The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Prognostic Impact of PPIC-Positive CTCs at Baseline
2.3. Prognostic Impact of PPIC-Positive CTCs at Follow-Up
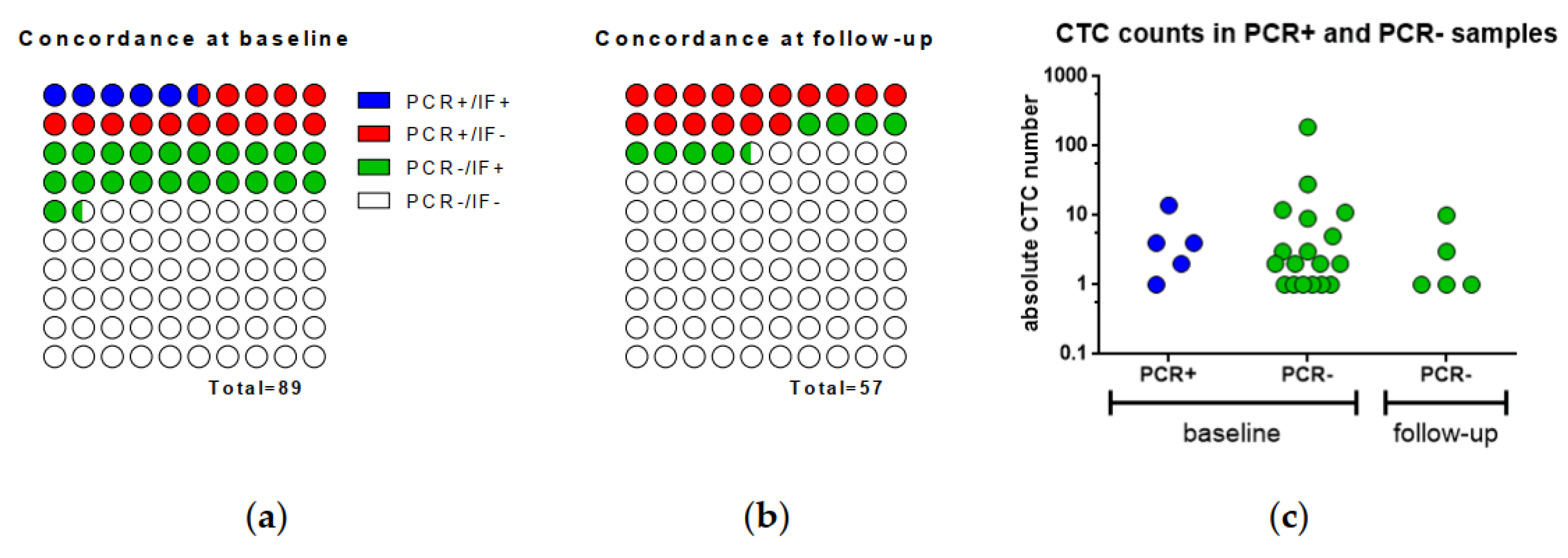
2.4. Concordance of qPCR and IF in CTCs
2.5. CTCcombo at Baseline and Follow-Up
2.6. PPIC in the Tumor Tissue
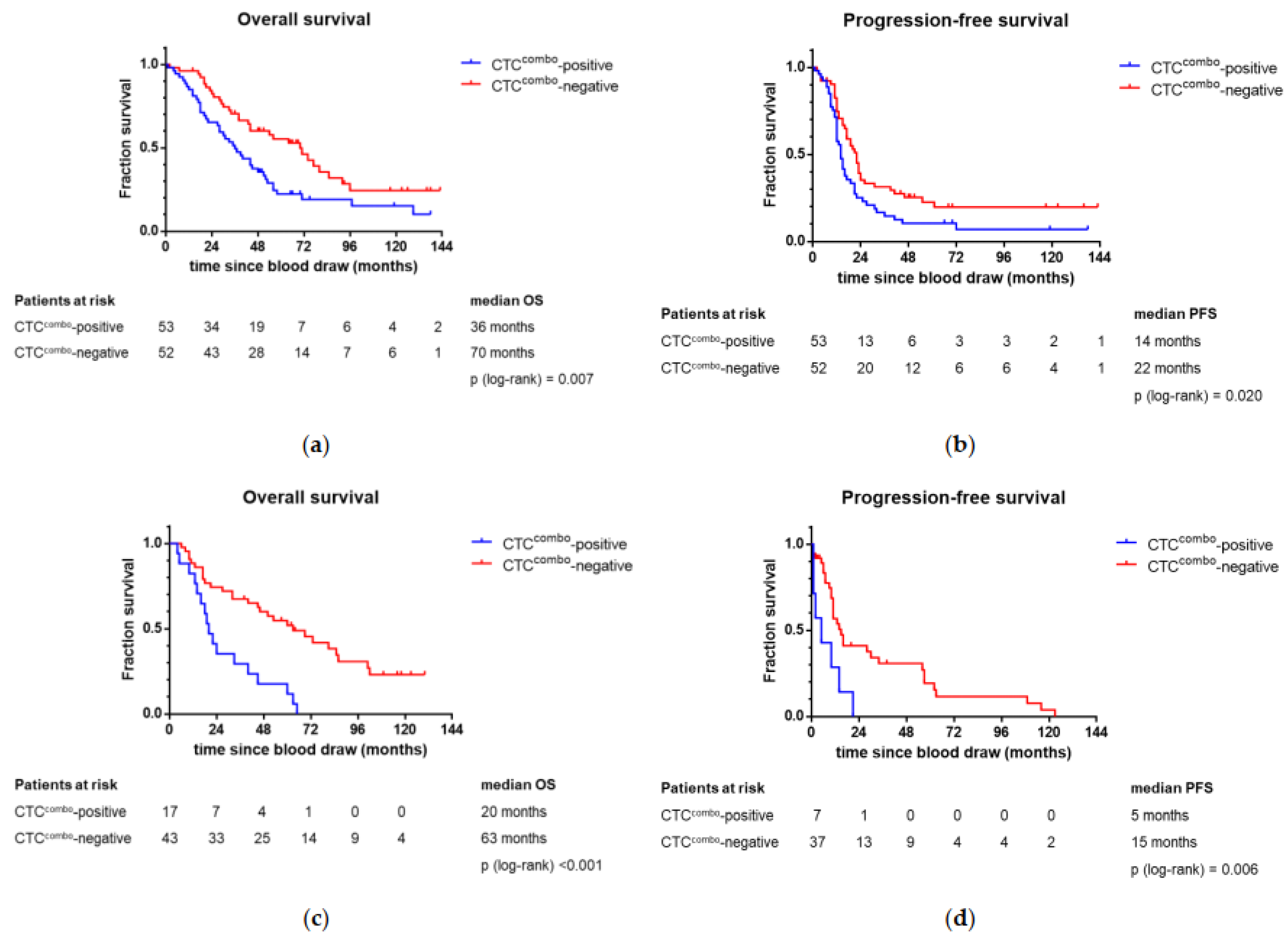
2.7. Association of CTCcombo and Patient Outcome
2.8. Prognostic Factors of Long-Term Survival
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. CTC Analysis
4.3. A Complementary CTC Approach by Combining IF and qPCR Results
4.4. PPIC Protein Expression in the Tumor Tissue
4.5. PPIC Gene Expression in the Tumor Tissue
4.6. Statistics
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999-2007 by country and age: Results of EUROCARE—5-a population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Baldwin, L.A.; Huang, B.; Miller, R.W.; Tucker, T.; Goodrich, S.T.; Podzielinski, I.; DeSimone, C.P.; Ueland, F.R.; van Nagell, J.R.; Seamon, L.G. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012, 120, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Hoppenot, C.; Eckert, M.A.; Tienda, S.M.; Lengyel, E. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol. Oncol. 2018, 148, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, L.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: Recent advances on circulating tumor cells and circulating tumor DNA. Clin. Chem. Lab. Med. 2018, 56, 186–197. [Google Scholar] [CrossRef]
- Obermayr, E.; Bednarz-Knoll, N.; Orsetti, B.; Weier, H.U.; Lambrechts, S.; Castillo-Tong, D.C.; Reinthaller, A.; Braicu, E.I.; Mahner, S.; Sehouli, J.; et al. Circulating tumor cells: Potential markers of minimal residual disease in ovarian cancer? A study of the OVCAD consortium. Oncotarget 2017, 8, 106415–106428. [Google Scholar] [CrossRef]
