Repetitively Administered Low-Dose Donor Lymphocyte Infusion for Prevention of Relapse after Allogeneic Stem Cell Transplantation in Patients with High-Risk Acute Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
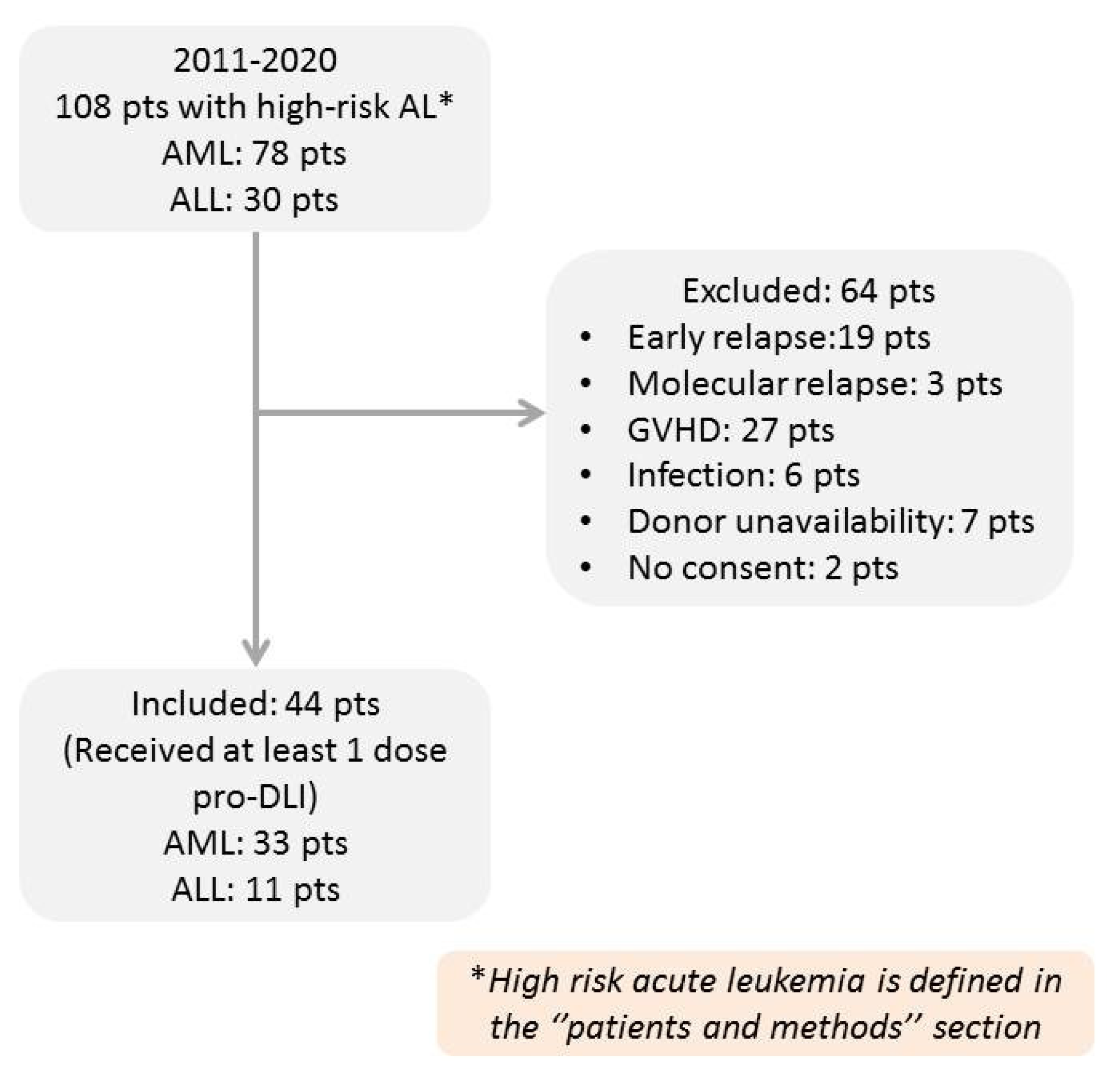
2. Patients and Methods
2.1. Inclusion and Exclusion Criteria
2.2. DLI Administration
2.3. GVHD and Chimerism Status Monitoring
2.4. Objectives and Statistical Analysis
3. Results
3.1. Patients and DLI
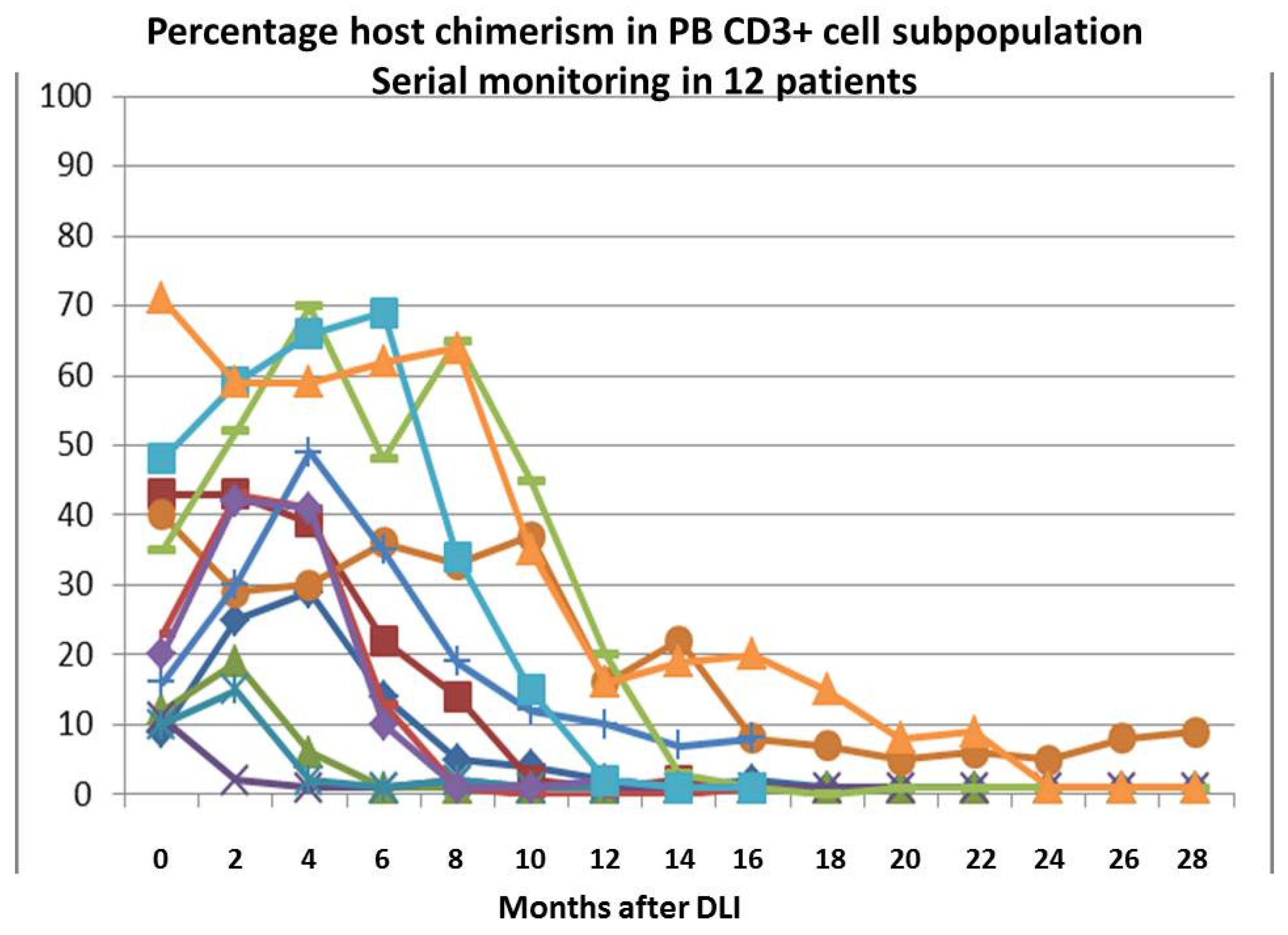
3.2. The Effect of DLI on Chimerism Status
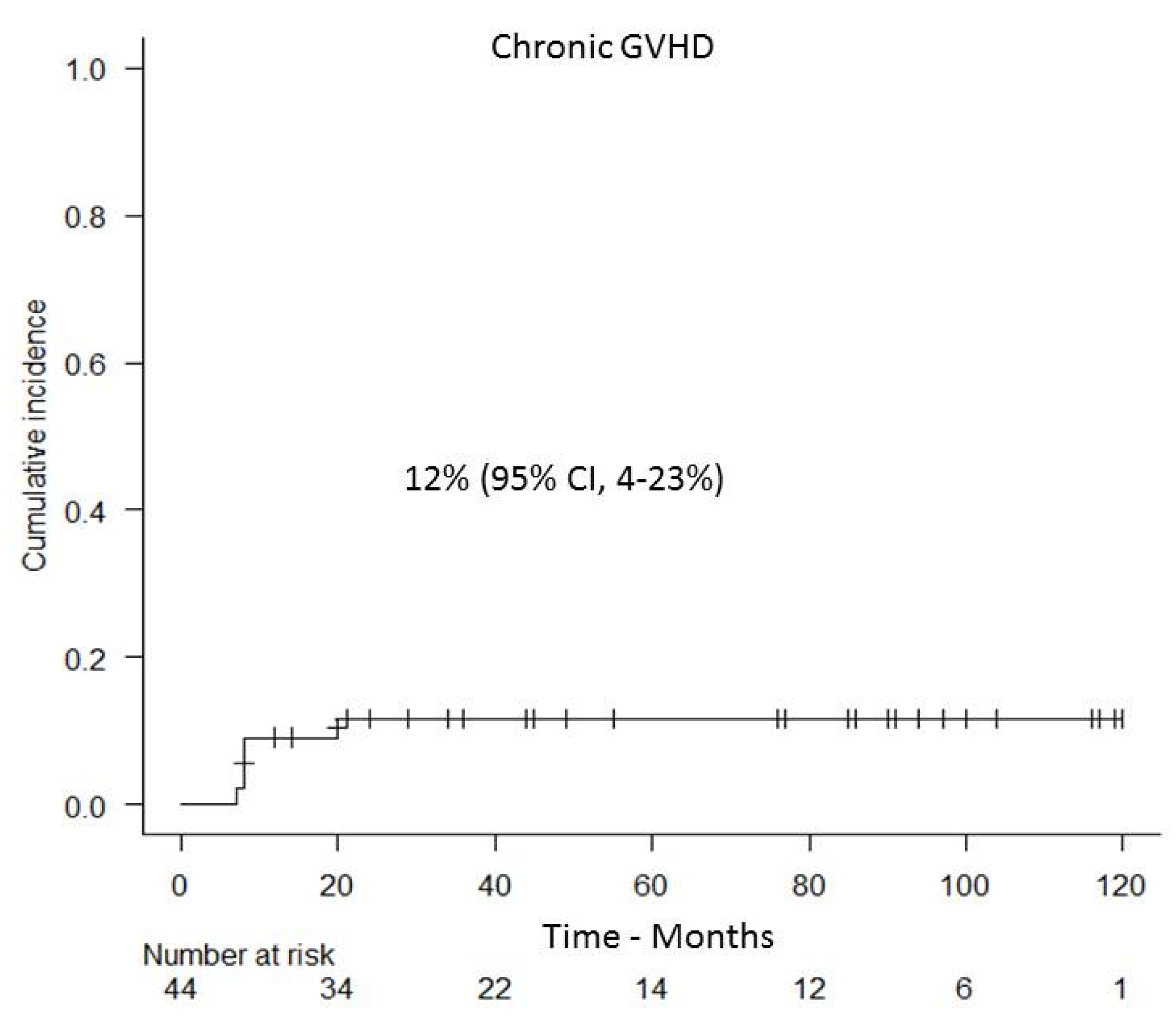
3.3. GVHD and Non-Relapse Mortality
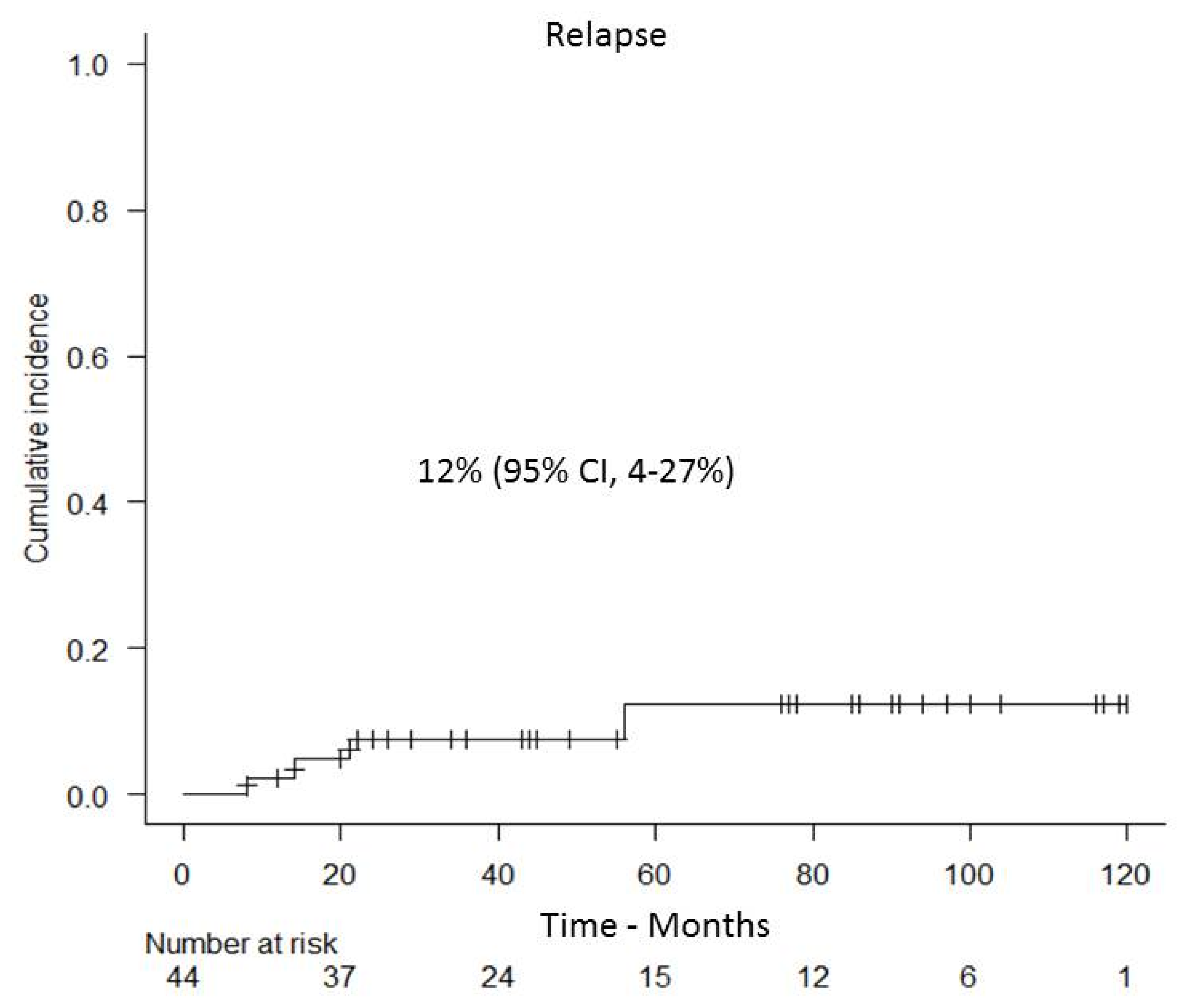
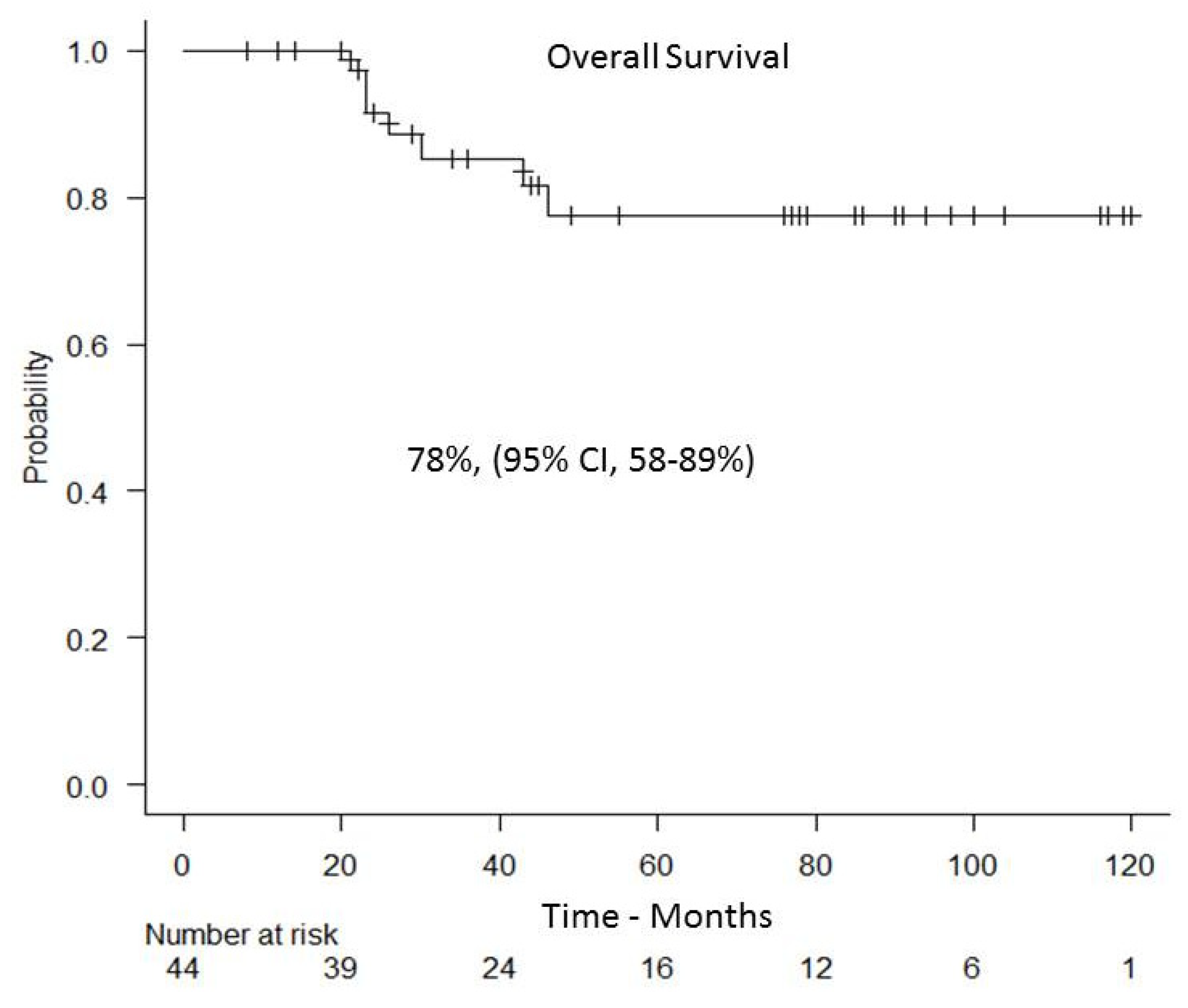
3.4. Relapse and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vyas, P.; Appelbaum, F.R.; Craddock, C. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Biol. Blood Marrow Transplant. 2015, 21, 8–15. [Google Scholar] [CrossRef]
- Baron, F.; Labopin, M.; Niederwieser, D.; Vigouroux, S.; Cornelissen, J.J.; Malm, C.; Vindelov, L.L.; Blaise, D.; Janssen, J.J.; Petersen, E.; et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia 2012, 26, 2462–2468. [Google Scholar] [CrossRef]
- Shouval, R.; Fein, J.A.; Labopin, M.; Cho, C.; Bazarbachi, A.; Baron, F.; Bug, G.; Ciceri, F.; Corbacioglu, S.; Galimard, J.E.; et al. Development and validation of a disease risk stratification system for patients with haematological malignancies: A retrospective cohort study of the European Society for Blood and Marrow Transplantation registry. Lancet Haematol. 