Prediction of Grade Reclassification of Prostate Cancer Patients on Active Surveillance through the Combination of a Three-miRNA Signature and Selected Clinical Variables
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohorts
2.2. Plasma Preparation, RNA Extraction and miRNA Profiling
2.3. Data Processing
2.4. Statistical Analysis
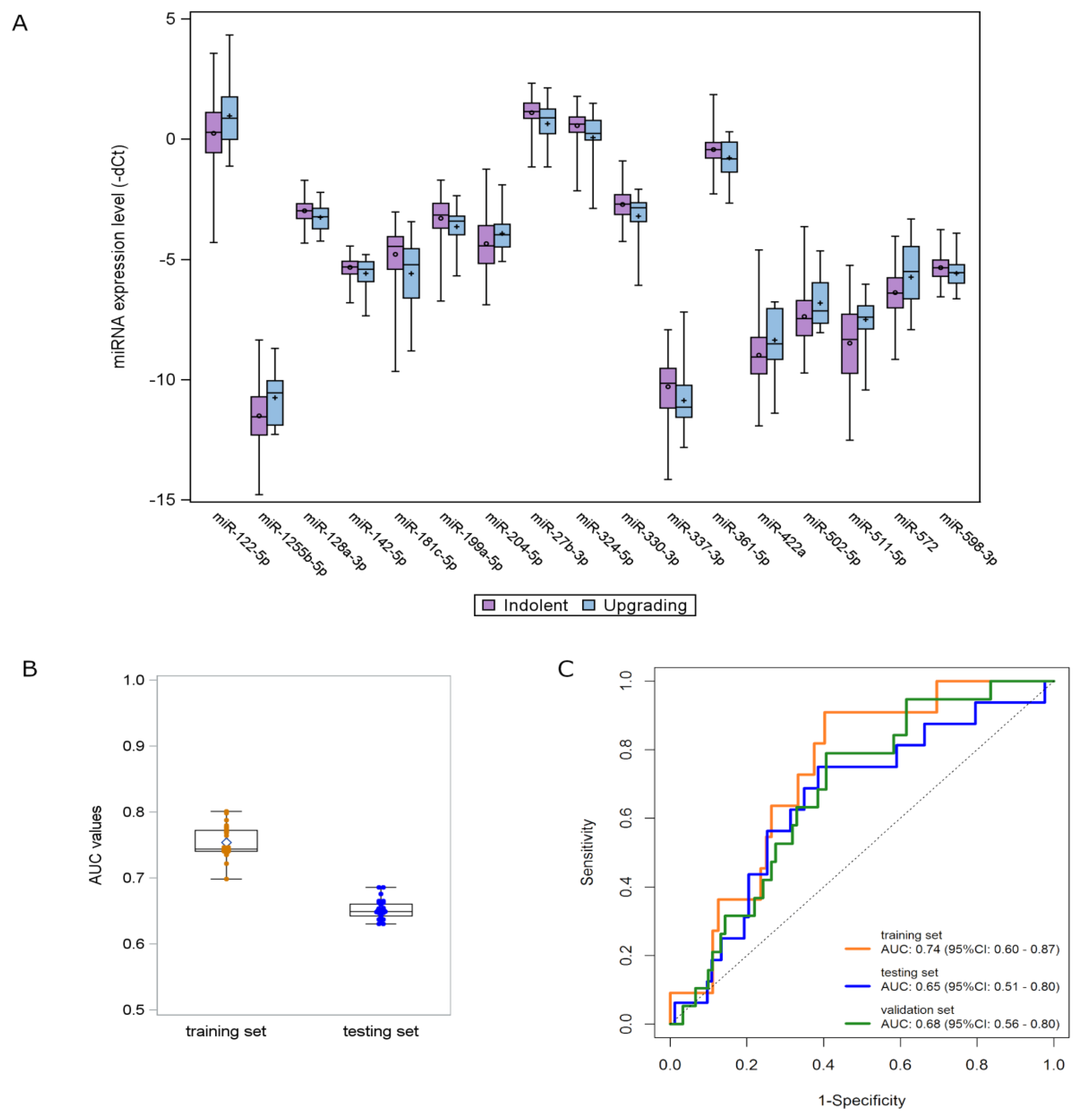
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertsen, P.C. Active Surveillance: A Ten-year Journey. Eur. Urol. 2017, 72, 542–543. [Google Scholar] [CrossRef]
- Klotz, L. Active surveillance for low-risk prostate cancer. Curr. Opin. Urol. 2017, 27, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Fossati, N.; Catto, J.W.; Cornford, P.; Montorsi, F.; Mottet, N.; Wirth, M.; Van Poppel, H. Active Surveillance for Low-risk Prostate Cancer: The European Association of Urology Position. Eur. Urol. 2018, 74, 357–368. [Google Scholar] [CrossRef]
- Ouzzane, A.; Renard-Penna, R.; Marliere, F.; Mozer, P.; Olivier, J.; Barkatz, J.; Puech, P.; Villers, A. Magnetic Resonance Imaging Targeted Biopsy Improves Selection of Patients Considered for Active Surveillance for Clinically Low Risk Prostate Cancer Based on Systematic Biopsies. J. Urol. 2015, 194, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.W.; Newcomb, L.F.; Brown, M.D.; Sjoberg, D.D.; Dong, Y.; Brooks, J.D.; Carroll, P.R.; Cooperberg, M.; Dash, A.; Ellis, W.J.; et al. Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. Eur. Urol. 2017, 72, 448–454. [Google Scholar] [CrossRef]
- Schwen, Z.R.; Mamawala, M.; Tosoian, J.J.; Druskin, S.C.; Ross, A.E.; Sokoll, L.J.; Epstein, J.I.; Carter, H.B.; Gorin, M.A.; Pavlovich, C.P. Prostate Health Index and multiparametric magnetic resonance imaging to predict prostate cancer grade reclassification in active surveillance. BJU Int. 2020, 126, 373–378. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Patel, H.D.; Mamawala, M.; Landis, P.; Wolf, S.; Elliott, D.J.; Epstein, J.I.; Carter, H.B.; Ross, A.E.; Sokoll, L.J.; et al. Longitudinal assessment of urinary PCA3 for predicting prostate cancer grade reclassification in favorable-risk men during active surveillance. Prostate Cancer Prostatic Dis. 2017, 20, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandellini, P.; Doldi, V.; Zaffaroni, N. microRNAs as players and signals in the metastatic cascade: Implications for the development of novel anti-metastatic therapies. Semin. Cancer Biol. 2017, 44, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Endzeliņš, E.; Melne, V.; Kalniņa, Z.; Lietuvietis, V.; Riekstiņa, U.; Llorente, A.; Line, A. Diagnostic, Prognostic and Predictive Value of Cell-Free miRNAs in Prostate Cancer: A Systematic Review. Mol. Cancer 2016, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marenghi, C.; Alvisi, M.F.; Palorini, F.; Avuzzi, B.; Badenchini, F.; Bedini, N.; Bellardita, L.; Biasoni, D.; Bosetti, D.; Casale, A.; et al. Eleven-year Management of Prostate Cancer Patients on Active Surveillance: What have We Learned? Tumor. J. 2017, 103, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Pizzamiglio, S.; Zanutto, S.; Ciniselli, C.M.; Belfiore, A.; Bottelli, S.; Gariboldi, M.; Verderio, P. A methodological procedure for evaluating the impact of hemolysis on circulating microRNAs. Oncol. Lett. 2017, 13, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Verderio, P.; Bottelli, S.; Ciniselli, C.M.; Pierotti, M.A.; Gariboldi, M.; Pizzamiglio, S. NqA: An R-based algorithm for the normalization and analysis of microRNA quantitative real-time polymerase chain reaction data. Anal. Biochem. 2014, 461, 7–9. [Google Scholar] [CrossRef]
- Verderio, P.; Bottelli, S.; Pizzamiglio, S.; Ciniselli, C.M. Developing miRNA signatures: A multivariate prospective. Br. J. Cancer 2016, 115, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.; Donders, A.R.T.; Steyerberg, E.; Harrell, F. Penalized maximum likelihood estimation to directly adjust diagnostic and prognostic prediction models for overoptimism: A clinical example. J. Clin. Epidemiol. 2004, 57, 1262–1270. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Tiberio, P.; Iorio, M.V.; Hilbers, F.; De Azambuja, E.; De La Peña, L.; Izquierdo, M. Ángel; Huober, J.; et al. Plasma miRNA Levels for Predicting Therapeutic Response to Neoadjuvant Treatment in HER2-positive Breast Cancer: Results from the NeoALTTO Trial. Clin. Cancer Res. 2019, 25, 3887–3895. [Google Scholar] [CrossRef] [Green Version]
- Artusi, R.; Verderio, P.; Marubini, E. Bravais-Pearson and Spearman Correlation Coefficients: Meaning, Test of Hypothesis and Confidence Interval. Int. J. Biol. Markers 2002, 17, 148–151. [Google Scholar] [CrossRef]
- Colton, T. Statistics in Medicine; Little, Brown and Company: New York, NY, USA, 1974. [Google Scholar]