- Pradeep, S.; Kim, S.W.; Wu, S.Y.; Nishimura, M.; Chaluvally-Raghavan, P.; Miyake, T.; Pecot, C.V.; Kim, S.J.; Choi, H.J.; Bischoff, F.Z.; et al. Hematogenous metastasis of ovarian cancer: Rethinking mode of spread. Cancer Cell 2014, 26, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Coffman, L.G.; Burgos-Ojeda, D.; Wu, R.; Cho, K.; Bai, S.; Buckanovich, R.J. New models of hematogenous ovarian cancer metastasis demonstrate preferential spread to the ovary and a requirement for the ovary for abdominal dissemination. Transl. Res. 2016, 175, 92–102.E2. [Google Scholar] [CrossRef] [Green Version]
- Chekerov, R.; Braicu, I.; Castillo-Tong, D.C.; Richter, R.; Cadron, I.; Mahner, S.; Woelber, L.; Marth, C.; Van Gorp, T.; Speiser, P.; et al. Outcome and Clinical Management of 275 Patients With Advanced Ovarian Cancer International Federation of Obstetrics and Gynecology II to IV Inside the European Ovarian Cancer Translational Research Consortium-OVCAD. Int. J. Gynecol. Cancer 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Obermayr, E.; Castillo-Tong, D.C.; Pils, D.; Speiser, P.; Braicu, I.; Van Gorp, T.; Mahner, S.; Sehouli, J.; Vergote, I.; Zeillinger, R. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance—A study of the OVCAD consortium. Gynecol. Oncol. 2013, 128, 15–21. [Google Scholar] [CrossRef]
- Blassl, C.; Kuhlmann, J.D.; Webers, A.; Wimberger, P.; Fehm, T.; Neubauer, H. Gene expression profiling of single circulating tumor cells in ovarian cancer—Establishment of a multi-marker gene panel. Mol. Oncol. 2016, 10, 1030–1042. [Google Scholar] [CrossRef] [Green Version]
- Chebouti, I.; Kasimir-Bauer, S.; Buderath, P.; Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017, 8, 48820–48831. [Google Scholar] [CrossRef] [Green Version]
- Mostert, B.; Sieuwerts, A.M.; Bolt-de Vries, J.; Kraan, J.; Lalmahomed, Z.; van Galen, A.; van der Spoel, P.; de Weerd, V.; Ramirez-Moreno, R.; Smid, M.; et al. mRNA expression profiles in circulating tumor cells of metastatic colorectal cancer patients. Mol. Oncol. 2015, 9, 920–932. [Google Scholar] [CrossRef] [Green Version]
- Tibbe, A.G.; Miller, M.C.; Terstappen, L.W. Statistical considerations for enumeration of circulating tumor cells. Cytom. A 2007, 71, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.M.; Slade, M.J.; English, J.; Graham, H.; Luchtenborg, M.; Sinnett, H.D.; Cross, N.C.; Coombes, R.C. Response of circulating tumor cells to systemic therapy in patients with metastatic breast cancer: Comparison of quantitative polymerase chain reaction and immunocytochemical techniques. J. Clin. Oncol. 2000, 18, 1432–1439. [Google Scholar] [CrossRef]
