | Dihydroxyflavonone | Naudin (Miconia chamissois) |
| Naringin | Flavanone Glycoside | Grapefruit (Citrus × paradisi) |
| Quercetin | Flavonol | Oak (Quercetus) |
| Resveratrol | Stilbenoid | Grape (Vitis) |
| Rutin | Flavonol Glycoside | Rue (Ruta graveolens) |
| Silymarin (Silibinin) | Flavonolignan | Milk thistle (Silybum marianum) |
| Tectorigenin | Methylated Isoflavone | Leopard lily (Iris domestica) |
| Xanthohumol | Prenylated Chalconoid | Hops (Humulus lupulus) |
| Lignans | ||
| Arctigenin | Lignan/Polyphenol | Greater burdock (Arctium lappa) |
| Magnolol | Biphenyl | Houpu magnolia (Magnolia officinalis) |
| Steroids | ||
| Diosgenin | Phytosteroid Sapogenin | Fenugreek (Trigonella foenum-graecum) |
| Gamabufotalin | Steroidal Lactone | Toad (Bufo) |
| N45 | Steroidal Saponin | Nan chong lou (Paris vietnamensis) |
| Withaferin A | Steroidal Lactone | Ashwa-gandha (Withania somnifera) |
| Tannins | ||
| Tannic Acid | Hydrolysable Tannin | Oak (Quercetus) |
| Terpenes | ||
| AM01-06 | Sesquiterpene Lactone | Sunflower (Eremanthus spp.) |
| Betulinic Acid | Triterpenoid | White birch (Betula pubescens) |
| Cedrol | Sesquiterpene Alcohol | Cypress (Cupressus); Juniper (Juniperus) |
| Coronarin D | Diterpene | White ginger lily (Hedychium coronarium) |
| Eucalyptal A | Monoterpenoid | Southern blue gum (Eucalyptus globulus) |
| Gossypol | Terpenoid Aldehyde | Cotton (Gossypium) |
| Paeoniflorin | Terpene Glycoside | Chinese peony (Paeonia lactiflora) |
| Paris saponin H | Triterpenoid Saponin | Chong Lou (Rhizoma paridis) |
| Pisosterol | Triterpene | Dead man’s foot (Pisolithus tinctorius) |
| Rupesin E | Iridoid (Monoterpenoid) | Indian valerian (Valeriana jatamansi) |
| Tubeimoside-1 | Triterpenoid Saponin | Tu bei mu (Rhizoma bolbostemmae) |
| Crude/Purified Plant Extracts | ||
| BcH, BcS | Extract-Food Supplement | Water hyssop (Bacopa monnieri) |
| CE70, CE95 | Ethanol Extract | Shaggy ink cap (Coprinus comatus) |
| CP | Chloroform Partition | Johnnyberry (Miconia chamissois) |
| CW | Aqueous Extract | Shaggy ink cap (Coprinus comatus) |
| KE70, KE95 | Ethanol Extract | Golden chanterelle (Cantherellus cibarius) |
| KW | Aqueous Extract | Golden chanterelle (Cantherellus cibarius) |
| PE70, PE95 | Ethanol Extract | Puffball (Lycoperdon perlatum) |
| PPE | Ethanol Extract | Polish propolis (bee glue) |
| PW | Aqueous Extract | Puffball (Lycoperdon perlatum) |
| RE70, RE95 | Ethanol Extract | Saffron milk cap (Lactarius delicious) |
| RW | Aqueous Extract | Saffron milk cap (Lactarius delicious) |
| Other | ||
| Carnosine | Dipeptide | Liebig’s meat extract |
| CrataBL | Protein: Lectin + Serine Protease Inhibitor | Beach block (Crataeva tapia) |