- Zanutto, S.; Ciniselli, C.M.; Belfiore, A.; Lecchi, M.; Masci, E.; Delconte, G.; Primignani, M.; Tosetti, G.; Fante, M.D.; Fazzini, L.; et al. Plasma miRNA-based signatures in CRC screening programs. Int. J. Cancer 2019, 146, 1164–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Olkhov-Mitsel, E.; Jeyapala, R.; Zhao, F.; Commisso, K.; Klotz, L.; Loblaw, A.; Liu, S.K.; Vesprini, D.; Fleshner, N.E.; et al. Assessment of Serum microRNA Biomarkers to Predict Reclassification of Prostate Cancer in Patients on Active Surveillance. J. Urol. 2018, 199, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Vesprini, D.; Liu, R.S.; Olkhov-Mitsel, E.; Klotz, L.H.; Loblaw, A.; Liu, S.K.; Bapat, B. Combining urinary DNA methylation and cell-free microRNA biomarkers for improved monitoring of prostate cancer patients on active surveillance. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 297.e9–297.e17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Shiboski, S.; Belair, C.D.; Cooperberg, M.R.; Simko, J.P.; Stoppler, H.; Cowan, J.; Carroll, P.R.; Blelloch, R. miR-19, miR-345, miR-519c-5p Serum Levels Predict Adverse Pathology in Prostate Cancer Patients Eligible for Active Surveillance. PLoS ONE 2014, 9, e98597. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Casiraghi, N.; Rancati, T.; Benelli, M.; Doldi, V.; Romanel, A.; Colecchia, M.; Marenghi, C.; Valdagni, R.; Demichelis, F.; et al. Core Biopsies from Prostate Cancer Patients in Active Surveillance Protocols Harbor PTEN and MYC Alterations. Eur. Urol. Oncol. 2019, 2, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.S.; Tosoian, J.J.; Nallandhighal, S.; Jones, T.A.; Brockman, S.; Elkhoury, F.F.; Bazzi, S.; Plouffe, K.R.; Siddiqui, J.; Liu, C.-J.; et al. Serial Molecular Profiling of Low-grade Prostate Cancer to Assess Tumor Upgrading: A Longitudinal Cohort Study. Eur. Urol. 2021, 79, 456–465. [Google Scholar] [CrossRef]
- Bruinsma, S.M.; Bangma, C.H.; Carroll, P.R.; Leapman, M.S.; Rannikko, A.; Petrides, N.; Weerakoon, M.; Bokhorst, L.P.; Roobol, M.J. The Movember GAP3 consortium Active surveillance for prostate cancer: A narrative review of clinical guidelines. Nat. Rev. Urol. 2016, 13, 151–167. [Google Scholar] [CrossRef]
- Liu, J.L.; Patel, H.D.; Haney, N.M.; Epstein, J.I.; Partin, A.W. Advances in the selection of patients with prostate cancer for active surveillance. Nat. Rev. Urol. 2021, 18, 197–208. [Google Scholar] [CrossRef]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, R.; Su, Z.; Zhang, M.; Xu, M.; Gong, J.; Chen, N.; Zeng, H.; Chen, X.; Zhou, Q. Downregulation of miR-199a-5p promotes prostate adeno-carcinoma progression through loss of its inhibition of HIF-1α. Oncotarget 2017, 8, 83523–83538. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, H.-Q.; Zhang, Y.-W. MiR-511-5p functions as a tumor suppressor and a predictive of prognosis in colorectal cancer by directly targeting GPR. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6119–6130. [Google Scholar] [PubMed]
- Fu, L.; Li, Z.; Zhu, J.; Wang, P.; Fan, G.; Dai, Y.; Zheng, Z.; Liu, Y. Serum expression levels of microRNA-382-3p, −598-3p, −1246 and −184 in breast cancer patients. Oncol. Lett. 2016, 12, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| TRS a | TES a | VAS a | ||||
|---|---|---|---|---|---|---|
| n = 121 | n = 111 | n = 127 | ||||
| Variable (at Diagnosis) | Median | IQR b | Median | IQR b | Median | IQR b |
| Age (years) | 64 | 59–70 | 62 | 58–66 | 63.4 | 58.1–69.2 |
| PSA (ng/mL) | 5.36 | 4.27–6.30 | 5.89 | 4.8–7.1 | 5.9 | 4.83–7.44 |
| Prostate volume (cm3) | 44 | 36–58 | 46 | 35–61 | 48 | 37–63 |
| PSA density (ng/mL/cm3) | 0.12 | 0.08–0.15 | 0.11 | 0.08–0.16 | 0.12 | 0.09–0.17 |
| Total biopsy cores (n) | 12 | 10–16 | 14 | 12–16 | 12 | 12–16 |
| Max PCa length (%) | 10 | 5–20 | 5 | 5–20 | 10 | 5–20.5 |
| Positive cores (n, %) | ||||||
| <10 | 62 (51.24%) | 64 (57.66%) | 52 (40.94%) | |||
| ≥10 | 59 (48.76%) | 47 (42.34%) | 75 (59.06%) | |||
| Positive cores (n, %) | ||||||
| ≤1 | 85 (70.25%) | 71 (63.96%) | 57 (44.88%) | |||
| >1 | 36 (29.75%) | 40 (36.04 %) | 70 (55.12%) | |||
| Gleason Pattern Score/Prognostic Grade Group (n, %) | ||||||
| GPS = /PGG1 | 121 (100%) | 111 (100%) | 127 (100%) | |||
| Clinical Stage (n, %) | ||||||
| T1b | - | - | 1 (0.79%) | |||
| T1c | 113 (93.39%) | 106 (95.5%) | 122 (96.06%) | |||
| T2a | 8 (6.61%) | 5 (4.5%) | 4 (3.15%) | |||
| miRNA | OR (95% CI) | p-Value | |
|---|---|---|---|
| miR-122-5p | 1.412 (1.007;1.979) | 0.046 | * |
| miR-1255b-5p | 1.590 (1.000;2.528) | 0.045 | † |
| miR-128a-3p | 0.380 (0.153;0.944) | 0.037 | * |
| miR-142-5p | 0.353 (0.138;0.903) | 0.030 | * |
| miR-181c-5p | 0.634 (0.439;0.916) | 0.015 | * |
| miR-199a-5p | 0.663 (0.402;1.095) | 0.043 | † |
| miR-204-5p | 1.428 (0.929;2.195) | 0.035 | ** |
| miR-27b-3p | 0.421 (0.219;0.809) | 0.010 | * |
| miR-324-5p | 0.471 (0.266;0.836) | 0.010 | * |
| miR-330-3p | 0.387 (0.172;0.873) | 0.022 | * |
| miR-337-3p | 0.694 (0.429;1.123) | 0.048 | † |
| miR-361-5p | 0.479 (0.236;0.973) | 0.042 | * |