- Gilje, B.; Nordgard, O.; Tjensvoll, K.; Borgen, E.; Synnestvedt, M.; Smaaland, R.; Naume, B. Comparison of molecular and immunocytochemical methods for detection of disseminated tumor cells in bone marrow from early breast cancer patients. BMC Cancer 2014, 14, 514. [Google Scholar] [CrossRef] [Green Version]
- Strati, A.; Kasimir-Bauer, S.; Markou, A.; Parisi, C.; Lianidou, E.S. Comparison of three molecular assays for the detection and molecular characterization of circulating tumor cells in breast cancer. Breast Cancer Res. 2013, 15, R20. [Google Scholar] [CrossRef] [Green Version]
- Van der Auwera, I.; Peeters, D.; Benoy, I.H.; Elst, H.J.; Van Laere, S.J.; Prove, A.; Maes, H.; Huget, P.; van Dam, P.; Vermeulen, P.B.; et al. Circulating tumour cell detection: A direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br. J. Cancer 2010, 102, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Markou, A.; Lazaridou, M.; Paraskevopoulos, P.; Chen, S.; Swierczewska, M.; Budna, J.; Kuske, A.; Gorges, T.M.; Joosse, S.A.; Kroneis, T.; et al. Multiplex Gene Expression Profiling of In Vivo Isolated Circulating Tumor Cells in High-Risk Prostate Cancer Patients. Clin. Chem. 2018, 64, 297–306. [Google Scholar] [CrossRef]
- Alpers, I. Detektion und Charakterisierung von zirkulierenden Tumorzellen im peripheren Blut von Patientinnen mit Ovarialkarzinom (Detection and Characterization of Circulating Tumor Cells in the Peripheral Blood of Ovarian Cancer Patients). Ph.D. Thesis, University Hamburg, Hamburg, Germany, 2012. [Google Scholar]
- Hu, X.; Zang, X.; Lv, Y. Detection of circulating tumor cells: Advances and critical concerns. Oncol. Lett. 2021, 21, 422. [Google Scholar] [CrossRef]
- Obermayr, E.; Agreiter, C.; Schuster, E.; Fabikan, H.; Weinlinger, C.; Baluchova, K.; Hamilton, G.; Hochmair, M.; Zeillinger, R. Molecular Characterization of Circulating Tumor Cells Enriched by A Microfluidic Platform in Patients with Small-Cell Lung Cancer. Cells 2019, 8, 880. [Google Scholar] [CrossRef] [Green Version]
