Recent Progress in Dendritic Cell-Based Cancer Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
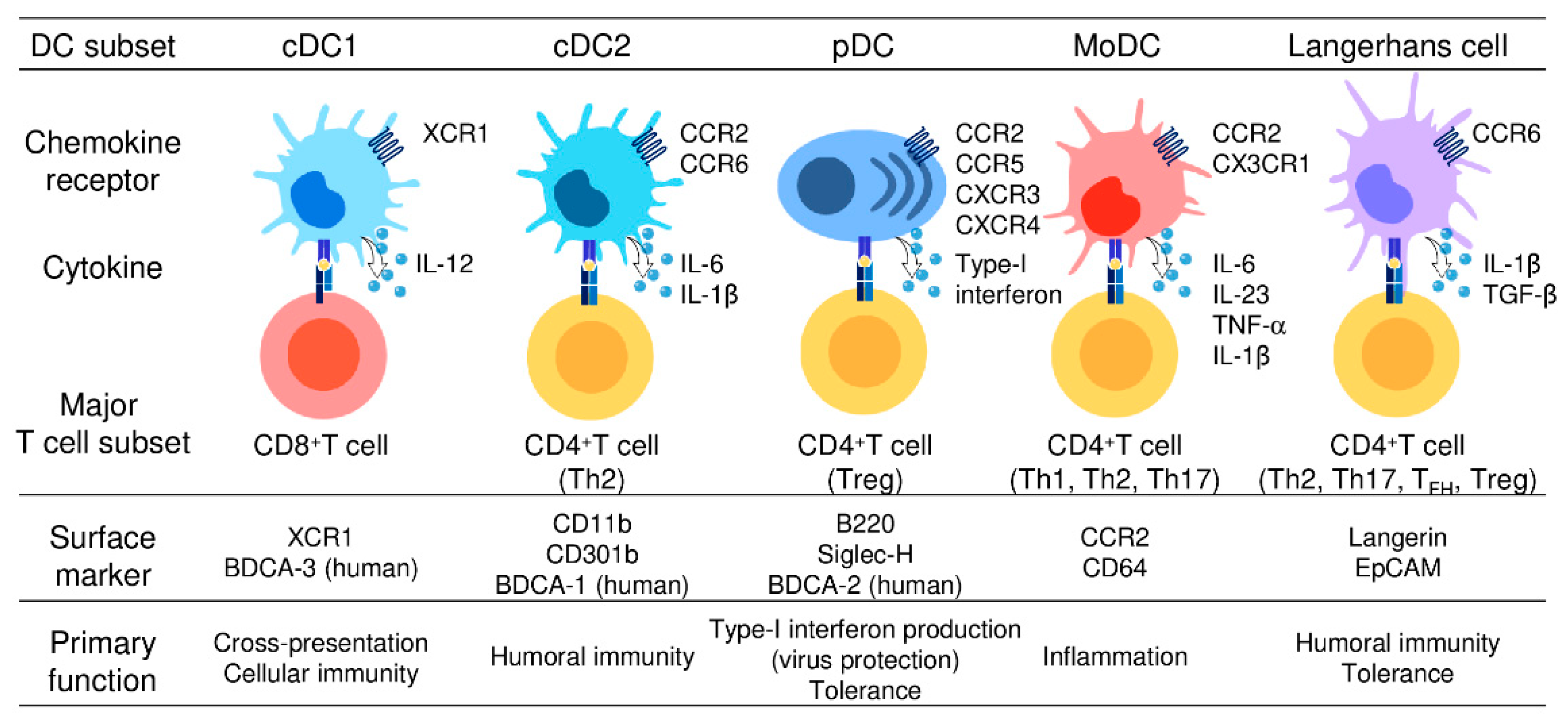
2. DC Subsets and Their Functions
2.1. cDC1
2.2. cDC2
2.3. pDC
2.4. moDC
2.5. LCs
3. Use of DEC205 and CLEC9A in cDC1-Targeting Vaccines
4. Differential Expression of Chemokine Receptors by DC Subsets
4.1. Use of Chemokines to Target DCs
4.2. The Role of the XCL1-XCR1 Axis in cDC1-CD8+ T-Cell Interactions
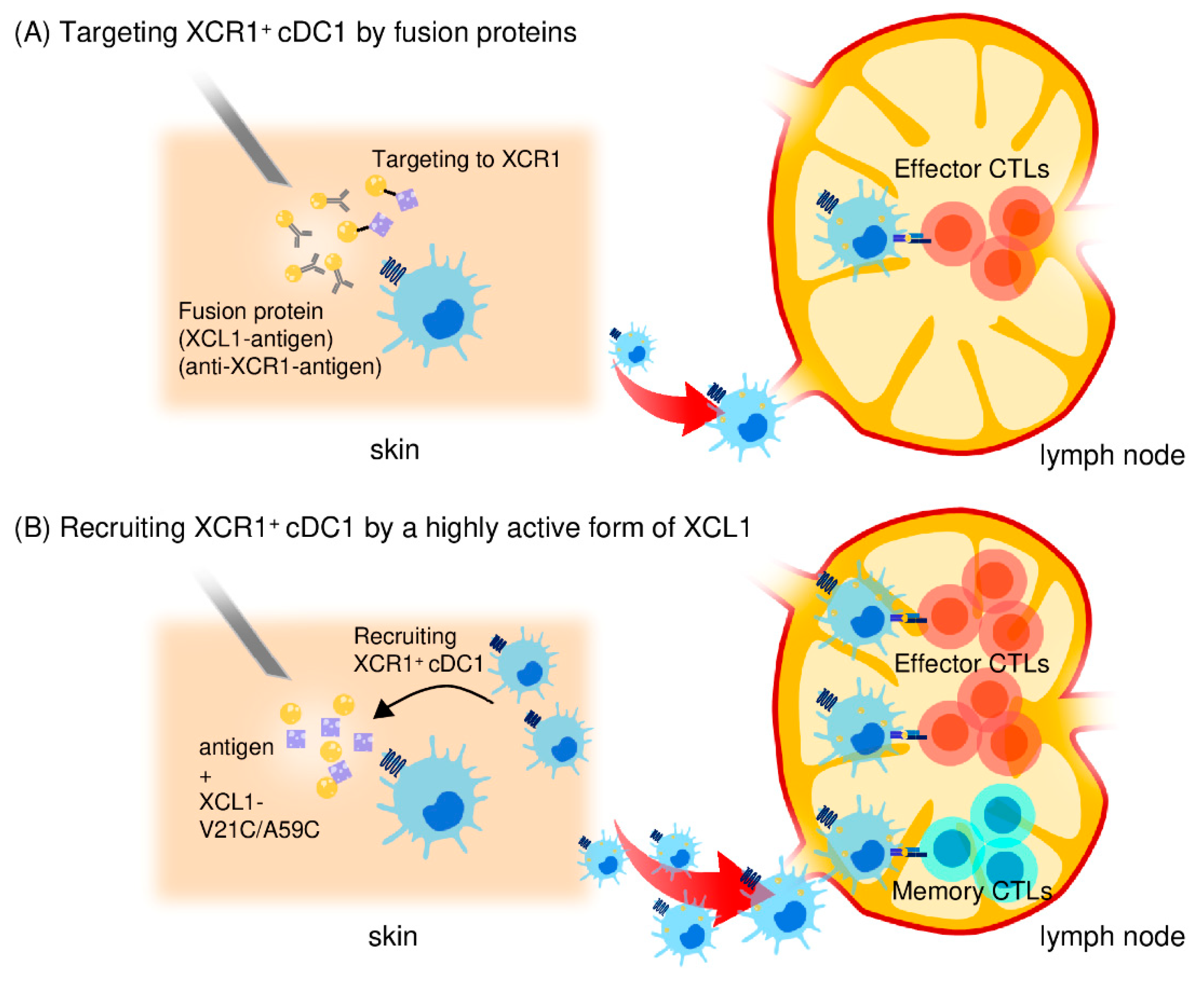
4.3. Use of Fusion Antigens Targeting XCR1 as CTL-inducing Vaccines
4.4. Generation of highly Active XCL1 and its Use as an Adjuvant for Antigen Delivery to cDC1s
4.5. Induction of Memory CD8+ CTLs by the Highly Active XCL1 Adjuvant
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganesh, K.; Massague, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, P.; Dzieciatkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.D.; Flickinger, J.C., Jr.; Snook, A.E. Talkin’ Toxins: From Coley’s to Modern Cancer Immunotherapy. Toxins (Basel) 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnet, F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970, 13, 1–27. [Google Scholar]
- Thomas, L. On immunosurveillance in human cancer. Yale J. Biol. Med. 1982, 55, 329–333. [Google Scholar]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 1–19. [Google Scholar] [CrossRef]
- Colbert, J.D.; Cruz, F.M.; Rock, K.L. Cross-presentation of exogenous antigens on MHC I molecules. Curr. Opin. Immunol. 2020, 64, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell. Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Anderson, D.A., 3rd; Dutertre, C.A.; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 2021, 21, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, J.P.; Reis e Sousa, C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- den Haan, J.M.; Lehar, S.M.; Bevan, M.J. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000, 192, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Pooley, J.L.; Heath, W.R.; Shortman, K. Cutting edge: Intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 2001, 166, 5327–5330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J. Exp. Med. 1974, 139, 380–397. [Google Scholar]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Thery, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [Green Version]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4. [Google Scholar] [CrossRef] [Green Version]
- Bachem, A.; Hartung, E.; Guttler, S.; Mora, A.; Zhou, X.; Hegemann, A.; Plantinga, M.; Mazzini, E.; Stoitzner, P.; Gurka, S.; et al. Expression of XCR1 Characterizes the Batf3-Dependent Lineage of Dendritic Cells Capable of Antigen Cross-Presentation. Front. Immunol. 2012, 3, 214. [Google Scholar] [CrossRef] [Green Version]
- Calabro, S.; Liu, D.; Gallman, A.; Nascimento, M.S.; Yu, Z.; Zhang, T.T.; Chen, P.; Zhang, B.; Xu, L.; Gowthaman, U.; et al. Differential Intrasplenic Migration of Dendritic Cell Subsets Tailors Adaptive Immunity. Cell Rep. 2016, 16, 2472–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura, E.; Durand, M.; Amigorena, S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 2013, 210, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Audsley, K.M.; McDonnell, A.M.; Waithman, J. Cross-Presenting XCR1(+) Dendritic Cells as Targets for Cancer Immunotherapy. Cells 2020, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Dorner, B.G.; Dorner, M.B.; Zhou, X.; Opitz, C.; Mora, A.; Guttler, S.; Hutloff, A.; Mages, H.W.; Ranke, K.; Schaefer, M.; et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 2009, 31, 823–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henri, S.; Poulin, L.F.; Tamoutounour, S.; Ardouin, L.; Guilliams, M.; de Bovis, B.; Devilard, E.; Viret, C.; Azukizawa, H.; Kissenpfennig, A.; et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 2010, 207, 189–206. [Google Scholar] [CrossRef] [Green Version]
- Contreras, V.; Urien, C.; Guiton, R.; Alexandre, Y.; Vu Manh, T.P.; Andrieu, T.; Crozat, K.; Jouneau, L.; Bertho, N.; Epardaud, M.; et al. Existence of CD8alpha-like dendritic cells with a conserved functional specialization and a common molecular signature in distant mammalian species. J. Immunol. 2010, 185, 3313–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozat, K.; Guiton, R.; Contreras, V.; Feuillet, V.; Dutertre, C.A.; Ventre, E.; Vu Manh, T.P.; Baranek, T.; Storset, A.K.; Marvel, J.; et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J. Exp. Med. 2010, 207, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huysamen, C.; Willment, J.A.; Dennehy, K.M.; Brown, G.D. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J. Biol. Chem. 2008, 283, 16693–16701. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Lafaille, F.G.; Pessach, I.M.; Zhang, S.Y.; Ciancanelli, M.J.; Herman, M.; Abhyankar, A.; Ying, S.W.; Keros, S.; Goldstein, P.A.; Mostoslavsky, G.; et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 2012, 491, 769–773. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochrein, H.; Shortman, K.; Vremec, D.; Scott, B.; Hertzog, P.; O’Keeffe, M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 2001, 166, 5448–5455. [Google Scholar] [CrossRef] [Green Version]