| GL-PP | Polysaccharide Peptide | Lingzhi (Ganoderma lucidum) |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Increases survival | Eucalyptal A | U87MG orthotopic implants, nude mice | [32] |
| Cedrol | DBTRG-05MG subcutaneous xenografts, nude mice | [33] | |
| Crocetin | Luc-U251MG orthotopic implants, CD1 mice | [34] | |
| Decreases tumor area/perimeter | Astaxanthin | GL261 orthotopic implants, C57BL/6J mice | [35] |
| Adonixanthin | GL261 orthotopic implants, C57BL/6J mice | [35] | |
| McC1 | U251 heterotopic xenograft, fertilized chicken eggs | [36] | |
| Decreases tumor volume | Astaxanthin | GL261 orthotopic implants, C57BL/6J mice | [35] |
| Adonixanthin | GL261 orthotopic implants, C57BL/6J mice | [35] | |
| Naringin | U87 subcutaneous xenograft, athymic mice | [37] | |
| Xanthohumol | U87, LN229 | [38] | |
| Tannic Acid | C6 orthotopic implants, Wistar rats | [39] | |
| Withaferin A | U87 subcutaneous xenografts, nude mice | [40] | |
| TBMS1 | U87 subcutaneous xenografts, NOD/SCID mice | [41] | |
| Decreases tumor weight | Xanthohumol | U87, LN229 | [38] |
| TBMS1 | U87 subcutaneous xenografts, nude mice | [41] | |
| Increases cell death/dec. viability | EGCG | U251, MO59J | [42] |
| Cinnamic Acid | LN-229 | [43] | |
| Ferulic Acid | LN-229 | [43] | |
| Astaxanthin | GL261, U251MG | [35] | |
| Adonixanthin | GL261, U251MG | [35] | |
| Cedrol | DBTRG-05MG, RG2 | [33] | |
| AM02 | U87MG, T98G | [44] | |
| AM04 | U87MG, T98G | [44] | |
| AM05 | U87MG, T98G | [44] | |
| AM06 | U87MG, T98G | [44] | |
| Naringin | U87 | [37] | |
| Xanthohumol | U87, T98G, LN229 | [38] | |
| Rupesin E | GSC-3#, GSC-12#, GSC-18# | [45] | |
| Diosmin | U87, GBM02, GBM95 | [46] | |
| Coronarin D | U251 | [47] | |
| CP | GAMG, U251 | [36] | |
| McC1 | GAMG, U251 | [36] | |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Tannic Acid | C6 | [39] | |
| Withaferin A | U87, U251 | [40] | |
| Betulinic Acid | U251, LN229 | [48] | |
| TBMS1 | U87, LN229 | [41] | |
| Carnosine | U87, T98G | [31] | |
| CrataBL | U87 | [49] | |
| Tectorigenin | GBM-8401, GBM-8901 | [50] | |
| Resveratrol | U87 | [51] | |
| Quercetin | U87 | [51] | |
| Curcumin | U87 | [52] | |
| Paeoniflorin | U251, T98G | [53] | |
| Diosgenin | C6, T98G | [54] | |
| CW | LN-18 | [55] | |
| CE70 | U87, LN-18 | [55] | |
| CE95 | U87, LN-18 | [55] | |
| KW | U87, LN-18 | [55] | |
| KE70 | U87, LN-18 | [55] | |
| KE95 | U87, LN-18 | [55] | |
| RW | U87, LN-18 | [55] | |
| RE70 | U87, LN-18 | [55] | |
| RE95 | U87, LN-18 | [55] | |
| PW | U87, LN-18 | [55] | |
| PE70 | U87, LN-18 | [55] | |
| PE95 | U87, LN-18 | [55] | |
| Silymarin | U118 | [56] | |
| BcS | U87, T98G, LN-18 | [57] | |
| BcH | U87, T98G, LN-19 | [57] | |
| BBR | U87 | [58] | |
| GL-PP | U251 | [59] | |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] | |