- Obermayr, E.; Maritschnegg, E.; Agreiter, C.; Pecha, N.; Speiser, P.; Helmy-Bader, S.; Danzinger, S.; Krainer, M.; Singer, C.; Zeillinger, R. Efficient leukocyte depletion by a novel microfluidic platform enables the molecular detection and characterization of circulating tumor cells. Oncotarget 2018, 9, 812–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadagni, S.; Clementi, M.; Masedu, F.; Fiorentini, G.; Sarti, D.; Deraco, M.; Kusamura, S.; Papasotiriou, I.; Apostolou, P.; Aigner, K.R.; et al. A Pilot Study of the Predictive Potential of Chemosensitivity and Gene Expression Assays Using Circulating Tumour Cells from Patients with Recurrent Ovarian Cancer. Int. J. Mol. Sci 2020, 21, 4813. [Google Scholar] [CrossRef] [PubMed]
- Doyle, V.; Virji, S.; Crompton, M. Evidence that cyclophilin-A protects cells against oxidative stress. BioChem. J. 1999, 341 Pt 1, 127–132. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhu, T.; Mao, C.X.; Liu, Z.X.; Wang, Z.B.; Mao, X.Y.; Li, L.; Yin, J.Y.; Zhou, H.H.; Liu, Z.Q. PPIC, EMP3 and CHI3L1 Are Novel Prognostic Markers for High Grade Glioma. Int. J. Mol. Sci 2016, 17, 1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlan, B.Y.; Dering, J.; Walsh, C.; Orsulic, S.; Lester, J.; Anderson, L.A.; Ginther, C.L.; Fejzo, M.; Slamon, D. POSTN/TGFBI-associated stromal signature predicts poor prognosis in serous epithelial ovarian cancer. Gynecol. Oncol. 2014, 132, 334–342. [Google Scholar] [CrossRef]
- Obermayr, E.; Sanchez-Cabo, F.; Tea, M.K.; Singer, C.F.; Krainer, M.; Fischer, M.B.; Sehouli, J.; Reinthaller, A.; Horvat, R.; Heinze, G.; et al. Assessment of a six gene panel for the molecular detection of circulating tumor cells in the blood of female cancer patients. BMC Cancer 2010, 10, 666. [Google Scholar] [CrossRef] [Green Version]
- Office for National Statistics (ONS). Cancer Survival in England: Patients Diagnosed 2005–2009 and Followed Up to 2010; Office for National Statistics (ONS): London, UK, 2011.