- Fukao, T.; Frucht, D.M.; Yap, G.; Gadina, M.; O’Shea, J.J.; Koyasu, S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J. Immunol. 2001, 166, 4446–4455. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molenkamp, B.G.; van Leeuwen, P.A.; Meijer, S.; Sluijter, B.J.; Wijnands, P.G.; Baars, A.; van den Eertwegh, A.J.; Scheper, R.J.; de Gruijl, T.D. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin. Cancer Res. 2007, 13, 2961–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tussiwand, R.; Everts, B.; Grajales-Reyes, G.E.; Kretzer, N.M.; Iwata, A.; Bagaitkar, J.; Wu, X.; Wong, R.; Anderson, D.A.; Murphy, T.L.; et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015, 42, 916–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, E.K.; Uronen-Hansson, H.; Semmrich, M.; Rivollier, A.; Hagerbrand, K.; Marsal, J.; Gudjonsson, S.; Hakansson, U.; Reizis, B.; Kotarsky, K.; et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013, 38, 958–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, C.; Han, J.A.; Choi, B.; Cho, Y.K.; Do, Y.; Ryu, S. Intrinsic features of the CD8alpha(-) dendritic cell subset in inducing functional T follicular helper cells. Immunol. Lett. 2016, 172, 21–28. [Google Scholar] [CrossRef]
- Sittig, S.P.; Bakdash, G.; Weiden, J.; Skold, A.E.; Tel, J.; Figdor, C.G.; de Vries, I.J.; Schreibelt, G. A Comparative Study of the T Cell Stimulatory and Polarizing Capacity of Human Primary Blood Dendritic Cell Subsets. Mediators Inflamm. 2016, 2016, 3605643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizzoli, G.; Larghi, P.; Paroni, M.; Crosti, M.C.; Moro, M.; Neddermann, P.; Caprioli, F.; Pagani, M.; De Francesco, R.; Abrignani, S.; et al. IL-10 promotes homeostatic proliferation of human CD8(+) memory T cells and, when produced by CD1c(+) DCs, shapes naive CD8(+) T-cell priming. Eur. J. Immunol. 2016, 46, 1622–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laoui, D.; Keirsse, J.; Morias, Y.; Van Overmeire, E.; Geeraerts, X.; Elkrim, Y.; Kiss, M.; Bolli, E.; Lahmar, Q.; Sichien, D.; et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat. Commun. 2016, 7, 13720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durai, V.; Murphy, K.M. Functions of Murine Dendritic Cells. Immunity 2016, 45, 719–736. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Matsumoto, T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar] [CrossRef]
- Piccioli, D.; Tavarini, S.; Borgogni, E.; Steri, V.; Nuti, S.; Sammicheli, C.; Bardelli, M.; Montagna, D.; Locatelli, F.; Wack, A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood 2007, 109, 5371–5379. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallee, V.P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863.e24. [Google Scholar] [CrossRef] [Green Version]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Sathe, P.; Vremec, D.; Wu, L.; Corcoran, L.; Shortman, K. Convergent differentiation: Myeloid and lymphoid pathways to murine plasmacytoid dendritic cells. Blood 2013, 121, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Shigematsu, H.; Reizis, B.; Iwasaki, H.; Mizuno, S.; Hu, D.; Traver, D.; Leder, P.; Sakaguchi, N.; Akashi, K. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity 2004, 21, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liu, Y.J. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell 2013, 4, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, P.; Palucka, A.K.; Gill, M.; Pascual, V.; Banchereau, J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science 2001, 294, 1540–1543. [Google Scholar] [CrossRef]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Faget, J.; Sisirak, V.; Blay, J.Y.; Caux, C.; Bendriss-Vermare, N.; Menetrier-Caux, C. ICOS is associated with poor prognosis in breast cancer as it promotes the amplification of immunosuppressive CD4(+) T cells by plasmacytoid dendritic cells. Oncoimmunology 2013, 2, e23185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, C.; Gregorio, J.; Wang, Y.H.; Ito, T.; Meller, S.; Hanabuchi, S.; Anderson, S.; Atkinson, N.; Ramirez, P.T.; Liu, Y.J.; et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012, 72, 5240–5249. [Google Scholar] [CrossRef] [Green Version]