| Decreases colony formation | Xanthohumol | U87, T98G, LN229 | [38] |
| Rupesin E | GSC-3#, GSC-18# | [45] | |
| CP | GAMG, U251 | [36] | |
| McC1 | U251, GAMG | [36] | |
| Tannic Acid | C6 | [39] | |
| Decreases cloning | Arctigenin | U87MG, T98G | [61] |
| AM01 | U87MG, T98G | [44] | |
| AM02 | U87MG, T98G | [44] | |
| AM03 | U87MG, T98G | [44] | |
| AM04 | U87MG, T98G | [44] | |
| AM05 | U87MG, T98G | [44] | |
| AM06 | U87MG, T98G | [44] | |
| TBMS1 | U87, LN229 | [41] | |
| Decreases sphere formation | Gossypol | TS13-20, TS13-18 | [30] |
| Decreases intracellular ATP | SLCP | U87, U251 | [29] |
| BBR | U87, U251 | [29] | |
| Gossypol | Diff13-20 | [30] | |
| Carnosine | U87, T98G | [31] | |
| Upregulates p53 (mRNA) | Pisosterol | U87, U343, AHOL1, 1231N1 | [60] |
| Upregulates p53 (protein) | BBR | U87, U251 | [29] |
| SLCP | U251 | [29] | |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Decreases proliferation/growth | Rutin | C6 | [63] |
| Quercetin | C6 | [63] | |
| Eucalyptal A | U87MG, LN229 | [32] | |
| Rupesin E | GSC-3#, GSC-18# | [45] | |
| Crocetin | U87, U138, U251, U373 | [34] | |
| Coronarin D | U251 | [47] | |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Tannic Acid | C6 | [39] | |
| Gossypol | Diff13-20, Diff13-18 | [30] | |
| Betulinic Acid | U251, LN229 | [48] | |
| CrataBL | U87 | [49] | |
| Galbanic Acid | U87 | [65] | |
| N45 | U87 | [66] | |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] | |
| Decreases DNA synthesis | CE95 | U87 | [55] |
| CE70 | U87, LN-18 | [55] | |
| KW | U87, LN-18 | [55] | |
| KE95 | U87, LN-18 | [55] | |
| KE70 | U87, LN-18 | [55] | |
| PW | U87 | [55] | |
| PE70 | U87 | [55] | |
| RW | U87, LN-18 | [55] | |
| PPE | U87, T98G, LN-18 | [57] | |
| BcH | U87, T98G, LN-18 | [57] | |
| Downregulates SRSF1 (mRNA) | Eucalyptal A | U87MG, LN229 | [32] |
| Downregulates SRSF1 (protein) | Eucalyptal A | U87MG, LN229 | [32] |
| Downregulates MYO1B-fl (protein) | Eucalyptal A | U87MG, LN229 | [32] |
| Downregulates p-PDK1 (protein) | Eucalyptal A | U87MG, LN229 | [32] |
| Downregulates TGF (mRNA) | Rutin | U251 orthotopic implants, WR | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Downregulates TGF-β (mRNA) | Rutin | C6 | [63] |
| Quercetin | C6 | [63] | |
| Downregulates IGF (mRNA) | Rutin | C6, WR-U251 orthotopic implants | [63] |
| Quercetin | C6, WR-U251 orthotopic implants | [63] | |
| Downregulates CCL2 (mRNA) | Rutin | U251 orthotopic implants, WR | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Upregulates CCL5 (mRNA) | Rutin | C6, WR-U251 orthotopic implants | [63] |
| Quercetin | C6, WR-U251 orthotopic implants | [63] | |
| Downregulates HDGF (mRNA) | Rutin | C6, WR-U251 orthotopic implants | [63] |