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Kotlum, J.E.; Olafsdottir, E.; Pukkala, E.; Storm, H.H. NORDCAN--a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef]
- Cress, R.D.; Chen, Y.S.; Morris, C.R.; Petersen, M.; Leiserowitz, G.S. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet. Gynecol. 2015, 126, 491–497. [Google Scholar] [CrossRef]
- Clarke, C.L.; Kushi, L.H.; Chubak, J.; Pawloski, P.A.; Bulkley, J.E.; Epstein, M.M.; Burnett-Hartman, A.N.; Powell, B.; Pearce, C.L.; Spencer Feigelson, H. Predictors of Long-Term Survival among High-Grade Serous Ovarian Cancer Patients. Cancer Epidemiol. Biomark. Prev 2019, 28, 996–999. [Google Scholar] [CrossRef] [Green Version]
- Fabbro, M.; Colombo, P.E.; Leaha, C.M.; Rouanet, P.; Carrere, S.; Quenet, F.; Gutowski, M.; Mourregot, A.; D’Hondt, V.; Coupier, I.; et al. Conditional Probability of Survival and Prognostic Factors in Long-Term Survivors of High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 2184. [Google Scholar] [CrossRef]
- Baum, J.; Braicu, E.I.; Hunsicker, O.; Vergote, I.; Concin, N.; Van Nieuwenhuysen, E.; Feldheiser, A.; Achimas-Cadariu, P.; Darb-Esfahani, S.; Berger, A.; et al. Impact of clinical factors and surgical outcome on long-term survival in high-grade serous ovarian cancer: A multicenter analysis. Int. J. Gynecol. Cancer 2021. [Google Scholar] [CrossRef]
- Brandt, B.; Griwatz, C. Two-layer buoyant density centrifugation gradient for enrichment of prostate-derived cells and cell clusters from peripheral blood. Clin. Chem. 1996, 42, 1881–1882. [Google Scholar] [CrossRef]
- Bachmayr-Heyda, A.; Aust, S.; Heinze, G.; Polterauer, S.; Grimm, C.; Braicu, E.I.; Sehouli, J.; Lambrechts, S.; Vergote, I.; Mahner, S.; et al. Prognostic impact of tumor infiltrating CD8+T cells in association with cell proliferation in ovarian cancer patients—A study of the OVCAD consortium. BMC Cancer 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Chui, X.; Egami, H.; Yamashita, J.; Kurizaki, T.; Ohmachi, H.; Yamamoto, S.; Ogawa, M. Immunohistochemical expression of the c-kit proto-oncogene product in human malignant and non-malignant breast tissues. Br. J. Cancer 1996, 73, 1233–1236. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, K.; Gluba, S.; Eidtmann, H.; Jonat, W. Overexpression of p53 and prognosis in breast cancer. Cancer 1993, 72, 3641–3647. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. B 1972, 34, 187. [Google Scholar] [CrossRef]
| Baseline Samples | Follow-Up Samples | |||||
|---|---|---|---|---|---|---|
| N | CTCcombo | p | N | CTCcombo | p | |
| Total | 105 | 53 (50.5%) | 61 | 18 (29.5%) | ||
| Patients’ characteristics at baseline | ||||||
| Age ≤55 >55 | 39 66 | 19 (48.7%) 34 (51.5%) | 0.782 | 23 38 | 4 (17.4%) 14 (36.8%) | 0.106 |