- Le Mercier, I.; Poujol, D.; Sanlaville, A.; Sisirak, V.; Gobert, M.; Durand, I.; Dubois, B.; Treilleux, I.; Marvel, J.; Vlach, J.; et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013, 73, 4629–4640. [Google Scholar] [CrossRef] [Green Version]
- Stary, G.; Bangert, C.; Tauber, M.; Strohal, R.; Kopp, T.; Stingl, G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 2007, 204, 1441–1451. [Google Scholar] [CrossRef]
- Kalb, M.L.; Glaser, A.; Stary, G.; Koszik, F.; Stingl, G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity. J. Immunol. 2012, 188, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Segura, E.; Amigorena, S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013, 34, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, A.; McGovern, N.; Ginhoux, F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin. Cell Dev. Biol. 2015, 41, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Serbina, N.V.; Salazar-Mather, T.P.; Biron, C.A.; Kuziel, W.A.; Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Kraft, S.; Hanau, D.; Bieber, T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J. Investig. Dermatol. 1996, 106, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Mommaas, M.; Oppel, T.; Schottdorf, E.M.; Gunther, S.; Moderer, M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J. Investig. Dermatol. 2002, 118, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Eguiluz-Gracia, I.; Bosco, A.; Dollner, R.; Melum, G.R.; Lexberg, M.H.; Jones, A.C.; Dheyauldeen, S.A.; Holt, P.G.; Baekkevold, E.S.; Jahnsen, F.L. Rapid recruitment of CD14(+) monocytes in experimentally induced allergic rhinitis in human subjects. J. Allergy Clin. Immunol. 2016, 137, 1872–1881.e12. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.C.; Pullman, W.E.; Bennett, G.M.; Sullivan, P.J.; Pavli, P.; Doe, W.F. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J. Gastroenterol. Hepatol. 1995, 10, 387–395. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Lin, K.L.; Yanagita, M.; Charbonneau, C.; Cook, D.N.; Kakiuchi, T.; Gunn, M.D. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 2009, 10, 394–402. [Google Scholar] [CrossRef]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, S.R.; Atif, S.M.; Gibbings, S.L.; Thomas, S.M.; Prabagar, M.G.; Danhorn, T.; Leach, S.M.; Henson, P.M.; Jakubzick, C.V. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Jeon, I.; Kim, B.S.; Kim, I.K.; Park, Y.J.; Koh, C.H.; Song, B.; Lee, J.M.; Lim, J.; Bae, E.A.; et al. Monocyte-Derived Dendritic Cells Dictate the Memory Differentiation of CD8(+) T Cells During Acute Infection. Front. Immunol. 2019, 10, 1887. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Corda, M.G.; Guidotti, A. On a brain polypeptide functioning as a putative effector for the recognition sites of benzodiazepine and beta-carboline derivatives. Neuropharmacology 1983, 22, 1481–1492. [Google Scholar] [CrossRef]
- Mody, N.; Dubey, S.; Sharma, R.; Agrawal, U.; Vyas, S.P. Dendritic cell-based vaccine research against cancer. Expert Rev. Clin. Immunol. 2015, 11, 213–232. [Google Scholar] [CrossRef]
- Osugi, Y.; Vuckovic, S.; Hart, D.N. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood 2002, 100, 2858–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.A.; Coleman, R.E.; Akabani, G.; Niehaus, N.; Coleman, D.; Lyerly, H.K. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999, 59, 56–58. [Google Scholar]
- Chorro, L.; Sarde, A.; Li, M.; Woollard, K.J.; Chambon, P.; Malissen, B.; Kissenpfennig, A.; Barbaroux, J.B.; Groves, R.; Geissmann, F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 2009, 206, 3089–3100. [Google Scholar] [CrossRef]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef] [Green Version]
- Sere, K.; Baek, J.H.; Ober-Blobaum, J.; Muller-Newen, G.; Tacke, F.; Yokota, Y.; Zenke, M.; Hieronymus, T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 2012, 37, 905–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, N.; Clausen, B.E.; Stoitzner, P. Langerhans cells and more: Langerin-expressing dendritic cell subsets in the skin. Immunol. Rev. 2010, 234, 120–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, K.; Ginhoux, F.; Leitner, W.W.; Motegi, S.; Bennett, C.L.; Clausen, B.E.; Merad, M.; Udey, M.C. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl. Acad. Sci. U S A 2009, 106, 3312–3317. [Google Scholar] [CrossRef] [Green Version]
- Matejuk, A. Skin Immunity. Arch. Immunol. Ther. Exp. (Warsz) 2018, 66, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef] [Green Version]