| Quercetin | C6, WR-U251 orthotopic implants | [63] | |
| Downregulates GDNF (mRNA) | Rutin | C6, WR-U251 orthotopic implants | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Downregulates PI3K (protein) | SLCP | U87 | [29] |
| BBR | U87 | [29] | |
| Diosgenin | C6 | [54] | |
| Downregulates (p-)PI3K (protein) | Osthole | MOGGCCM, T98 | [64] |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Upregulates AMPK (protein) | Metformin | U87 | [67] |
| Downregulates Akt (mRNA) | Arctigenin | U87MG | [61] |
| Downregulates Akt (protein) | Cedrol | RG2 | [33] |
| Metformin | U87, U251 | [68] | |
| SLCP | U87 | [29] | |
| BBR | U87 | [29] | |
| Downregulates p-Akt (mRNA) | Arctigenin | U87MG, T98G | [61] |
| Downregulates p-Akt (protein) | Eucalyptal A | U87MG, LN229 | [32] |
| Astaxanthin | GL261 | [35] | |
| Adonixanthin | GL261 | [35] | |
| Cedrol | DBTRG-05MG, RG2 | [33] | |
| Arctigenin | U87MG, T98G | [61] | |
| Xanthohumol | U87 | [38] | |
| CP | GAMG | [36] | |
| McC1 | GAMG, U251 | [36] | |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Diosgenin | C6 | [54] | |
| Downregulates mTOR (protein) | Metformin | U87 | [67] |
| SLCP | U87 | [29] | |
| BBR | U87, U251 | [29] | |
| Downregulates p-mTOR (mRNA) | Arctigenin | U87MG, T98G | [61] |
| Downregulates p-mTOR (protein) | Arctigenin | U87MG, T98G | [61] |
| SLCP | U87 | [29] | |
| BBR | U87, U251 | [29] | |
| Diosgenin | T98G | [54] | |
| Downregulates Raf (protein) | Osthole | MOGGCCM, T98 | [64] |
| Downregulates c-Myc | Eucalyptal A | U87MG, LN229 | [32] |
| Xanthohumol | U87, T98G, LN229 | [38] | |
| SLCP | U87 | [29] | |
| BBR | U87 | [29] | |
| Downregulates ROS | Astaxanthin | GL261 | [35] |
| Adonixanthin | GL261 | [35] | |
| Tannic Acid | C6 | [39] | |
| Upregulates CAT activity | Tannic Acid | C6 | [39] |
| BBR | U87 | [58] | |
| Upregulates SOD activity | Tannic Acid | C6 | [39] |
| BBR | U87 | [58] | |
| Downregulates JNK (protein) | Cedrol | DBTRG-05MG, RG2 | [33] |
| Downregulates p-JNK (protein) | Cedrol | RG2 | [33] |
| Downregulates p-MEK (protein) | TBMS1 | U87, LN229 | [41] |
| Downregulates p-ERK (protein) | Astaxanthin | GL261 | [35] |
| Adonixanthin | GL261 | [35] | |
| TBMS1 | LN229 | [41] | |
| Downregulates p38 (protein) | Diosgenin | T98G | [54] |
| Upregulates p-p38 MAPK (protein) | Astaxanthin | GL261 | [35] |
| Adonixanthin | GL261 | [35] | |
| Downregulates HIF-1α activity | Metformin | U251 | [68] |
| Downregulates NF-κB | Diosgenin | C6, T98G | [54] |
| Downregulates MET (protein) | TBMS1 | U87, LN229 | [41] |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Causes apoptosis | Arctigenin | U87MG, T98G | [61] |
| Osthole | MOGGCCM, T98 | [64] | |
| Xanthohumol | U87 | [38] | |
| Rupesin E | GSC-3#, GSC-18# | [45] | |