2021, 8, e205–e215. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Schmid, C.; Labopin, M.; Beelen, D.; Wolfgang-Blau, I.; Potter, V.; Niittyvuopio, R.; Socié, G.; Blaise, D.; Sanz, J.; et al. Evaluation of Trends and Prognosis Over Time in Patients with AML Relapsing After Allogeneic Hematopoietic Cell Transplant Reveals Improved Survival for Young Patients in Recent Years. Clin. Cancer Res. 2020, 26, 6475–6482. [Google Scholar] [CrossRef]
- Bejanyan, N.; Weisdorf, D.J.; Logan, B.R.; Wang, H.L.; Devine, S.M.; de Lima, M.; Bunjes, D.W.; Zhang, M.-J. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: A center for international blood and marrow transplant research study. Biol. Blood Marrow Transplant. 2015, 21, 454–459. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—A matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2019, 184, 782–787. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Dignan, F.L.; Tulpule, S.; Gupta, E.D.; Kolade, S.; Shaw, B.; Evison, F.; Shah, G.; Tholouli, E.; Mufti, G.; et al. A multicentre UK study of GVHD following DLI: Rates of GVHD are high but mortality from GVHD is infrequent. Bone Marrow Transplant. 2015, 50, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhäuser, M.; Finke, J.; Fassas, A.; Volin, L.; Gürman, G.; Maertens, J.; Bordigoni, P.; et al. EBMT Acute Leukemia Working Party. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar] [PubMed]
