NMR-Based Lipid Metabolite Profiles to Predict Outcomes in Patients Undergoing Interventional Therapy for a Hepatocellular Carcinoma (HCC): A Substudy of the SORAMIC Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
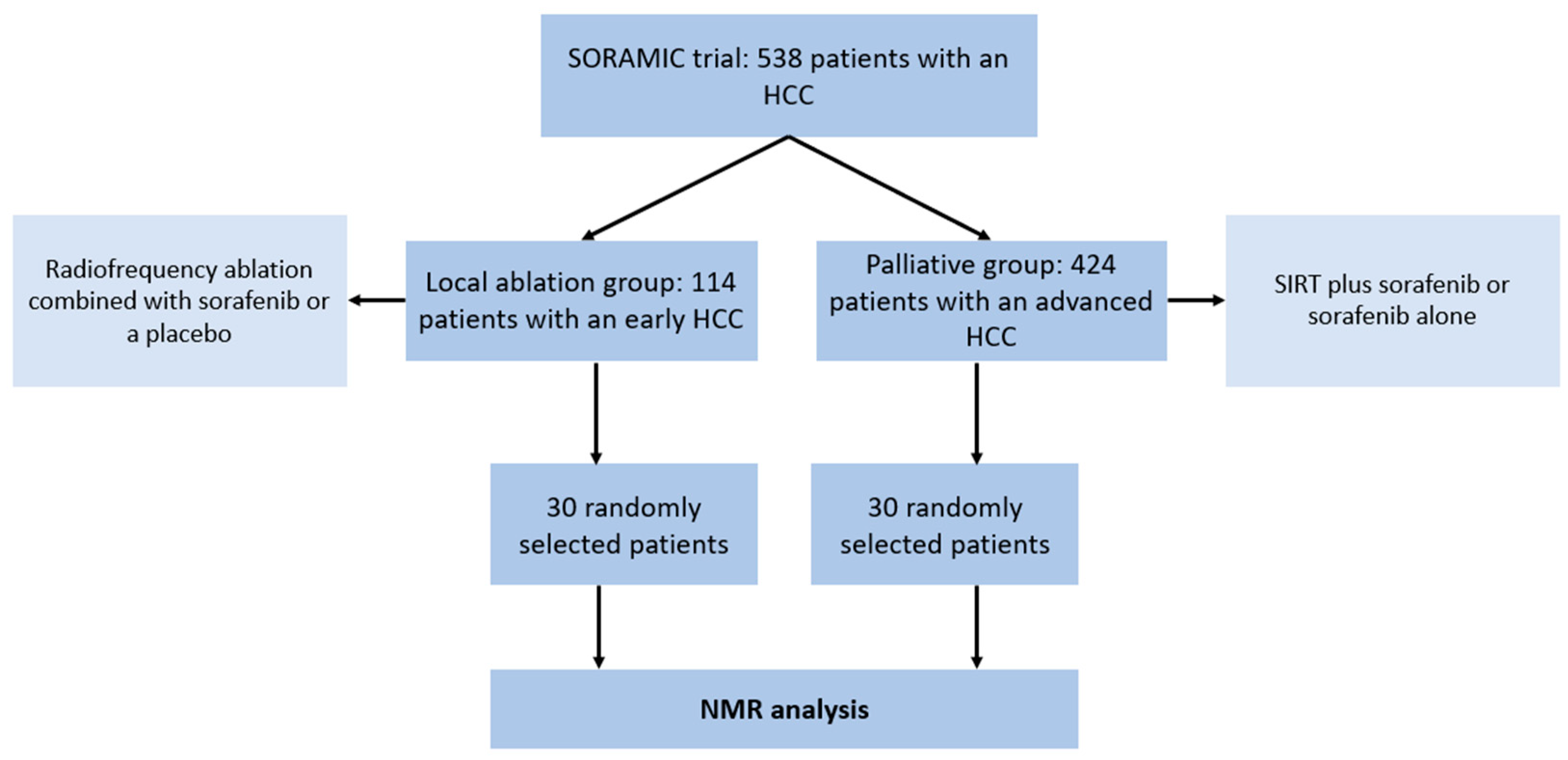
2.1. Study Design
- A total of 30 patients (24 male, 6 female; mean age 67, range: 53–83) with an early HCC (BCLC A) and liver cirrhosis who underwent a local ablation within the SORAMIC trial (radiofrequency ablation combined with sorafenib or a placebo)
- A total of 30 patients (28 male, 2 female; mean age 66, range: 41–79) with an advanced HCC (BCLC B or C) and liver cirrhosis who underwent palliative treatment within the SORAMIC trial (selective internal radiation therapy (SIRT) with yttrium-90 (90Y) resin microspheres plus sorafenib vs. sorafenib alone).