| Diosmin | GBM02, GBM95 | [46] | |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Gossypol | TS13-20, Diff13-20 | [30] | |
| Withaferin A | U87, U251 | [40] | |
| Tectorigenin | GBM-8401, GBM-8901 | [50] | |
| Diosgenin | C6, T98G | [54] | |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] | |
| Causes DNA fragmentation | SLCP | U87, U251 | [29] |
| BBR | U87, U251 | [29] | |
| Upregulates (c-)caspase 9 (protein) | Cedrol | RG2 | [33] |
| Coronarin D | U251 | [47] | |
| CP | GAMG | [36] | |
| McC1 | GAMG | [36] | |
| Withaferin A | U87, U251 | [40] | |
| Upregulates caspase 3 (mRNA) | Pisosterol | U87, U343, AHOL1, 1231N1 | [60] |
| Upregulates (c-)caspase 3 (protein) | EGCG | MO59J, U251 | [42] |
| Cedrol | DBTRG-05MG, RG2 | [33] | |
| Osthole | T98 | [64] | |
| Xanthohumol | U87, T98G, LN229 | [38] | |
| Rupesin E | GSC-3#, GSC-18# | [45] | |
| Crocetin | U87, U138, U251, U373 | [34] | |
| Diosmin | GBM02, GBM95 | [46] | |
| Coronarin D | U251 | [47] | |
| CP | GAMG | [36] | |
| McC1 | GAMG, U251 | [36] | |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Withaferin A | U87, U251 | [40] | |
| Betulinic Acid | U251, LN229 | [48] | |
| Resveratrol | U87 | [51] | |
| Quercetin | U87 | [51] | |
| GL-PP | U251 | [59] | |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] | |
| Upregulates (c-)PARP (protein) | Cedrol | RG2 | [33] |
| Xanthohumol | U87 | [38] | |
| Coronarin D | U251 | [47] | |
| CP | U251 | [36] | |
| McC1 | GAMG, U251 | [36] | |
| Gossypol | TS13-20, Diff13-20 | [30] | |
| Withaferin A | U87, U251 | [40] | |
| Downregulates PARP-1 (protein) | Diosgenin | C6, T98G | [54] |
| Downregulates ICAD (protein) | Diosgenin | C6, T98G | [54] |
| Upregulates Bax (protein) | SLCP | U87, U251 | [29] |
| BBR | U87, U251 | [29] | |
| Diosgenin | C6, T98G | [54] | |
| Downregulates Bcl-2 (mRNA) | Pisosterol | U87, U343, AHOL1, 1231N1 | [60] |
| Downregulates Bcl-2 (protein) | Diosgenin | C6, T98G | [54] |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] | |
| Upregulates Bad (protein) | Withaferin A | U87, U251 | [40] |
| Upregulates Bim (protein) | Withaferin A | U87, U251 | [40] |
| Depolarizes MMP | Coronarin D | U251 | [47] |
| CP | U251 | [36] | |
| McC1 | U251 | [36] | |
| Gossypol | TS13-20 | [30] | |
| Withaferin A | U87, U251 | [40] | |
| Upregulates ROS | Coronarin D | U251 | [47] |
| SLCP | U87, U251 | [29] | |
| BBR | U87, U251 | [29] | |
| Upregulates cytochrome c (protein) | SLCP | U87, U251 | [29] |
| BBR | U87, U251 | [29] | |
| Upregulates GRP78 (mRNA) | Withaferin A | U87, U251 | [40] |
| Upregulates GRP78 (protein) | EGCG | MO59J | [42] |
| Upregulates ATF4 (mRNA) | Withaferin A | U87, U251 | [40] |
| Upregulates ATF4 (protein) | Withaferin A | U87, U251 | [40] |