- Peggs, K.S.; Mackinnon, S. Cellular therapy: Donor lymphocyte infusion. Curr. Opin. Hematol. 2001, 8, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotis, P.; Byrne, M.; Schmid, C.; Baron, F.; Ciceri, F.; Esteve, J.; Gorin, N.C.; Giebel, S.; Mohty, M.; Savani, B.; et al. Relapse of AML after hematopoietic stem cell transplantation: Methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016, 51, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Rujkijyanont, P.; Morris, C.; Kang, G.; Gan, K.; Hartford, C.; Triplett, B.; Dallas, M.; Srinivasan, A.; Shook, D.; Pillai, A.; et al. Risk-adapted donor lymphocyte infusion based on chimerism and donor source in pediatric leukemia. Blood Cancer J. 2013, 3, e137. [Google Scholar] [CrossRef]
- Yan, C.H.; Liu, D.H.; Liu, K.Y.; Xu, L.P.; Liu, Y.R.; Chen, H.; Han, W.; Wang, Y.; Qin, Y.-Z.; Huang, X.-J. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012, 119, 3256–3262. [Google Scholar] [CrossRef]
- Schmid, C.; Schleuning, M.; Tischer, J.; Holler, E.; Haude, K.H.; Braess, J.; Haferlach, C.; Baurmann, H.; Oruzio, D.; Hahn, J.; et al. Early allo-SCT for AML with a complex aberrant karyotype--results from a prospective pilot study. Bone Marrow Transplant. 2012, 47, 46–53. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Liu, D.H.; Xu, L.P.; Liu, K.Y.; Chen, H.; Zhang, X.H.; Chen, Y.-H.; Han, W.; Wang, F.-R.; Wang, J.-Z.; et al. Prevention of relapse using granulocyte CSF-primed PBPCs following HLA-mismatched/haploidentical, T-cell-replete hematopoietic SCT in patients with advanced-stage acute leukemia: A retrospective risk-factor analysis. Bone Marrow Transplant. 2012, 47, 1099–1104. [Google Scholar] [CrossRef]