2.2. NMR AXINON® Platform
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment Groups
3.2. NMR Data
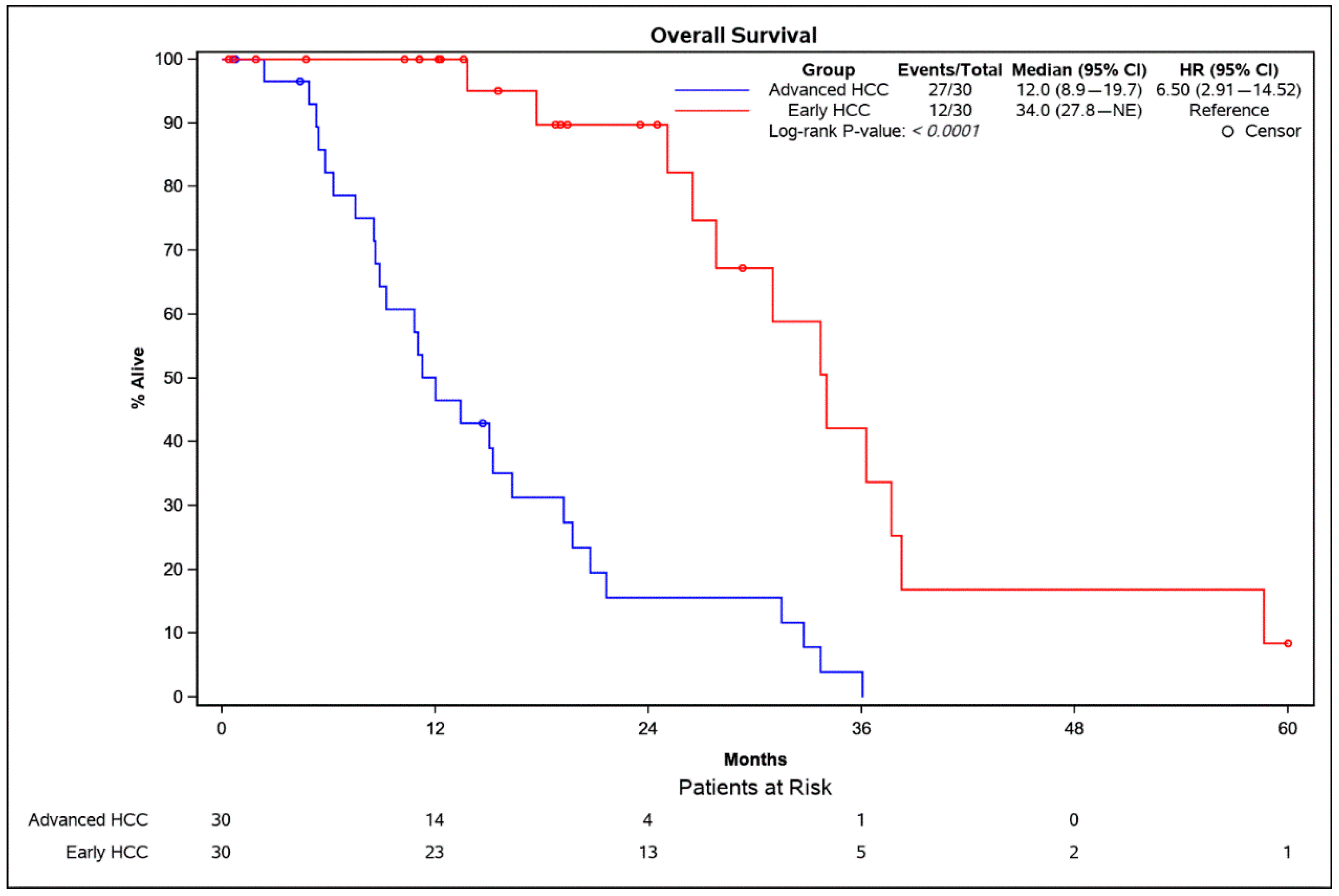
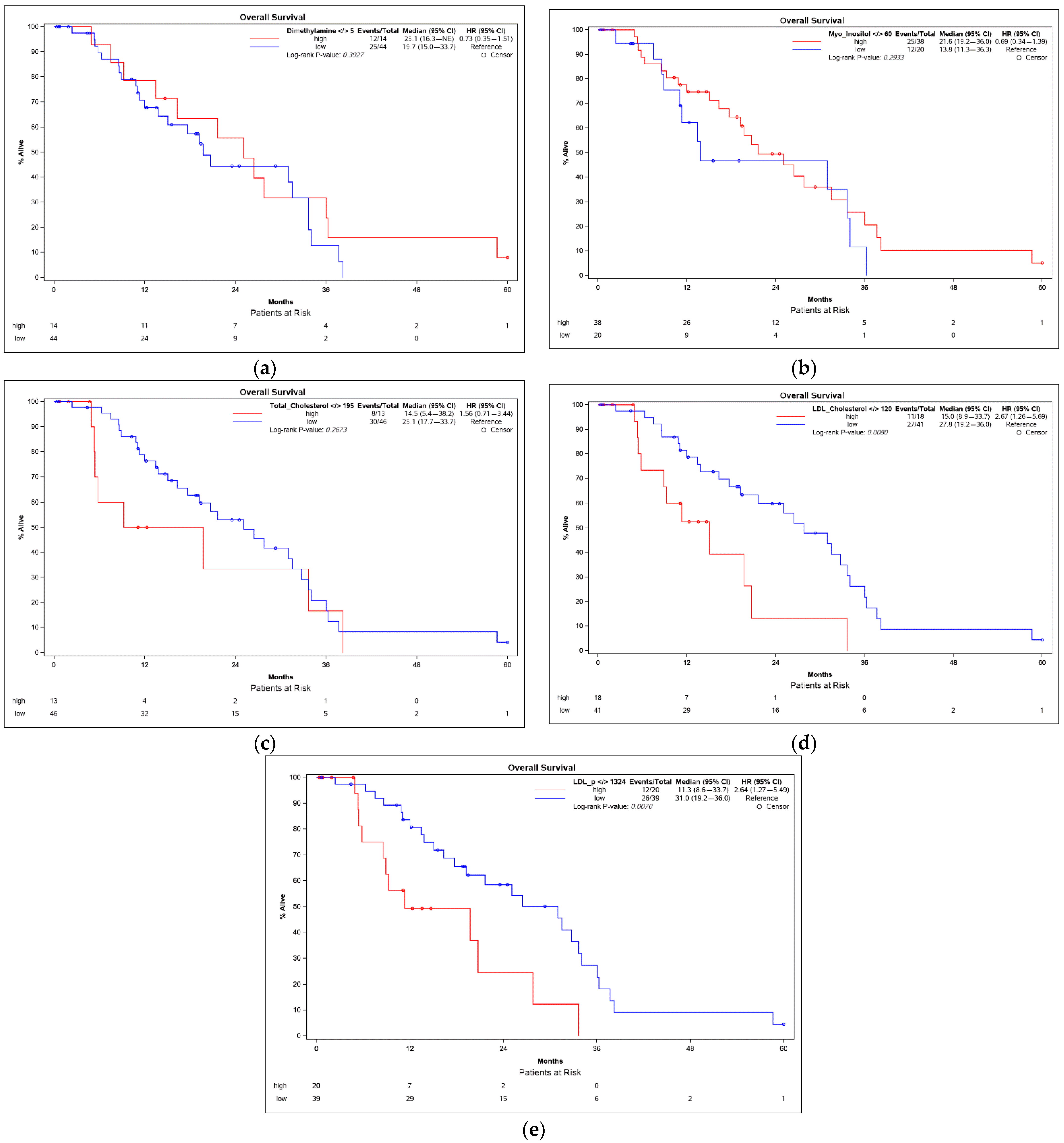
3.3. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhang, A.; Sun, H. Power of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinoma. Hepatology 2013, 57, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Addissie, B.D.; Roberts, L.R. Update on biomarkers of hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2015, 13, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Tan, H.Y.; Wang, N.; Wang, X.; Feng, Y. Deciphering hepatocellular carcinoma through metabolomics: From biomarker discovery to therapy evaluation. Cancer Manag. Res. 2018, 10, 715–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, Y.; Zhang, Y.; Cheng, L.; Ma, J.; Xi, Y.; Yang, L.; Su, C.; Shao, B.; Huang, A.; Xiang, R.; et al. Hepatitis B virus X protein (HBx)-induced abnormalities of nucleic acid metabolism revealed by (1)H-NMR-based metabonomics. Sci. Rep. 2016, 6, 24430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.; Dewaele, S.; Zhao, Y.P.; Stärkel, P.; Vanhooren, V.; Chen, Y.M.; Ji, X.; Luo, M.; Sun, B.M.; Horsmans, Y.; et al. Serum N-glycome biomarker for monitoring development of DENA-induced hepatocellular carcinoma in rat. Mol. Cancer 2010, 9, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Kohjima, M.; Tanaka, M.; Goya, T.; Itoh, S.; Yoshizumi, T.; Mori, M.; Tsuda, M.; Takahashi, M.; Kurokawa, M.; et al. Metabolic Alteration in Hepatocellular Carcinoma: Mechanism of Lipid Accumulation in Well-Differentiated Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8813410. [Google Scholar] [CrossRef] [PubMed]
- Armitage, E.G.; Southam, A.D. Monitoring cancer prognosis, diagnosis and treatment efficacy using metabolomics and lipidomics. Metabolomics 2016, 12, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganti, S.; Weiss, R.H. Urine metabolomics for kidney cancer detection and biomarker discovery. Urol. Oncol. 2011, 29, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Stratmann, B.; Krepak, Y.; Schiffer, E.; Jarick, I.; Hauber, M.; Lee-Barkey, Y.H.; Fischer, M.; Tschoepe, D. Beneficial Metabolic Effects of Duodenal Jejunal Bypass Liner for the Treatment of Adipose Patients with Type 2 Diabetes Mellitus: Analysis of Responders and Non-Responders. Horm. Metab. Res. 2016, 48, 630–637. [Google Scholar] [CrossRef]