| EGCG | U251 | [42] | |
| Upregulates ATF6 (mRNA) | Withaferin A | U251 | [40] |
| Upregulates XBP1 (mRNA) | Withaferin A | U87, U251 | [40] |
| Upregulates XBP1 (protein) | Withaferin A | U87, U251 | [40] |
| Upregulates CHOP (mRNA) | Withaferin A | U87, U251 | [40] |
| Upregulates CHOP (protein) | Withaferin A | U87, U251 | [40] |
| Upregulates Bax (protein) | Cedrol | DBTRG-05MG | [33] |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
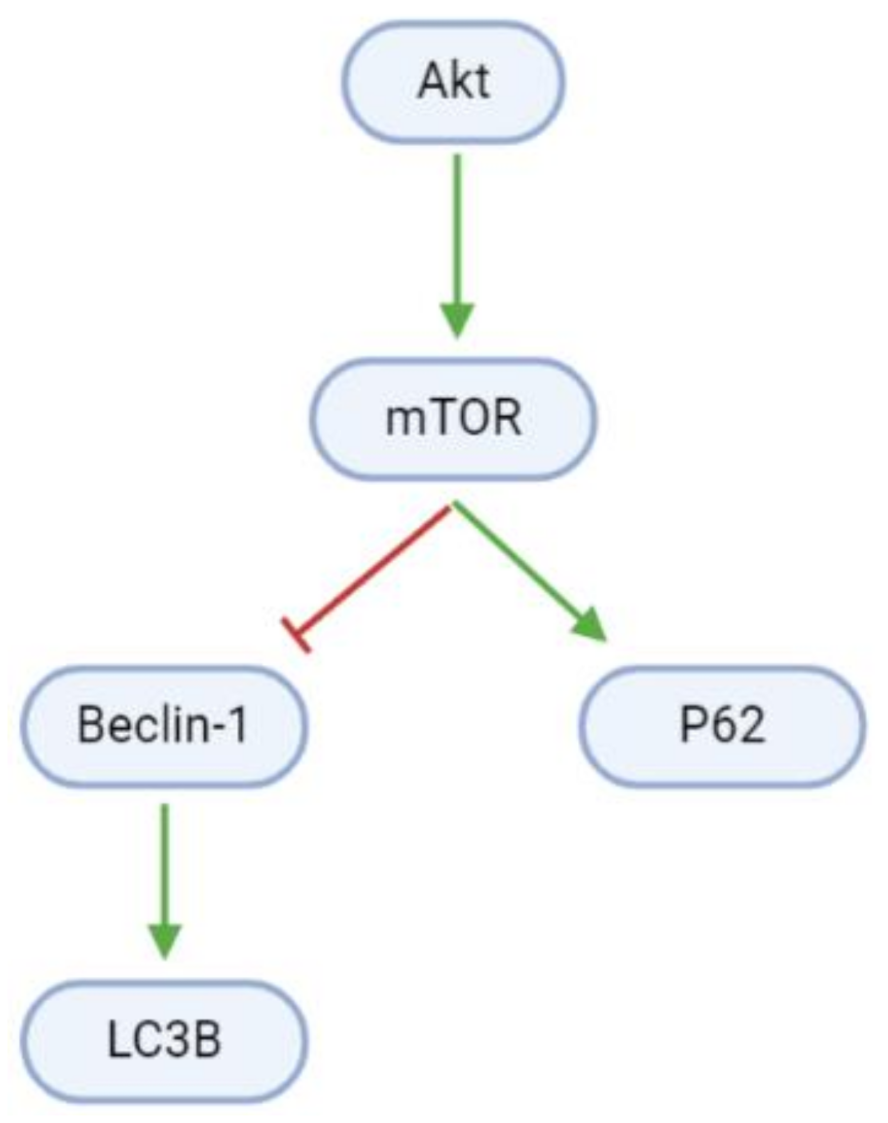
| Causes autophagy | Osthole | MOGGCCM | [64] |
| Upregulates Beclin-1 (mRNA) | Arctigenin | U87MG, T98G | [61] |
| Upregulates Beclin-1 (protein) | Arctigenin | U87MG, T98G | [61] |
| Osthole | MOGGCCM | [64] | |
| Upregulates LC3B-II (mRNA) | Arctigenin | U87MG, T98G | [61] |
| Upregulates LC3B-II (protein) | Arctigenin | U87MG | [61] |
| Downregulates P62 (mRNA) | Arctigenin | U87MG, T98G | [61] |
| Downregulates P62 (protein) | Arctigenin | U87MG, T98G | [61] |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Causes G0/G1 phase cell cycle arrest | Cedrol | DBTRG-05MG, RG2 | [33] |
| Coronarin D | U251 | [47] | |
| Tannic Acid | C6 | [39] | |
| Tectorigenin | GBM-8401 | [50] | |
| BBR | U87 | [58] | |
| GL-PP | U251 | [59] | |
| Causes G2/M phase cell cycle arrest | Eucalyptal A | U87MG, LN229 | [32] |
| Withaferin A | U87, U251 | [40] | |
| TBMS1 | U87, LN229 | [41] | |
| Pisosterol | U87, U343, AHOL1, 1231N1 | [60] | |
| Downregulates Cyclin D1 (protein) | Astaxanthin | GL261 | [35] |
| Adonixanthin | GL261 | [35] | |
| Cedrol | DBTRG-05MG | [33] | |
| Downregulates CDK1 (protein) | Withaferin A | U87, U251 | [40] |
| TBMS1 | U87 | [41] | |
| Downregulates CDK2 (protein) | Cedrol | DBTRG-05MG, RG2 | [33] |
| Downregulates CDK4 (protein) | Tectorigenin | GBM-8401 | [50] |
| Downregulates Cyclin A (protein) | Cedrol | DBTRG-05MG, RG2 | [33] |
| TBMS1 | U87, LN229 | [41] | |
| Downregulates Cyclin B1 (protein) | Cedrol | DBTRG-05MG, RG2 | [33] |
| TBMS1 | U87, LN229 | [41] | |
| Upregulates (p-)H2AX (protein) | Coronarin D | U251 | [47] |
| CP | U251 | [36] | |
| McC1 | GAMG, U251 | [36] | |
| Downregulates (p-)RB (protein) | Tectorigenin | GBM-8401 | [50] |
| Upregulates p21 (protein) | Coronarin D | U251 | [47] |
| Paris saponin H | U251 | [69] | |
| Withaferin A | U87, U251 | [40] | |
| Tectorigenin | GBM-8401 | [50] | |
| Upregulates p27 (protein) | Astaxanthin | GL261 | [35] |
| Paris saponin H | U251 | [69] | |
| Adonixanthin | GL261 | [35] | |
| AM05 | T98G | [44] |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Activates microglia | Rutin | C6 | [63] |
| Quercetin | C6 | [63] | |
| Upregulates IL-1 (mRNA) | Rutin | U251 orthotopic implants, WR | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Upregulates IL-1β (mRNA) | Rutin | C6 | [63] |