- Hansen, D.K.; Kim, J.; Thompson, Z.; Hussaini, M.; Nishihori, T.; Ahmad, A.; Elmariah, H.; Faramand, R.; Mishra, A.; Davila, M.L.; et al. ELN 2017 Genetic Risk Stratification Predicts Survival of Acute Myeloid Leukemia Patients Receiving Allogeneic Hematopoietic Stem Cell Transplantation. Transplant. Cell Ther. 2021, 27, e1–e256. [Google Scholar] [CrossRef]
- Rettig, A.R.; Ihorst, G.; Bertz, H.; Lübbert, M.; Marks, R.; Waterhouse, M.; Wäsch, R.; Zeiser, R.; Duyster, J.; Finke, J. Donor lymphocyte infusions after first allogeneic hematopoietic stem-cell transplantation in adults with acute myeloid leukemia: A single-center landmark analysis. Ann. Hematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotis, P.; Liga, M.; Gkirkas, K.; Stamouli, M.; Triantafyllou, E.; Marangos, M.; Pessach, I.; Sarantopoulos, A.; Spyridis, N.; Spyridonidis, A. Low-dose alemtuzumab for GvHD prevention followed by prophylactic donor lymphocyte infusions in high-risk leukemia. Bone Marrow Transplant. 2017, 52, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4e10. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Nagler, A.; Baron, F.; Labopin, M.; Polge, E.; Esteve, J.; Bazarbachi, A.; Brissot, E.; Bug, G.; Ciceri, F.; Giebel, S.; et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: A survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant. 2021, 56, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Kim, H.T.; Logan, B.R.; Wang, Z.; Alyea, E.P.; Kalaycio, M.E.; Maziarz, R.T.; Antin, J.H.; Soiffer, R.J.; Weisdorf, D.J.; et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.H.; Fan, Z.P.; Sun, J.; Wu, X.J.; Ma, X.; Xu, L.P.; Liu, K.Y.; Liu, Q.F.; Wu, D.P.; et