- Chan, S.Y.; Capitão, L.; Probert, F.; Klinge, C.; Hoeckner, S.; Harmer, C.J.; Cowen, P.J.; Anthony, D.C.; Burnet, P.W.J. A single administration of the antibiotic, minocycline, reduces fear processing and improves implicit learning in healthy volunteers: Analysis of the serum metabolome. Transl. Psychiatry 2020, 10, 148. [Google Scholar] [CrossRef]
- Baumstark, D.; Kremer, W.; Boettcher, A.; Schreier, C.; Sander, P.; Schmitz, G.; Kirchhoefer, R.; Huber, F.; Kalbitzer, H.R. (1)H NMR spectroscopy quantifies visibility of lipoproteins, subclasses, and lipids at varied temperatures and pressures. J. Lipid Res. 2019, 60, 1516–1534. [Google Scholar] [CrossRef] [PubMed]
- Degoricija, V.; Potočnjak, I.; Gastrager, M.; Pregartner, G.; Berghold, A.; Scharnagl, H.; Stojakovic, T.; Tiran, B.; Marsche, G.; Frank, S. HDL subclasses and mortality in acute heart failure patients. Clin. Chim. Acta 2019, 490, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Schettler, V.J.; Muellendorff, F.; Schettler, E.; Platzer, C.; Norkauer, S.; Julius, U.; Neumann, C.L. NMR-based lipoprotein analysis for patients with severe hypercholesterolemia undergoing lipoprotein apheresis or PCSK9-inhibitor therapy (NAPALI-Study). Ther. Apher. Dial. 2019, 23, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banas, M.C.; Neumann, S.; Pagel, P.; Putz, F.J.; Krämer, B.K.; Böhmig, G.A.; Eiglsperger, J.; Schiffer, E.; Ruemmele, P.; Banas, B. A urinary metabolite constellation to detect acute rejection in kidney allografts. EBioMedicine 2019, 48, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Banas, M.; Neumann, S.; Eiglsperger, J.; Schiffer, E.; Putz, F.J.; Reichelt-Wurm, S.; Krämer, B.K.; Pagel, P.; Banas, B. Identification of a urine metabolite constellation characteristic for kidney allograft rejection. Metabolomics 2018, 14, 116. [Google Scholar] [CrossRef] [Green Version]
- Ehrich, J.; Dubourg, L.; Hansson, S.; Pape, L.; Steinle, T.; Fruth, J.; Höckner, S.; Schiffer, E. Serum Myo-Inositol, Dimethyl Sulfone, and Valine in Combination with Creatinine Allow Accurate Assessment of Renal Insufficiency-A Proof of Concept. Diagnostics 2021, 11, 234. [Google Scholar] [CrossRef]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef]