| Quercetin | C6 | [63] | |
| Downregulates IL-4 (mRNA) | Rutin | U251 orthotopic implants, WR | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Upregulates IL-6 (mRNA) | Rutin | C6 | [63] |
| Quercetin | C6, TG1 | [63] | |
| Downregulates IL-6 (mRNA) | Rutin | U251, TG1, WR-U251 orthotopic implants | [63] |
| Quercetin | U251, WR-U251 orthotopic implants | [63] | |
| Downregulates IL-6 (protein) | Rutin | C6 | [63] |
| CrataBL | U87 | [49] | |
| Downregulates IL-8 (protein) | CrataBL | U87 | [49] |
| Downregulates IL-10 (mRNA) | Rutin | C6, U251, TG1, WR-U251 orthotopic implants | [63] |
| Quercetin | C6, U251, TG1, WR-U251 orthotopic implants | [63] | |
| Downregulates IL-10 (protein) | Rutin | C6 | [63] |
| Upregulates IL-18 (mRNA) | Rutin | U251 orthotopic implants, WR | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Upregulates TNF (mRNA) | Rutin | U251, TG1 | [63] |
| Quercetin | U251 | [63] | |
| Downregulates TNF (mRNA) | Rutin | U251 orthotopic implants, WR | [63] |
| Quercetin | U251 orthotopic implants, WR | [63] | |
| Upregulates TNF (protein) | Rutin | C6 | [63] |
| Upregulates TNF-α (mRNA) | Rutin | C6 | [63] |
| Quercetin | C6 | [63] | |
| Upregulates CX3CL1 (mRNA) | Rutin | C6, WR-U251 orthotopic implants | [63] |
| Quercetin | C6 | [63] | |
| Downregulates (p-)STAT3 (protein) | Curcumin | U87 | [52] |
| Effect | Substance | Cell Line | Source |
|---|---|---|---|
| Decreases angiogenesis area | McC1 | U251 heterotopic xenograft, fertilized chicken eggs | [36] |
| Decreases blood vessel junctions | McC1 | U251 heterotopic xenograft, fertilized chicken eggs | [36] |
| Decreases tube formation | Diosgenin | C6, T98G | [54] |
| Upregulates ADAMTS1 (protein) | AM04 | U87MG, T98G | [44] |
| Downregulates CD31 (mRNA) | Naringin | U87 subcutaneous xenograft, athymic mice | [37] |
| Downregulates CD105 (mRNA) | Naringin | U87 subcutaneous xenograft, athymic mice | [37] |
| Downregulates tumor hemoglobin | Naringin | U87 subcutaneous xenograft, athymic mice | [37] |
| Downregulates VEGF (protein) | Metformin | U251 | [68] |
| Paris saponin H | U251 | [69] | |
| CrataBL | U87 | [49] | |
| Diosgenin | C6 | [54] |
| Effect | Substance | Cell Line(s) | Source |
|---|---|---|---|
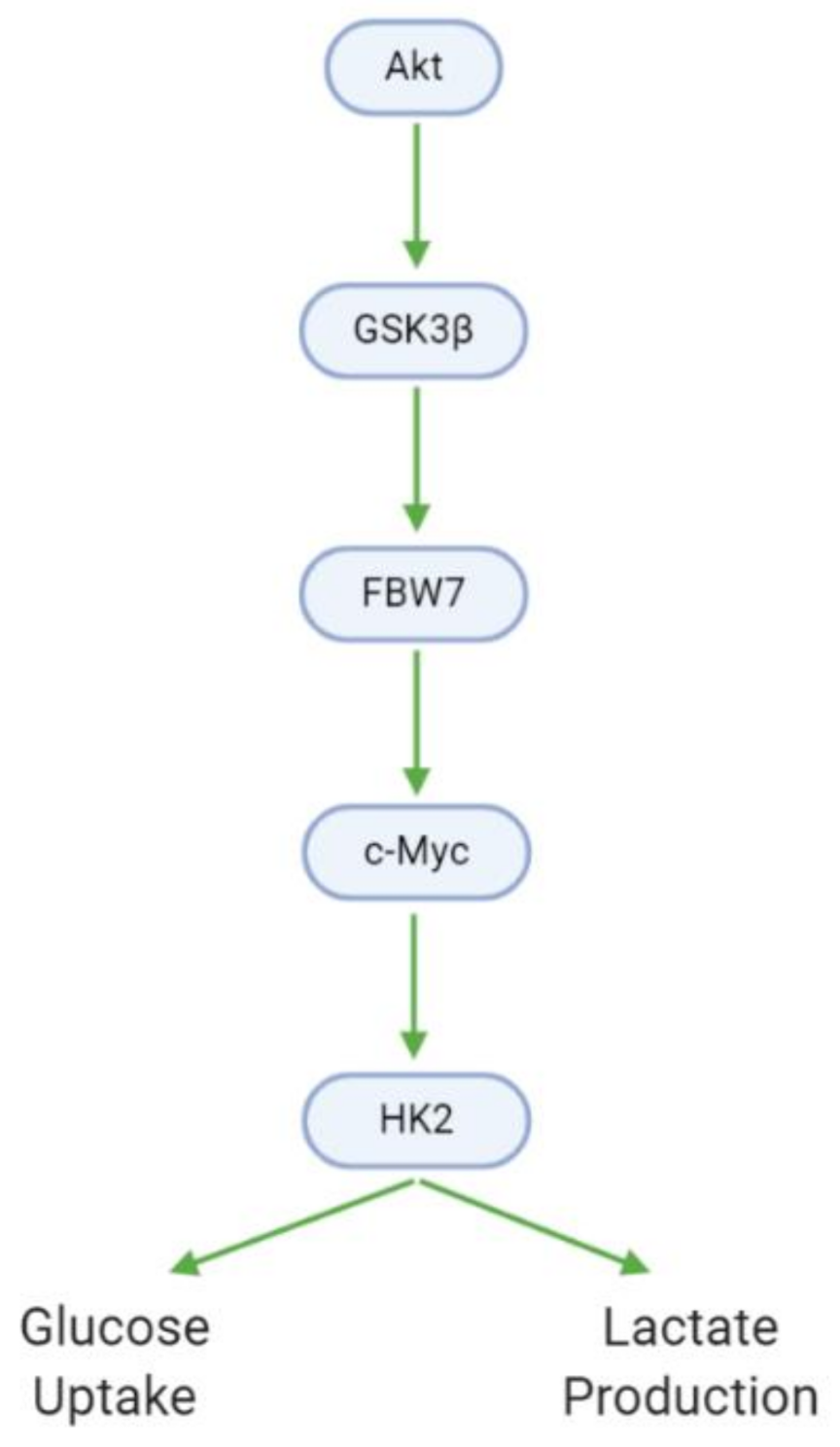
| Downregulates HK2 (protein) | Xanthohumol | U87, T98G, LN229 | [38] |
| Decreases glucose consumption | Xanthohumol | U87, T98G, LN229 | [38] |
| Decreases lactate production | Xanthohumol | U87, T98G, LN229 | [38] |
| Downregulates (p-)GSK3β (protein) | Xanthohumol | U87 | [38] |
| Upregulates PDK4 (mRNA) | Carnosine | U87, T98G | [31] |
| Downregulates FASN (protein) | Crocetin | U87, U138, U251, U373 | [34] |