al. Prevention of relapse using DLI can increase survival following HLA identical transplantation in patients with advanced-stage acute leukemia: A multi-center study. Clin. Transpl. 2012, 26, 635–643. [Google Scholar] [CrossRef]
- Jedlickova, Z.; Schmid, C.; Koenecke, C.; Hertenstein, B.; Baurmann, H.; Schwerdtfeger, R.; Tischer, J.; Kolb, H.J.; Schleuning, M. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 663–667. [Google Scholar] [CrossRef]
- Eefting, M.; Halkes, C.J.; de Wreede, L.C.; van Pelt, C.M.; Kersting, S.; Marijt, E.W.; von dem Borne, P.A.; Willemze, R.; Veelken, H.; Falkenburg, J.H. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. 2014, 49, 287–291. [Google Scholar] [CrossRef]
- Yan, C.-H.; Liu, Q.; Wu, D.; Zhang, X.; Xu, L.-P.; Zhang, X.-H.; Wang, Y.; Huang, H.; Bai, H.; Huang, F.; et al. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft-versus-Host Disease–Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia. Biol Blood Marrow Transplant. 2017, 23, 1311–1319. [Google Scholar]
- Schmid, C.; Schleuning, M.; Ledderose, G.; Tischer, J.; Kolb, H.J. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J. Clin. Oncol. 2005, 23, 5675–5687. [Google Scholar] [CrossRef]
- Schaap, N.; Schattenberg, A.; Bär, B.; Preijers, F.; van Kemenade, E.V.D.W.; De Witte, T. Induction of graft-versus-leukemia to prevent relapse after partially lymphocyte depleted allogeneic bone marrow transplantation by pre-emptive donor leukocyte infusions. Leukemia 2001, 15, 1339–1346. [Google Scholar] [CrossRef][Green Version]