- Ricke, J.; Bulla, K.; Kolligs, F.; Peck-Radosavljevic, M.; Reimer, P.; Sangro, B.; Schott, E.; Schütte, K.; Verslype, C.; Walecki, J.; et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: Analysis of the European multicentre trial SORAMIC. Liver Int. 2015, 35, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Nilsson-Ehle, P.; Xu, N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Arain, S.Q.; Talpur, F.N.; Channa, N.A. A comparative study of serum lipid contents in pre and post IFN-alpha treated acute hepatitis C patients. Lipids Health Dis. 2015, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Arain, S.Q.; Talpur, F.N.; Channa, N.A.; Ali, M.S.; Afridi, H.I. Serum lipid profile as a marker of liver impairment in hepatitis B Cirrhosis patients. Lipids Health Dis. 2017, 16, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, S.; Smuts, C.M.; de Villiers, C.; Gelderblom, W.C. Changes in essential fatty acid patterns associated with normal liver regeneration and the progression of hepatocyte nodules in rat hepatocarcinogenesis. Carcinogenesis 2001, 22, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Beyoğlu, D.; Imbeaud, S.; Maurhofer, O.; Bioulac-Sage, P.; Zucman-Rossi, J.; Dufour, J.F.; Idle, J.R. Tissue metabolomics of hepatocellular carcinoma: Tumor energy metabolism and the role of transcriptomic classification. Hepatology 2013, 58, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Nderitu, P.; Bosco, C.; Garmo, H.; Holmberg, L.; Malmström, H.; Hammar, N.; Walldius, G.; Jungner, I.; Ross, P.; Van Hemelrijck, M. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: A study in the Swedish AMORIS cohort. Int. J. Cancer 2017, 141, 1148–1160. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Ding, Y.; Wang, Y.; Wang, Z.; Yin, X.; Yan, G.; Zhang, L.; Yang, P.; Shen, H. Functional lipidomics: Palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology 2017, 66, 432–448. [Google Scholar] [CrossRef]
- Sauer, L.A.; Dauchy, R.T.; Blask, D.E. Dietary linoleic acid intake controls the arterial blood plasma concentration and the rates of growth and linoleic acid uptake and metabolism in hepatoma 7288CTC in Buffalo rats. J. Nutr. 1997, 127, 1412–1421. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, Q.; Halim, A.; Song, G. Targeting lipid metabolism of cancer cells: A promising therapeutic strategy for cancer. Cancer Lett. 2017, 401, 39–45. [Google Scholar] [CrossRef]