| FIGO stage IIA–IIIB IIIC IV | 8 68 29 | 3 (37.5%) 32 (47.1%) 18 (62.1%) | 0.301 * | 8 42 11 | 0 12 (28.6%) 6 (54.5%) | 0.038 * |
| Histotype LGSOC HGSOC other | 6 80 15 a | 2 (33.3%) 39 (48.8%) 8 (53.3%) | 0.754 | 4 48 6 b | 0 13 (27.1%) 3 (50.0%) | 0.248 |
| Grade 1–2 3 | 23 82 | 11 (47.8%) 42 (51.2%) | 0.774 | 13 48 | 4 (30.8%) 14 (29.2%) | 1.000 * |
| Residual disease yes no | 37 68 | 20 (56.0%) 33 (48.5%) | 0.589 | 21 40 | 9 (42.9%) 9 (22.5%) | 0.098 |
| Peritoneal carcinosis yes no | 75 30 | 42 (56.0%) 11 (36.7%) | 0.073 | 45 16 | 16 (35.6%) 2 (12.5%) | 0.114 * |
| Ascites yes no | 77 28 | 45 (58.4%) 8 (28.6%) | 0.007 | 49 12 | 16 (32.7%) 2 (16.7%) | 0.481 * |
| Response to adjuvant treatment | ||||||
| At completion cCR cPD, cSD, cPR | 78 27 | 37 (47.4%) 16 (59.3%) | 0.290 | 55 6 | 13 (23.6%) 5 (83.3%) | 0.007 * |
| Six months after completion cCR cPD, cSD, cPR | 66 39 | 30 (45.5%) 23 (59.0%) | 0.181 | 40 21 | 6 (15.0%) 12 (57.1%) | 0.001 |
| Progression-free interval <18 months ≥18 months | 58 47 | 36 (62.1%) 17 (36.2%) | 0.008 | 29 32 | 15 (51.7%) 3 (9.4%) | <0.001 |
| Long-term survival LTS (OS ≥ 5y) non-LTS | 33 72 | 10 (30.3%) 43 (59.7%) | 0.005 | 25 36 | 3 (12.0%) 15 (41.7%) | 0.012 |
| Non-Long-Term Survivors | Long-Term Survivors | |||||
|---|---|---|---|---|---|---|
| N | OS < 5y | OS ≥ 5y | p | OS ≥ 10y | p | |
| Total Cases | 215 | 139 (64.7%) | 76 (35.3%) | 27 (12.6%) | ||
| Median PFS (95% CI) | 14 Months (12.5–15.5) | 39 Months (24.1–53.9) | <0.001 | Not Reached | <0.001 | |
| Baseline characteristics | ||||||
| Age ≤55 >55 | 87 128 | 41 (29.5%) 98 (70.5%) | 46 (60.5%) 30 (39.5%) | <0.001 | 19 (70.4%) 9 (29.6%) | <0.001 |
| FIGO stage IIA–IIIB IIIC IV | 24 153 38 | 10 (7.2%) 96 (69.1%) 33 (23.7%) | 14 (18.4%) 57 (75.0%) 5 (6.6%) | 0.001 | 10 (37.0%) 17 (63.0%) 0 (0%) | <0.001 * |
| Histotype LGSOC HGSOC other | 15 163 28 | 7 (5.3%) 107 (81.1%) 18a (13.6%) | 8 (10.8%) 56 (75.7%) 10b (13.5%) | 0.352 | 4 (15.4%) 18 (69.2%) 4c (15.4%) | 0.153 * |
| Peritoneal carcinosis yes no | 147 68 | 105 (75.5%) 34 (24.5%) | 42 (55.3%) 34 (44.7%) | 0.002 | 8 (29.6%) 19 (70.4%) | <0.001 * |
| Ascites yes no | 160 55 | 109 (78.4%) 30 (21.6%) | 51 (55.3%) 25 (44.7%) | 0.069 | 13 (48.1%) 14 (51.9%) | 0.002 |
| Residual disease yes no | 70 145 | 55 (39.6%) 84 (60.4%) | 15 (19.7%) 61 (80.3%) | 0.003 | 3 (11.1%) 24 (88.9%) | 0.005 |
| Response to adjuvant treatment | ||||||
| At completion cCR cPD, cSD, cPR | 173 42 | 99 (71.2%) 40 (28.8%) | 74 (97.4%) 2 (2.6%) | <0.001 | 27 (100.0%) 0 (0%) | <0.001 |
| Six months after completion cCR cPD, cSD, cPR | 142 73 | 72 (51.8%) 67 (48.2%) | 70 (92.1%) 6 (7.9%) | <0.001 | 27 (100.0%) 0 (0%) | <0.001 |