- Montero, A.; Savani, B.N.; Shenoy, A.; Read, E.J.; Carter, C.S.; Leitman, S.F.; Mielke, S.; Rezvani, K.; Childs, R.; Barrett, A.J. T-cell depleted peripheral blood stem cell allotransplantation with T-cell add-back for patients with hematological malignancies: Effect of chronic GvHD on outcome. Biol Blood Marrow Transplant. 2006, 12, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; de Magalhaes-Silverman, M.; Hohl, R.J.; Hayashi, M.; Buatti, J.; Wen, B.C.; Schlueter, A.; Strauss, R.G.; Gingrich, R.D. Prophylactic T cell infusion after T cell-depleted bone marrow transplantation in patients with refractory lymphoma. Bone Marrow Transplant. 2002, 29, 615–620. [Google Scholar] [CrossRef]
- Su, Q.; Fan, Z.; Huang, F.; Xu, N.; Nie, D.; Lin, D.; Guo, Z.; Shi, P.; Wang, Z.; Jiang, L.; et al. Comparison of Two Strategies for Prophylactic Donor Lymphocyte Infusion in Patients With Refractory/Relapsed Acute Leukemia. Front. Oncol. 2021, 11, 554503. [Google Scholar] [CrossRef]
- Johnson, B.D.; Becker, E.E.; Truitt, R.L. Graft-vs.-host and graft-vs.-leukemia reactions after delayed infusions of donor T-subsets. Biol. Blood Marrow Transplant. 1999, 5, 123–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raiola, A.M.; Van Lint, M.T.; Valbonesi, M.; Lamparelli, T.; Gualandi, F.; Occhini, D.; Bregante, S.; di Grazia, C.; Dominietto, A.; Soracco, M.; et al. Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: A study on 593 infusions. Bone Marrow Transplant. 2003, 31, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, C.; Arcese, W.; Dazzi, F.; Brand, R.; Bunjes, D.; Verdonck, L.F.; Schattenberg, A. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: Prognostic relevance of the initial cell dose. Blood 2002, 100, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hale, G.; Rebello, P.; Brettman, L.R.; Fegan, C.; Kennedy, B.; Kimby, E.; Leach, M.; Lundin, J.; Mellstedt, H.; Moreton, P.; et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 2004, 104, 948–955. [Google Scholar] [CrossRef] [PubMed]
| Age, Median, (Range) | 53 Years (20–67) |
|---|---|
| Sex, Male/Female | 24/20 |
| Acute Myeloid Leukemia Good risk genetics * Intermediate risk genetics * Poor risk genetics * CR1 ≥CR2 Refractory disease MRD positive ** | 33 1/33 15/33 17/33 17/33 8/33 8/33 4/33 |
| Acute Lymphoblastic Leukemia Standard risk Poor risk CR1 ≥CR2 Refractory disease MRD positive ** | 11 6/11 5/11 6/11 4/11 1/11 5/11 |
| Disease Risk Index Intermediate High | 21 23 |
| Donor Matched related donor Matched unrelated donor | 38 6 |
| Conditioning regimen Myeloablative Reduced intensity | 36 8 |
| GVHD prophylaxis Cyclosporine plus low dose Alemtuzumab Cyclosporine plus short course MTX | 39 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsirigotis, P.; Gkirkas, K.; Kitsiou, V.; Chondropoulos, S.; Athanassiades, T.; Thomopoulos, T.; Tsirogianni, A.; Stamouli, M.; Karagiannidi, A.; Siafakas, N.; et al. Repetitively Administered Low-Dose Donor Lymphocyte Infusion for Prevention of Relapse after Allogeneic Stem Cell Transplantation in Patients with High-Risk Acute Leukemia. Cancers 2021, 13, 2699. https://doi.org/10.3390/cancers13112699