- Igyarto, B.Z.; Haley, K.; Ortner, D.; Bobr, A.; Gerami-Nejad, M.; Edelson, B.T.; Zurawski, S.M.; Malissen, B.; Zurawski, G.; Berman, J.; et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 2011, 35, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Mathers, A.R.; Janelsins, B.M.; Rubin, J.P.; Tkacheva, O.A.; Shufesky, W.J.; Watkins, S.C.; Morelli, A.E.; Larregina, A.T. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J. Immunol. 2009, 182, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Levin, C.; Bonduelle, O.; Nuttens, C.; Primard, C.; Verrier, B.; Boissonnas, A.; Combadiere, B. Critical Role for Skin-Derived Migratory DCs and Langerhans Cells in TFH and GC Responses after Intradermal Immunization. J. Invest. Dermatol. 2017, 137, 1905–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, T.; Kubo, A.; Yokouchi, M.; Adachi, T.; Kobayashi, T.; Kitashima, D.Y.; Fujii, H.; Clausen, B.E.; Koyasu, S.; Amagai, M.; et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 2011, 208, 2607–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, S.; Igyarto, B.Z.; Honda, T.; Egawa, G.; Otsuka, A.; Hara-Chikuma, M.; Watanabe, N.; Ziegler, S.F.; Tomura, M.; Inaba, K.; et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 2012, 129, 1048–1055.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshiki, R.; Kabashima, K.; Sugita, K.; Atarashi, K.; Shimauchi, T.; Tokura, Y. IL-10-producing Langerhans cells and regulatory T cells are responsible for depressed contact hypersensitivity in grafted skin. J. Invest. Dermatol. 2009, 129, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez de Aguero, M.; Vocanson, M.; Hacini-Rachinel, F.; Taillardet, M.; Sparwasser, T.; Kissenpfennig, A.; Malissen, B.; Kaiserlian, D.; Dubois, B. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J. Clin. Investig. 2012, 122, 1700–1711. [Google Scholar] [CrossRef]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Wang, B.; Zaidi, N.; He, L.Z.; Zhang, L.; Kuroiwa, J.M.; Keler, T.; Steinman, R.M. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012, 14, R39. [Google Scholar] [CrossRef]
- Idoyaga, J.; Lubkin, A.; Fiorese, C.; Lahoud, M.H.; Caminschi, I.; Huang, Y.; Rodriguez, A.; Clausen, B.E.; Park, C.G.; Trumpfheller, C.; et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc. Natl. Acad. Sci. USA 2011, 108, 2384–2389. [Google Scholar] [CrossRef] [Green Version]
- Charalambous, A.; Oks, M.; Nchinda, G.; Yamazaki, S.; Steinman, R.M. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J. Immunol. 2006, 177, 8410–8421. [Google Scholar] [CrossRef] [Green Version]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Sancho, D.; Mourao-Sa, D.; Joffre, O.P.; Schulz, O.; Rogers, N.C.; Pennington, D.J.; Carlyle, J.R.; Reis e Sousa, C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J. Clin. Invest. 2008, 118, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Tullett, K.M.; Leal Rojas, I.M.; Minoda, Y.; Tan, P.S.; Zhang, J.G.; Smith, C.; Khanna, R.; Shortman, K.; Caminschi, I.; Lahoud, M.H.; et al. Targeting CLEC9A delivers antigen to human CD141(+) DC for CD4(+) and CD8(+)T cell recognition. JCI Insight 2016, 1, e87102. [Google Scholar] [CrossRef] [PubMed]
- Masterman, K.A.; Haigh, O.L.; Tullett, K.M.; Leal-Rojas, I.M.; Walpole, C.; Pearson, F.E.; Cebon, J.; Schmidt, C.; O’Brien, L.; Rosendahl, N.; et al. Human CLEC9A antibodies deliver NY-ESO-1 antigen to CD141(+) dendritic cells to activate naive and memory NY-ESO-1-specific CD8(+) T cells. J. Immunother. Cancer 2020, 8, e000691. [Google Scholar] [CrossRef] [PubMed]
- Pearson, F.E.; Tullett, K.M.; Leal-Rojas, I.M.; Haigh, O.L.; Masterman, K.A.; Walpole, C.; Bridgeman, J.S.; McLaren, J.E.; Ladell, K.; Miners, K.; et al. Human CLEC9A antibodies deliver Wilms’ tumor 1 (WT1) antigen to CD141(+) dendritic cells to activate naive and memory WT1-specific CD8(+) T cells. Clin. Transl. Immunol. 2020, 9, e1141. [Google Scholar] [CrossRef]
- Joffre, O.P.; Sancho, D.; Zelenay, S.; Keller, A.M.; Reis e Sousa, C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur. J. Immunol. 2010, 40, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ahmet, F.; Sullivan, L.C.; Brooks, A.G.; Kent, S.J.; De Rose, R.; Salazar, A.M.; Reis e Sousa, C.; Shortman, K.; Lahoud, M.H.; et al. Antibodies targeting Clec9A promote strong humoral immunity without adjuvant in mice and non-human primates. Eur. J. Immunol. 2015, 45, 854–864. [Google Scholar] [CrossRef]
- Caminschi, I.; Proietto, A.I.; Ahmet, F.; Kitsoulis, S.; Shin Teh, J.; Lo, J.C.; Rizzitelli, A.; Wu, L.; Vremec, D.; van Dommelen, S.L.; et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 2008, 112, 3264–3273. [Google Scholar] [CrossRef]