| Progression-free interval <18 months ≥18 months | 105 110 | 90 (64.7%) 49 (35.3%) | 15 (19.7%) 61 (80.3%) | <0.001 | 27 (100.0%) 0 (0%) | <0.001 |
| Laboratory parameters assessed at baseline | ||||||
| CA-125 <35 U/mL ≥35 U/mL Not assessed | 19 176 20 | 14 (11.0%) 113 (89.0%) | 5 (7.4%) 63 (92.6%) | 0.410 | 2 (7.7%) 24 (92.3%) | 1.000 * |
| HE-4 < median ≥ median not assessed | 101 100 14 | 58 (43.6%) 75 (56.4%) | 43 (63.2%) 25 (36.8%) | 0.008 | 21 (80.8%) 5 (19.2%) | 0.001 |
| CTCcombo positive negative not assessed | 52 53 110 | 43 (81.1%) 29 (18.9%) | 10 (30.3%) 23 (69.7%) | 0.005 | 3 (37.5%) 5 (62.5%) | 0.488 * |
| Laboratory parameters assessed at follow-up | ||||||
| CA-125 <35 U/mL ≥35 U/mL Not assessed | 62 34 119 | 24 (46.2%) 28 (53.8%) | 38 (86.4%) 6 (13.6%) | <0.001 | 15 (100.0%) 0 (0%) | <0.001 |
| CTCcombo positive negative not assessed | 18 43 154 | 15 (41.7%) 21 (58.3%) | 3 (12.0%) 22 (88.0%) | 0.012 | 0 (0%) 5 (100.0%) | 0.309 * |
| Univariate | Multiple | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Age | 1.364 | 0.675 | 2.753 | 0.387 | * | |||
| FIGO | 2.713 | 1.196 | 6.153 | 0.017 | 1.349 | 0.362 | 5.021 | 0.655 |
| Residual disease | 2.084 | 0.949 | 4.572 | 0.067 | * | |||
| Peritoneal carcinosis | 2.210 | 1.069 | 4.568 | 0.032 | 0.724 | 0.225 | 2.325 | 0.587 |
| PFI | 10.074 | 3.800 | 26.706 | <0.001 | 11.341 | 1.069 | 120.293 | 0.044 |
| CTCcombo at baseline | 0.710 | 0.189 | 2.661 | 0.612 | * | |||
| CTCcombo at follow-up | 6.168 | 1.326 | 28.697 | 0.020 | 16.588 | 1.542 | 178.477 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obermayr, E.; Reiner, A.; Brandt, B.; Braicu, E.I.; Reinthaller, A.; Loverix, L.; Concin, N.; Woelber, L.; Mahner, S.; Sehouli, J.; et al. The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium. Cancers 2021, 13, 2613. https://doi.org/10.3390/cancers13112613
Obermayr E, Reiner A, Brandt B, Braicu EI, Reinthaller A, Loverix L, Concin N, Woelber L, Mahner S, Sehouli J, et al. The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium. Cancers. 2021; 13(11):2613. https://doi.org/10.3390/cancers13112613
Chicago/Turabian StyleObermayr, Eva, Angelika Reiner, Burkhard Brandt, Elena Ioana Braicu, Alexander Reinthaller, Liselore Loverix, Nicole Concin, Linn Woelber, Sven Mahner, Jalid Sehouli, and et al. 2021. "The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium" Cancers 13, no. 11: 2613. https://doi.org/10.3390/cancers13112613
APA StyleObermayr, E., Reiner, A., Brandt, B., Braicu, E. I., Reinthaller, A., Loverix, L., Concin, N., Woelber, L., Mahner, S., Sehouli, J., Vergote, I., & Zeillinger, R. (2021). The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium. Cancers, 13(11), 2613. https://doi.org/10.3390/cancers13112613