- Wang, Q.; Lau, W.Y.; Zhang, B.; Zhang, Z.; Huang, Z.; Luo, H.; Chen, X. Preoperative total cholesterol predicts postoperative outcomes after partial hepatectomy in patients with chronic hepatitis B- or C-related hepatocellular carcinoma. Surgery 2014, 155, 263–270. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, S.S.; Chen, H.; Wang, Y.H.; Yuan, C.H.; Wang, F.B. Systemic Hepatic-Damage Index for Predicting the Prognosis of Hepatocellular Carcinoma after Curative Resection. Front. Physiol. 2017, 8, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.S.; Weng, D.S.; Jiang, L.; Zhang, Y.J.; Pan, K.; Pan, Q.Z.; Chen, C.L.; Zhao, J.J.; Zhang, X.F.; Zhang, H.X.; et al. The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. J. Cancer 2016, 7, 626–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.X.; Tang, Z.Y. Hepatocellular carcinoma with obstructive jaundice: Diagnosis, treatment and prognosis. World J. Gastroenterol. 2003, 9, 385–391. [Google Scholar] [CrossRef]
- Bhattarai, S.; Graham, R.P.; Sigel, C.S.; Shi, J.; Gonzalez, R.S.; Xue, Y.; Krasinskas, A.M.; HooKim, K.; Adsay, V.; Reid, M.D. Bile duct involvement by hepatocellular carcinoma: A rare occurrence and poor prognostic indicator in bile duct brushing samples. Cancer Cytopathol. 2019, 127, 691–699. [Google Scholar] [CrossRef]
- Strohmaier, S.; Edlinger, M.; Manjer, J.; Stocks, T.; Bjørge, T.; Borena, W.; Häggström, C.; Engeland, A.; Nagel, G.; Almquist, M.; et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS ONE 2013, 8, e54242. [Google Scholar] [CrossRef] [Green Version]
- Iso, H.; Ikeda, A.; Inoue, M.; Sato, S.; Tsugane, S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int. J. Cancer 2009, 125, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Trompet, S.; Jukema, J.W.; Katan, M.B.; Blauw, G.J.; Sattar, N.; Buckley, B.; Caslake, M.; Ford, I.; Shepherd, J.; Westendorp, R.G.; et al. Apolipoprotein e genotype, plasma cholesterol, and cancer: A Mendelian randomization study. Am. J. Epidemiol. 2009, 170, 1415–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicognani, C.; Malavolti, M.; Morselli-Labate, A.M.; Zamboni, L.; Sama, C.; Barbara, L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch. Intern. Med. 1997, 157, 792–796. [Google Scholar] [CrossRef]
