Response and Toxicity to the Second Course of 3 Cycles of 177Lu-PSMA Therapy Every 4 Weeks in Patients with Metastatic Castration-Resistant Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Medical Care of the Patients and the Applied PSMA-RLT Protocol
2.3. Statistical Analysis
3. Results
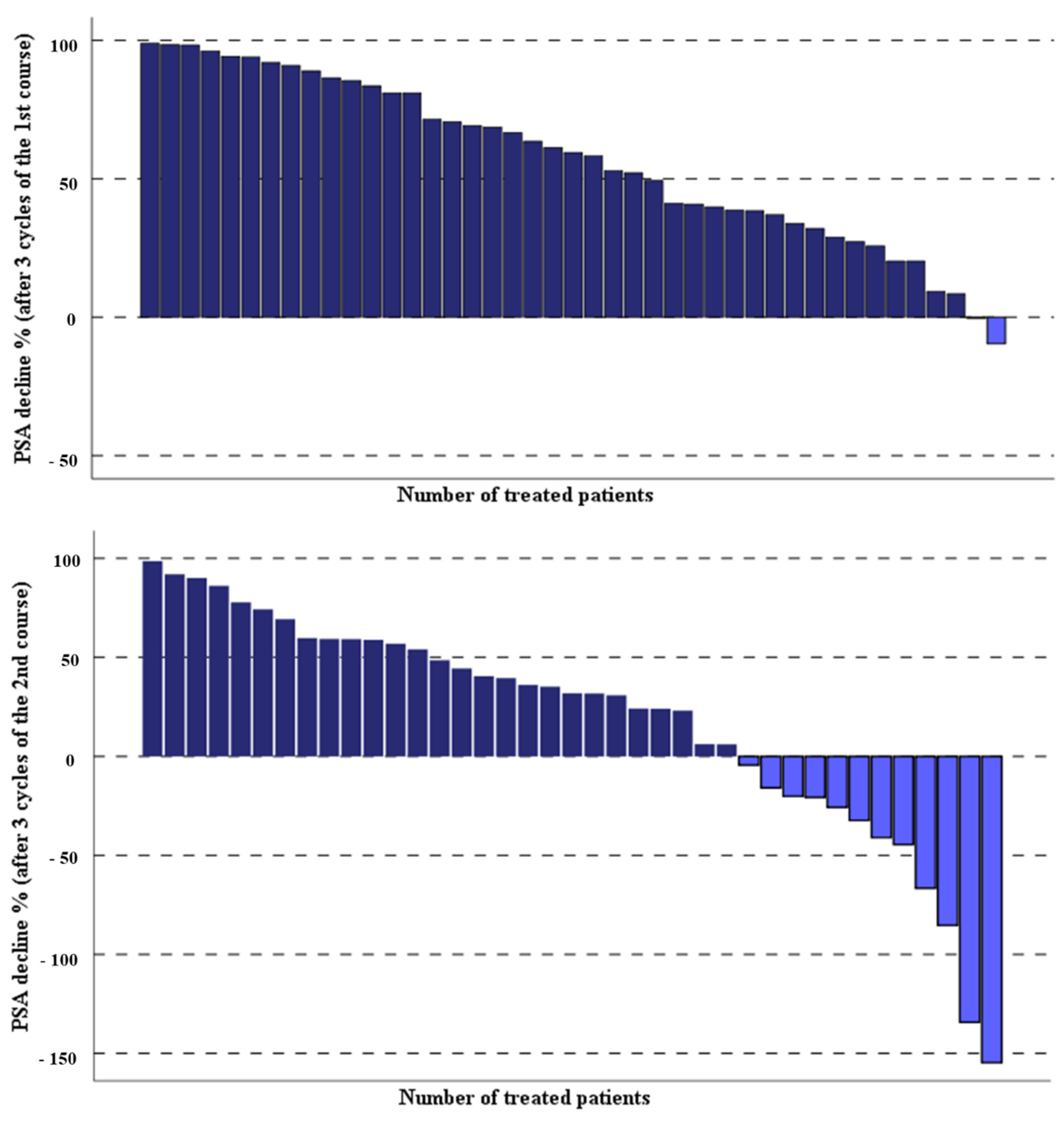
3.1. Response Rate and Clinical Effects of Second PSMA-RLT Course
3.2. Overall Survival of Patients Treated with Second PSMA-RLT Course
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Rasul, S.; Hacker, M.; Kretschmer-Chott, E.; Leisser, A.; Grubmuller, B.; Kramer, G.; Shariat, S.; Wadsak, W.; Mitterhauser, M.; Hartenbach, M.; et al. Clinical outcome of standardized (177)Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadzadehfar, H.; Rahbar, K.; Kurpig, S.; Bogemann, M.; Claesener, M.; Eppard, E.; Gartner, F.; Rogenhofer, S.; Schafers, M.; Essler, M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015, 5, 114. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadehfar, H.; Eppard, E.; Kurpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.D.; Gartner, F.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477–12488. [Google Scholar] [CrossRef]
- McBean, R.; O’Kane, B.; Parsons, R.; Wong, D. Lu177-PSMA therapy for men with advanced prostate cancer: Initial 18 months experience at a single Australian tertiary institution. J. Med. Imaging Radiat. Oncol. 2019, 63, 538–545. [Google Scholar] [CrossRef]
- Rahbar, K.; Boegemann, M.; Yordanova, A.; Eveslage, M.; Schafers, M.; Essler, M.; Ahmadzadehfar, H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Boning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017, 8, 3581–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdinandus, J.; Eppard, E.; Gaertner, F.C.; Kurpig, S.; Fimmers, R.; Yordanova, A.; Hauser, S.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. Predictors of Response to Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with 177Lu-PSMA-617. J. Nucl. Med. 2017, 58, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Grubmuller, B.; Senn, D.; Kramer, G.; Baltzer, P.; D’Andrea, D.; Grubmuller, K.H.; Mitterhauser, M.; Eidherr, H.; Haug, A.R.; Wadsak, W.; et al. Response assessment using (68)Ga-PSMA ligand PET in patients undergoing (177)Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Rasul, S.; Hartenbach, M.; Wollenweber, T.; Kretschmer-Chott, E.; Grubmuller, B.; Kramer, G.; Shariat, S.; Wadsak, W.; Mitterhauser, M.; Pichler, V.; et al. Prediction of response and survival after standardized treatment with 7400 MBq (177)Lu-PSMA-617 every 4 weeks in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Chau, C.H.; Figg, W.D. Challenges to improved therapeutics for metastatic castrate resistant prostate cancer: From recent successes and failures. J. Hematol. Oncol. 2012, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of (177)Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Retz, M.; Knorr, K.; Heck, M.; Wester, H.J.; D’Alessandria, C.; Weber, W.A.; Eiber, M.; Tauber, R. Early Experience of Rechallenge (177)Lu-PSMA Radioligand Therapy After an Initial Good Response in Patients with Advanced Prostate Cancer. J. Nucl. Med. 2019, 60, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.J.; Smith-Jones, P.M.; Wongvipat, J.; Navarro, V.; Kim, S.; Bander, N.H.; Larson, S.M.; Sawyers, C.L. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl. Acad. Sci. USA 2011, 108, 9578–9582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Ravi Kumar, A.S.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Yordanova, A.; Linden, P.; Hauser, S.; Meisenheimer, M.; Kurpig, S.; Feldmann, G.; Gaertner, F.C.; Essler, M.; Ahmadzadehfar, H. Outcome and safety of rechallenge [(177)Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1073–1080. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Schlolaut, S.; Fimmers, R.; Yordanova, A.; Hirzebruch, S.; Schlenkhoff, C.; Gaertner, F.C.; Awang, Z.H.; Hauser, S.; Essler, M. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [(177)Lu]Lu-PSMA-617 radioligand therapy. Oncotarget 2017, 8, 103108–103116. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bogemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [(177)Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.R.; Singh, A.; Schuchardt, C.; Niepsch, K.; Sayeg, M.; Leshch, Y.; Wester, H.J.; Baum, R.P. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J. Nucl. Med. 2016, 57 (Suppl. 3), 97S–104S. [Google Scholar] [CrossRef] [Green Version]
- Von Eyben, F.E.; Kiljunen, T.; Joensuu, T.; Kairemo, K.; Uprimny, C.; Virgolini, I. (177)Lu-PSMA-617 radioligand therapy for a patient with lymph node metastatic prostate cancer. Oncotarget 2017, 8, 66112–66116. [Google Scholar] [CrossRef] [Green Version]
- Von Eyben, F.E.; Singh, A.; Zhang, J.; Nipsch, K.; Meyrick, D.; Lenzo, N.; Kairemo, K.; Joensuu, T.; Virgolini, I.; Soydal, C.; et al. (177)Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget 2019, 10, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Barna, S.; Haug, A.R.; Hartenbach, M.; Rasul, S.; Grubmuller, B.; Kramer, G.; Blaickner, M. Dose Calculations and Dose-Effect Relationships in 177Lu-PSMA I&T Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Clin. Nucl. Med. 2020, 45, 661–667. [Google Scholar] [PubMed]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of (177)Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paganelli, G.; Sarnelli, A.; Severi, S.; Sansovini, M.; Belli, M.L.; Monti, M.; Foca, F.; Celli, M.; Nicolini, S.; Tardelli, E.; et al. Dosimetry and safety of (177)Lu PSMA-617 along with polyglutamate parotid gland protector: Preliminary results in metastatic castration-resistant prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the Vision of Radioligand Therapy for Prostate Cancer Becoming a Reality? An Overview of the Phase III VISION Trial and Its Importance for the Future of Theranostics. J. Nucl. Med. 2019, 60, 1504–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameters | Values |
|---|---|
| Patients (n) | 43 |
| Age (mean ± SD) years | 71.4 ± 6.6 |
| Weight (mean ± SD) kilogram | 83.1 ± 11.4 |
| [177Lu]Lu-PSMA-617 MBq | 7351 ± 647 |
| ≥50% PSA decline after 1st PSMA-RLT (n) % | (26) 60.5 |
| Karnofsky score (n) % | |