| miR-422a | 1.451 (0.980;2.147) | 0.039 | † |
| miR-502-5p | 1.497 (0.991;2.262) | 0.049 | † |
| miR-511-5p | 1.568 (0.976;2.518) | 0.044 | † |
| miR-572 | 1.720 (1.034;2.860) | 0.037 | * |
| miR-598-3p | 0.395 (0.151;1.036) | 0.048 | † |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | UPG * | IND ** | OR † | 95% CI | OR | 95% CI |
| Age (years) | 65 | 294 | 1.074 | 1.029–1.120 | 1.081 | 1.033–1.132 |
| PSA density (ng/mL/cm3) | 65 | 294 | 2.599 | 1.392–4.853 | 2.677 | 1.418–5.053 |
| Prostate volume (cm3) | 65 | 294 | 0.469 | 0.264–0.833 | – | |
| Positive cores (n) | 65 | 294 | 1.923 | 1.119–3.306 | – | |
| Positive cores (%) | 65 | 294 | 2.036 | 1.166–3.556 | – | |
| Max PCa length (%) | 62 | 285 | 1.024 | 1.009–1.040 | 1.024 | 1.008–1.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandellini, P.; Ciniselli, C.M.; Rancati, T.; Marenghi, C.; Doldi, V.; El Bezawy, R.; Lecchi, M.; Claps, M.; Catanzaro, M.; Avuzzi, B.; et al. Prediction of Grade Reclassification of Prostate Cancer Patients on Active Surveillance through the Combination of a Three-miRNA Signature and Selected Clinical Variables. Cancers 2021, 13, 2433. https://doi.org/10.3390/cancers13102433
Gandellini P, Ciniselli CM, Rancati T, Marenghi C, Doldi V, El Bezawy R, Lecchi M, Claps M, Catanzaro M, Avuzzi B, et al. Prediction of Grade Reclassification of Prostate Cancer Patients on Active Surveillance through the Combination of a Three-miRNA Signature and Selected Clinical Variables. Cancers. 2021; 13(10):2433. https://doi.org/10.3390/cancers13102433
Chicago/Turabian StyleGandellini, Paolo, Chiara Maura Ciniselli, Tiziana Rancati, Cristina Marenghi, Valentina Doldi, Rihan El Bezawy, Mara Lecchi, Melanie Claps, Mario Catanzaro, Barbara Avuzzi, and et al. 2021. "Prediction of Grade Reclassification of Prostate Cancer Patients on Active Surveillance through the Combination of a Three-miRNA Signature and Selected Clinical Variables" Cancers 13, no. 10: 2433. https://doi.org/10.3390/cancers13102433
APA StyleGandellini, P., Ciniselli, C. M., Rancati, T., Marenghi, C., Doldi, V., El Bezawy, R., Lecchi, M., Claps, M., Catanzaro, M., Avuzzi, B., Campi, E., Colecchia, M., Badenchini, F., Verderio, P., Valdagni, R., & Zaffaroni, N. (2021). Prediction of Grade Reclassification of Prostate Cancer Patients on Active Surveillance through the Combination of a Three-miRNA Signature and Selected Clinical Variables. Cancers, 13(10), 2433. https://doi.org/10.3390/cancers13102433