Tsirigotis P, Gkirkas K, Kitsiou V, Chondropoulos S, Athanassiades T, Thomopoulos T, Tsirogianni A, Stamouli M, Karagiannidi A, Siafakas N, et al. Repetitively Administered Low-Dose Donor Lymphocyte Infusion for Prevention of Relapse after Allogeneic Stem Cell Transplantation in Patients with High-Risk Acute Leukemia. Cancers. 2021; 13(11):2699. https://doi.org/10.3390/cancers13112699
Chicago/Turabian StyleTsirigotis, Panagiotis, Konstantinos Gkirkas, Vassiliki Kitsiou, Spiros Chondropoulos, Theofilos Athanassiades, Thomas Thomopoulos, Alexandra Tsirogianni, Maria Stamouli, Aggeliki Karagiannidi, Nikolaos Siafakas, and et al. 2021. "Repetitively Administered Low-Dose Donor Lymphocyte Infusion for Prevention of Relapse after Allogeneic Stem Cell Transplantation in Patients with High-Risk Acute Leukemia" Cancers 13, no. 11: 2699. https://doi.org/10.3390/cancers13112699
APA StyleTsirigotis, P., Gkirkas, K., Kitsiou, V., Chondropoulos, S., Athanassiades, T., Thomopoulos, T., Tsirogianni, A., Stamouli, M., Karagiannidi, A., Siafakas, N., Pappa, V., & Nagler, A. (2021). Repetitively Administered Low-Dose Donor Lymphocyte Infusion for Prevention of Relapse after Allogeneic Stem Cell Transplantation in Patients with High-Risk Acute Leukemia. Cancers, 13(11), 2699. https://doi.org/10.3390/cancers13112699