- Mittal, D.; Vijayan, D.; Putz, E.M.; Aguilera, A.R.; Markey, K.A.; Straube, J.; Kazakoff, S.; Nutt, S.L.; Takeda, K.; Hill, G.R.; et al. Interleukin-12 from CD103(+) Batf3-Dependent Dendritic Cells Required for NK-Cell Suppression of Metastasis. Cancer Immunol. Res. 2018, 5, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, Y.O.; Ghilas, S.; Sanchez, C.; Le Bon, A.; Crozat, K.; Dalod, M. XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J. Exp. Med. 2016, 213, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.L.; Berk, E.; Edwards, R.P.; Kalinski, P. IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res. 2013, 73, 4653–4662. [Google Scholar] [CrossRef] [Green Version]
- Vu Manh, T.P.; Elhmouzi-Younes, J.; Urien, C.; Ruscanu, S.; Jouneau, L.; Bourge, M.; Moroldo, M.; Foucras, G.; Salmon, H.; Marty, H.; et al. Defining Mononuclear Phagocyte Subset Homology Across Several Distant Warm-Blooded Vertebrates Through Comparative Transcriptomics. Front. Immunol. 2015, 6, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartung, E.; Becker, M.; Bachem, A.; Reeg, N.; Jakel, A.; Hutloff, A.; Weber, H.; Weise, C.; Giesecke, C.; Henn, V.; et al. Induction of potent CD8 T cell cytotoxicity by specific targeting of antigen to cross-presenting dendritic cells in vivo via murine or human XCR1. J. Immunol. 2015, 194, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, Y.; Hemmi, H.; Katsuda, M.; Miyazawa, M.; Kitahata, Y.; Miyamoto, A.; Nakamori, M.; Ojima, T.; Matsuda, K.; Nakamura, M.; et al. Anticancer effects of chemokine-directed antigen delivery to a cross-presenting dendritic cell subset with immune checkpoint blockade. Br. J. Cancer 2020, 122, 1185–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.K.; Wall, K.A. Use of Dendritic Cell Receptors as Targets for Enhancing Anti-Cancer Immune Responses. Cancers (Basel) 2019, 11, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Swiggard, W.J.; Heufler, C.; Peng, M.; Mirza, A.; Steinman, R.M.; Nussenzweig, M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 1995, 375, 151–155. [Google Scholar] [CrossRef]
- Shrimpton, R.E.; Butler, M.; Morel, A.S.; Eren, E.; Hue, S.S.; Ritter, M.A. CD205 (DEC-205): A recognition receptor for apoptotic and necrotic self. Mol. Immunol. 2009, 46, 1229–1239. [Google Scholar] [CrossRef]
- Guo, M.; Gong, S.; Maric, S.; Misulovin, Z.; Pack, M.; Mahnke, K.; Nussenzweig, M.C.; Steinman, R.M. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum. Immunol. 2000, 61, 729–738. [Google Scholar] [CrossRef]
- Kato, M.; Neil, T.K.; Fearnley, D.B.; McLellan, A.D.; Vuckovic, S.; Hart, D.N. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int. Immunol. 2000, 12, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Inaba, K.; Swiggard, W.J.; Inaba, M.; Meltzer, J.; Mirza, A.; Sasagawa, T.; Nussenzweig, M.C.; Steinman, R.M. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell. Immunol. 1995, 163, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sancho, D.; Joffre, O.P.; Keller, A.M.; Rogers, N.C.; Martinez, D.; Hernanz-Falcon, P.; Rosewell, I.; Reis e Sousa, C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009, 458, 899–903. [Google Scholar] [CrossRef]
- Ahrens, S.; Zelenay, S.; Sancho, D.; Hanc, P.; Kjaer, S.; Feest, C.; Fletcher, G.; Durkin, C.; Postigo, A.; Skehel, M.; et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 2012, 36, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.G.; Czabotar, P.E.; Policheni, A.N.; Caminschi, I.; Wan, S.S.; Kitsoulis, S.; Tullett, K.M.; Robin, A.Y.; Brammananth, R.; van Delft, M.F.; et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 2012, 36, 646–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullett, K.M.; Lahoud, M.H.; Radford, K.J. Harnessing Human Cross-Presenting CLEC9A(+)XCR1(+) Dendritic Cells for Immunotherapy. Front. Immunol. 2014, 5, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iborra, S.; Izquierdo, H.M.; Martinez-Lopez, M.; Blanco-Menendez, N.; Reis e Sousa, C.; Sancho, D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J. Clin. Investig. 2012, 122, 1628–1643. [Google Scholar] [CrossRef] [Green Version]
- Murray, D.P.; Watson, R.D.; Zezulka, A.V.; Murray, R.G.; Littler, W.A. Plasma catecholamine levels in acute myocardial infarction: Influence of beta-adrenergic blockade and relation to central hemodynamics. Am. Heart J. 1988, 115, 38–44. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2013, 66, 1–79. [Google Scholar]