| Effect | Cell Line | Subs. 1 | Subs. 1 Conc. | Subs. 2 | Subs. 2 Conc. | Source |
|---|---|---|---|---|---|---|
| Increases cell death/dec viability | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| T98G | PPE | 30 µg/mL | BcH | 5, 10, 25, 50, 100 µg/mL | [57] | |
| LN-18 | PPE | 30 µg/mL | BcH | 50, 100 µg/mL | [57] | |
| U87 | PPE | 30 µg/mL | BcH | 50, 100 µg/mL | [57] | |
| Decreases proliferation | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Causes apoptosis | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Causes DNA fragmentation | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Decreases DNA synthesis | T98G | PPE | 30 µg/mL | BcH | 25, 50 µg/mL | [57] |
| LN-18 | PPE | 30 µg/mL | BcH | 25, 50 µg/mL | [57] | |
| U87 | PPE | 30 µg/mL | BcH | 25, 50 µg/mL | [57] | |
| Decreases intracellular ATP | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Upregulates ROS | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Upregulates Bax (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Upregulates cytochrome c (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Upregulates (c-)caspase 3 | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Downregulates c-Myc (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Upregulates p53 (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Downregulates Akt (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| Downregulates (p-)Akt (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Downregulates PI3K (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| Downregulates (p-)PI3K (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Downregulates mTOR (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] | |
| Downregulates (p-)mTOR (protein) | U87 | SLCP | 20 µM | BBR | 100 µM | [29] |
| U251 | SLCP | 20 µM | BBR | 100 µM | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, K.; Siddiqui, M.; Abdellatif, B.; Liskova, A.; Kubatka, P.; Büsselberg, D. Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook. Cancers 2021, 13, 2317. https://doi.org/10.3390/cancers13102317
Zhai K, Siddiqui M, Abdellatif B, Liskova A, Kubatka P, Büsselberg D. Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook. Cancers. 2021; 13(10):2317. https://doi.org/10.3390/cancers13102317
Chicago/Turabian StyleZhai, Kevin, Manaal Siddiqui, Basma Abdellatif, Alena Liskova, Peter Kubatka, and Dietrich Büsselberg. 2021. "Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook" Cancers 13, no. 10: 2317. https://doi.org/10.3390/cancers13102317
APA StyleZhai, K., Siddiqui, M., Abdellatif, B., Liskova, A., Kubatka, P., & Büsselberg, D. (2021). Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook. Cancers, 13(10), 2317. https://doi.org/10.3390/cancers13102317