| <80% | (16) 37.2 |
| ≥80% | (27) 62.8 |
| ECOG index (n) % | |
| 0 | (8) 18.6 |
| 1 | (26) 60.5 |
| 2 | (9) 20.9 |
| Previous treatments (n) % | |
| Enzalutamide/abiraterone | (27) 62.8 |
| Docetaxel/cabazitaxel | (30) 69.7 |
| Ra-223 (Xofigo®) | (12) 27.9 |
| Metastatic lesions (n) % | |
| M1a | (8) 18.6 |
| M1b | (27) 62.8 |
| M1c | (8) 18.6 |
| Parameters | Before Therapy | After Therapy | q -Value |
|---|---|---|---|
| * PSA µg/L | 40.8 (0.87–1358) | 20.2 (0.60–1962) | 0.002 |
| Hemoglobin g/dL (mean ± SD) | 11.5 ± 1.7 | 11 ± 1.6 | 0.006 |
| Thrombocyte g/L (mean ± SD) | 208 ± 63 | 185 ± 63 | 0.002 |
| * Leucocyte g/L | 5.4 (1.17–14.3) | 4.8 (2.1–14.1) | n.s. |
| * Creatinine mg/dL | 0.96 (0.54–2.24) | 0.94 (0.61–2.6) | n.s. |
| * Alkaline phosphatase U/L | 78 (42–995) | 84 (47–1345) | n.s. |
| * LDH U/L | 205 (96–278) | 194 (86–551) | n.s. |
| Parameters | Before Therapy | After Therapy | ||||
|---|---|---|---|---|---|---|
| Toxicity (n) | Toxicity (n) | |||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Hemoglobin g/dL | 18 | 7 | 0 | 15 | 9 | 2 |
| Thrombocyte g/L | 8 | 0 | 0 | 8 | 0 | 1 |
| Leucocyte g/L | 2 | 0 | 1 | 3 | 1 | 0 |
| Creatinine mg/dL | 3 | 0 | 0 | 1 | 0 | 0 |
| Type of Metastasis | OS Calculated from 1st Course (Weeks) | OS Calculated from 2nd Course (Weeks) | PFS after 1st Course (Weeks) | PFS after 2nd Course (Weeks) |
|---|---|---|---|---|
| Total population | 188 | 136 | 27 | 31 |
| M1a | >169 * | >147 * | 41 | 32 |
| M1b | 176 | 123 | 25 | 24 |
| M1c | 119 | 106 | 44 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasul, S.; Wollenweber, T.; Zisser, L.; Kretschmer-Chott, E.; Grubmüller, B.; Kramer, G.; Shariat, S.F.; Eidherr, H.; Mitterhauser, M.; Vraka, C.; et al. Response and Toxicity to the Second Course of 3 Cycles of 177Lu-PSMA Therapy Every 4 Weeks in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 2489. https://doi.org/10.3390/cancers13102489
Rasul S, Wollenweber T, Zisser L, Kretschmer-Chott E, Grubmüller B, Kramer G, Shariat SF, Eidherr H, Mitterhauser M, Vraka C, et al. Response and Toxicity to the Second Course of 3 Cycles of 177Lu-PSMA Therapy Every 4 Weeks in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers. 2021; 13(10):2489. https://doi.org/10.3390/cancers13102489
Chicago/Turabian StyleRasul, Sazan, Tim Wollenweber, Lucia Zisser, Elisabeth Kretschmer-Chott, Bernhard Grubmüller, Gero Kramer, Shahrokh F. Shariat, Harald Eidherr, Markus Mitterhauser, Chrysoula Vraka, and et al. 2021. "Response and Toxicity to the Second Course of 3 Cycles of 177Lu-PSMA Therapy Every 4 Weeks in Patients with Metastatic Castration-Resistant Prostate Cancer" Cancers 13, no. 10: 2489. https://doi.org/10.3390/cancers13102489
APA StyleRasul, S., Wollenweber, T., Zisser, L., Kretschmer-Chott, E., Grubmüller, B., Kramer, G., Shariat, S. F., Eidherr, H., Mitterhauser, M., Vraka, C., Langsteger, W., Hacker, M., & Haug, A. R. (2021). Response and Toxicity to the Second Course of 3 Cycles of 177Lu-PSMA Therapy Every 4 Weeks in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers, 13(10), 2489. https://doi.org/10.3390/cancers13102489