- Yoshie, O.; Imai, T.; Nomiyama, H. Chemokines in immunity. Adv. Immunol. 2001, 78, 57–110. [Google Scholar]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Dieu, M.C.; Vanbervliet, B.; Vicari, A.; Bridon, J.M.; Oldham, E.; Ait-Yahia, S.; Briere, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.J.; Esserman, L.; Byars, N.E.; Allison, A.C.; Levy, R. Idiotype vaccination against murine B cell lymphoma. Humoral and cellular requirements for the full expression of antitumor immunity. J. Immunol. 1990, 145, 1029–1036. [Google Scholar] [PubMed]
- Bosteels, C.; Fierens, K.; De Prijck, S.; Van Moorleghem, J.; Vanheerswynghels, M.; De Wolf, C.; Chalon, A.; Collignon, C.; Hammad, H.; Didierlaurent, A.M.; et al. CCR2- and Flt3-Dependent Inflammatory Conventional Type 2 Dendritic Cells Are Necessary for the Induction of Adaptive Immunity by the Human Vaccine Adjuvant System AS01. Front. Immunol. 2021, 11, 606805. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Vulcano, M.; Roncari, A.; Facchetti, F.; Sozzani, S.; Adorini, L. Cutting edge: Differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 2002, 169, 6673–6676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, A.; Uppaluri, R.; Facchetti, F.; Dorner, B.G.; Sheehan, K.C.; Schreiber, R.D.; Cella, M.; Colonna, M. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J. Immunol. 2002, 169, 6079–6083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megjugorac, N.J.; Young, H.A.; Amrute, S.B.; Olshalsky, S.L.; Fitzgerald-Bocarsly, P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 2004, 75, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, K.V.; Lew, A.M.; Sutherland, R.M.; Zhan, Y. Monocyte-Derived Dendritic Cells Promote Th Polarization, whereas Conventional Dendritic Cells Promote Th Proliferation. J. Immunol. 2016, 196, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Sutti, S.; Bruzzi, S.; Heymann, F.; Liepelt, A.; Krenkel, O.; Toscani, A.; Ramavath, N.N.; Cotella, D.; Albano, E.; Tacke, F. CX3CR1 Mediates the Development of Monocyte-Derived Dendritic Cells during Hepatic Inflammation. Cells 2019, 8, 1099. [Google Scholar] [CrossRef] [Green Version]
- Biragyn, A.; Tani, K.; Grimm, M.C.; Weeks, S.; Kwak, L.W. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat. Biotechnol. 1999, 17, 253–258. [Google Scholar] [CrossRef]
- Biragyn, A.; Surenhu, M.; Yang, D.; Ruffini, P.A.; Haines, B.A.; Klyushnenkova, E.; Oppenheim, J.J.; Kwak, L.W. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J. Immunol. 2001, 167, 6644–6653. [Google Scholar] [CrossRef] [Green Version]
- Toumbis, M.; Zervas, J.; Anagnostopoulou, O.; Konstantopoulos, K.; Krimbeni, G.; Kotsovoulou, V.; Fessas, F. HLA antigens and bronchogenic carcinoma in the Greek population. Acta Oncol. 1991, 30, 575–578. [Google Scholar] [CrossRef]
- Kelner, G.S.; Kennedy, J.; Bacon, K.B.; Kleyensteuber, S.; Largaespada, D.A.; Jenkins, N.A.; Copeland, N.G.; Bazan, J.F.; Moore, K.W.; Schall, T.J.; et al. Lymphotactin: A cytokine that represents a new class of chemokine. Science 1994, 266, 1395–1399. [Google Scholar] [CrossRef]
- Yoshida, T.; Imai, T.; Kakizaki, M.; Nishimura, M.; Yoshie, O. Molecular cloning of a novel C or gamma type chemokine, SCM-1. FEBS Lett. 1995, 360, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, J.A.; Saylor, V.; Figueroa, D.; Mizoue, L.; Xu, Y.; Menon, S.; Abrams, J.; Handel, T.; Zlotnik, A. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J. Immunol. 1997, 158, 1533–1540. [Google Scholar]
- Kennedy, J.; Kelner, G.S.; Kleyensteuber, S.; Schall, T.J.; Weiss, M.C.; Yssel, H.; Schneider, P.V.; Cocks, B.G.; Bacon, K.B.; Zlotnik, A. Molecular cloning and functional characterization of human lymphotactin. J. Immunol. 1995, 155, 203–209. [Google Scholar] [PubMed]
- Yoshida, T.; Imai, T.; Kakizaki, M.; Nishimura, M.; Takagi, S.; Yoshie, O. Identification of single C motif-1/lymphotactin receptor XCR1. J. Biol. Chem. 1998, 273, 16551–16554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Izawa, D.; Nakayama, T.; Nakahara, K.; Kakizaki, M.; Imai, T.; Suzuki, R.; Miyasaka, M.; Yoshie, O. Molecular cloning of mXCR1, the murine SCM-1/lymphotactin receptor. FEBS Lett. 1999, 458, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Crozat, K.; Tamoutounour, S.; Vu Manh, T.P.; Fossum, E.; Luche, H.; Ardouin, L.; Guilliams, M.; Azukizawa, H.; Bogen, B.; Malissen, B.; et al. Cutting edge: Expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8alpha+ type. J. Immunol. 2011, 187, 4411–4415. [Google Scholar] [CrossRef] [Green Version]
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef] [Green Version]
- Bodder, J.; Zahan, T.; van Slooten, R.; Schreibelt, G.; de Vries, I.J.M.; Florez-Grau, G. Harnessing the cDC1-NK Cross-Talk in the Tumor Microenvironment to Battle Cancer. Front. Immunol. 2021, 11, 631713. [Google Scholar] [CrossRef] [PubMed]
- Fossum, E.; Grodeland, G.; Terhorst, D.; Tveita, A.A.; Vikse, E.; Mjaaland, S.; Henri, S.; Malissen, B.; Bogen, B. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur. J. Immunol. 2015, 45, 624–635. [Google Scholar] [CrossRef]
- Terhorst, D.; Fossum, E.; Baranska, A.; Tamoutounour, S.; Malosse, C.; Garbani, M.; Braun, R.; Lechat, E.; Crameri, R.; Bogen, B.; et al. Laser-assisted intradermal delivery of adjuvant-free vaccines targeting XCR1+ dendritic cells induces potent antitumoral responses. J. Immunol. 2015, 194, 5895–5902. [Google Scholar] [CrossRef] [Green Version]
- Lysen, A.; Braathen, R.; Gudjonsson, A.; Tesfaye, D.Y.; Bogen, B.; Fossum, E. Dendritic cell targeted Ccl3- and Xcl1-fusion DNA vaccines differ in induced immune responses and optimal delivery site. Sci. Rep. 2019, 9, 1820. [Google Scholar] [CrossRef]
- Matsuo, K.; Kitahata, K.; Kawabata, F.; Kamei, M.; Hara, Y.; Takamura, S.; Oiso, N.; Kawada, A.; Yoshie, O.; Nakayama, T. A Highly Active Form of XCL1/Lymphotactin Functions as an Effective Adjuvant to Recruit Cross-Presenting Dendritic Cells for Induction of Effector and Memory CD8(+) T Cells. Front. Immunol. 2018, 9, 2775. [Google Scholar] [CrossRef]
- Tuinstra, R.L.; Peterson, F.C.; Kutlesa, S.; Elgin, E.S.; Kron, M.A.; Volkman, B.F. Interconversion between two unrelated protein folds in the lymphotactin native state. Proc. Natl. Acad. Sci. USA 2008, 105, 5057–5062. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.C.; Nakayama, T.; Tyler, R.C.; Sander, T.L.; Yoshie, O.; Volkman, B.F. Structural and agonist properties of XCL2, the other member of the C-chemokine subfamily. Cytokine 2015, 71, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.C.; Thomas, M.A.; Dishman, A.F.; Larsen, O.; Nakayama, T.; Yoshie, O.; Rosenkilde, M.M.; Volkman, B.F. Structure-function guided modeling of chemokine-GPCR specificity for the chemokine XCL1 and its receptor XCR1. Sci. Signal. 2019, 12, eaat4128. [Google Scholar] [CrossRef]
- Kamei, M.; Matsuo, K.; Imanishi, H.; Hara, Y.; Quen, Y.S.; Kamiyama, F.; Oiso, N.; Kawada, A.; Okada, N.; Nakayama, T. Transcutaneous immunization with a highly active form of XCL1 as a vaccine adjuvant using a hydrophilic gel patch elicits long-term CD8(+) T cell responses. J. Pharmacol. Sci. 2020, 143, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Roussel, H.; Diniz, M.O.; Karaki, S.; Tran, T.; Voron, T.; Dransart, E.; Sandoval, F.; Riquet, M.; Rance, B.; et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 2017, 8, 15221. [Google Scholar] [CrossRef]
- Klebanoff, C.A.; Gattinoni, L.; Torabi-Parizi, P.; Kerstann, K.; Cardones, A.R.; Finkelstein, S.E.; Palmer, D.C.; Antony, P.A.; Hwang, S.T.; Rosenberg, S.A.; et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA 2005, 102, 9571–9576. [Google Scholar] [CrossRef] [Green Version]
- Manjarrez-Orduno, N.; Menard, L.C.; Kansal, S.; Fischer, P.; Kakrecha, B.; Jiang, C.; Cunningham, M.; Greenawalt, D.; Patel, V.; Yang, M.; et al. Circulating T Cell Subpopulations Correlate With Immune Responses at the Tumor Site and Clinical Response to PD1 Inhibition in Non-Small Cell Lung Cancer. Front. Immunol. 2018, 9, 1613. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Joshi, N.S.; Cui, W.; Chandele, A.; Lee, H.K.; Urso, D.R.; Hagman, J.; Gapin, L.; Kaech, S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 2007, 27, 281–295. [Google Scholar] [CrossRef] [Green Version]
- Pipkin, M.E.; Sacks, J.A.; Cruz-Guilloty, F.; Lichtenheld, M.G.; Bevan, M.J.; Rao, A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 2010, 32, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.A.; Tyznik, A.J.; Bevan, M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006, 441, 890–893. [Google Scholar] [CrossRef]
- Hermans, I.F.; Ritchie, D.S.; Yang, J.; Roberts, J.M.; Ronchese, F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J. Immunol. 2000, 164, 3095–3101. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Huck, S.P.; McHugh, R.S.; Hermans, I.F.; Ronchese, F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Glenn, G.M.; O’Hagan, D.T. Adjuvants: Progress, regress and pandemic preparedness. Expert Rev. Vaccines 2007, 6, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Ishii, K.J.; Akira, S. Toll or toll-free adjuvant path toward the optimal vaccine development. J. Clin. Immunol. 2007, 27, 363–371. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef] [Green Version]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Mastelic-Gavillet, B.; Balint, K.; Boudousquie, C.; Gannon, P.O.; Kandalaft, L.E. Personalized Dendritic Cell Vaccines-Recent Breakthroughs and Encouraging Clinical Results. Front. Immunol. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldin, A.V.; Savvateeva, L.V.; Bazhin, A.V.; Zamyatnin, A.A., Jr. Dendritic Cells in Anticancer Vaccination: Rationale for Ex Vivo Loading or In Vivo Targeting. Cancers (Basel) 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Bobanga, I.D.; Petrosiute, A.; Huang, A.Y. Chemokines as Cancer Vaccine Adjuvants. Vaccines (Basel) 2013, 1, 444–462. [Google Scholar] [CrossRef] [Green Version]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Rice, A.E.; Latchman, Y.E.; Balint, J.P.; Lee, J.H.; Gabitzsch, E.S.; Jones, F.R. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther. 2015, 22, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Antonios, J.P.; Soto, H.; Everson, R.G.; Orpilla, J.; Moughon, D.; Shin, N.; Sedighim, S.; Yong, W.H.; Li, G.; Cloughesy, T.F.; et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight 2016, 1, e87059. [Google Scholar] [CrossRef] [Green Version]
- Curran, M.A.; Glisson, B.S. New Hope for Therapeutic Cancer Vaccines in the Era of Immune Checkpoint Modulation. Annu. Rev. Med. 2019, 70, 409–424. [Google Scholar] [CrossRef]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A., Jr.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; Kudchadkar, R.R.; Yu, B.; Gallenstein, D.; Horak, C.E.; Inzunza, H.D.; Zhao, X.; Martinez, A.J.; Wang, W.; Gibney, G.; et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013, 31, 4311–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibney, G.T.; Kudchadkar, R.R.; DeConti, R.C.; Thebeau, M.S.; Czupryn, M.P.; Tetteh, L.; Eysmans, C.; Richards, A.; Schell, M.J.; Fisher, K.J.; et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin. Cancer Res. 2015, 21, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Mougel, A.; Terme, M.; Tanchot, C. Therapeutic Cancer Vaccine and Combinations With Antiangiogenic Therapies and Immune Checkpoint Blockade. Front. Immunol. 2019, 10, 467. [Google Scholar] [CrossRef] [Green Version]
| Target Molecule | Type | Expressing Cell | Function | Application (Ref) |
|---|---|---|---|---|
| DEC-205 | C-type lectin receptor | cDC1s, cDC2s, B cells, T cells, NK cells | Antigen recognition (apoptotic and necrotic cells) | [106,107,108,109,110] |
| CLEC9A | C-type lectin receptor | cDC1s, some pDCs, monocytes | Antigen recognition/endocytosis (cross-presentation) | [111,112,113,114,115,116,117] |
| XCR1 | Chemokine receptor | cDC1s | Cell migration | [118,119,120,121,122,123] |
| Chemokine | Chemokine Receptor | Immune Response | Ref. |
|---|---|---|---|
| CCL7 | CCR1, CCR2, CCR3 | Antigen-specific IgG response Antitumor effect | [149,150] |
| CCL20 | CCR6 | Antigen-specific IgG response Antitumor effect | [149,150] |
| CCL21 | CCR7 | Antigen-specific IgG response | [150] |
| XCL1 | XCR1 | CTL response, Antitumor effect Antiviral effect | [118,119,120,121,122,123] |
| Type | Immunization Method | Immune Response | Ref |
|---|---|---|---|
| Targeting cDC1 | |||
| Antigen-XCL1 fusion protein | Injection | CTL responses combined with LPS | [118] |
| Antigen-conjugated anti-XCR1 antibody | Injection | CTL responses combined with LPS | [118] |
| Antigen peptide-XCL1 fusion protein | Injection | CTL responses, antitumor immunity, enhance CTL responses with anti-PD1 | [119] |
| Antigen-XCL1 dimeric fusion protein | DNA vaccine | CTL responses, Influenza virus protection | [120,122] |
| Laser-assisted intradermal delivery | CD4+/CD8+ T-cell responses, antitumor immunity | [121] | |
| Recruiting cDC1 | |||
| Mixture of antigen and a highly active form of XCL1 | Injection | Effector and memory CTL responses, antitumor immunity | [123] |
| Transcutaneous device delivery | Enhance memory CTL responses, antitumor immunity | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, K.; Yoshie, O.; Kitahata, K.; Kamei, M.; Hara, Y.; Nakayama, T. Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers 2021, 13, 2495. https://doi.org/10.3390/cancers13102495
Matsuo K, Yoshie O, Kitahata K, Kamei M, Hara Y, Nakayama T. Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers. 2021; 13(10):2495. https://doi.org/10.3390/cancers13102495
Chicago/Turabian StyleMatsuo, Kazuhiko, Osamu Yoshie, Kosuke Kitahata, Momo Kamei, Yuta Hara, and Takashi Nakayama. 2021. "Recent Progress in Dendritic Cell-Based Cancer Immunotherapy" Cancers 13, no. 10: 2495. https://doi.org/10.3390/cancers13102495
APA StyleMatsuo, K., Yoshie, O., Kitahata, K., Kamei, M., Hara, Y., & Nakayama, T. (2021). Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers, 13(10), 2495. https://doi.org/10.3390/cancers13102495