- Cromwell, W.C.; Otvos, J.D.; Keyes, M.J.; Pencina, M.J.; Sullivan, L.; Vasan, R.S.; Wilson, P.W.; D’Agostino, R.B. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study—Implications for LDL Management. J. Clin. Lipidol. 2007, 1, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, S.; Buring, J.E.; Ridker, P.M. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation 2014, 129, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.P.; Mills, S.J.; Potter, B.V. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in myo-Inositol Chemistry). Angew. Chem. Int. Ed. Engl. 2016, 55, 1614–1650. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; McWilliams, A.; LeRiche, J.; MacAulay, C.; Wattenberg, L.; Szabo, E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1526–1531. [Google Scholar] [CrossRef] [Green Version]
- Stentz, R.; Osborne, S.; Horn, N.; Li, A.W.; Hautefort, I.; Bongaerts, R.; Rouyer, M.; Bailey, P.; Shears, S.B.; Hemmings, A.M.; et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014, 6, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Antonsson, B. Phosphatidylinositol synthase from mammalian tissues. Biochim. Biophys. Acta 1997, 1348, 179–186. [Google Scholar] [CrossRef]
- Alcázar-Román, A.R.; Wente, S.R. Inositol polyphosphates: A new frontier for regulating gene expression. Chromosoma 2008, 117, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad Spectrum Anticancer Activity of Myo-Inositol and Inositol Hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vucenik, I.; Shamsuddin, A.M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: From laboratory to clinic. J. Nutr. 2003, 133, 3778s–3784s. [Google Scholar] [CrossRef]
- Lam, S.; Mandrekar, S.J.; Gesthalter, Y.; Allen Ziegler, K.L.; Seisler, D.K.; Midthun, D.E.; Mao, J.T.; Aubry, M.C.; McWilliams, A.; Sin, D.D.; et al. A Randomized Phase IIb Trial of myo-Inositol in Smokers with Bronchial Dysplasia. Cancer Prev. Res. 2016, 9, 906–914. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Song, Y.; Cui, L.; Wen, Z.; Lu, X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int. J. Clin. Exp. Pathol. 2015, 8, 1402–1410. [Google Scholar] [PubMed]
- Dinicola, S.; Fabrizi, G.; Masiello, M.G.; Proietti, S.; Palombo, A.; Minini, M.; Harrath, A.H.; Alwasel, S.H.; Ricci, G.; Catizone, A.; et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp. Cell Res. 2016, 345, 37–50. [Google Scholar] [CrossRef]
- Shafie, N.H.; Mohd Esa, N.; Ithnin, H.; Md Akim, A.; Saad, N.; Pandurangan, A.K. Preventive inositol hexaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/beta-catenin and COX-2 pathways. Biomed Res. Int. 2013, 2013, 681027. [Google Scholar] [CrossRef] [Green Version]
- Di Sabatino, A.; Jackson, C.L.; Pickard, K.M.; Buckley, M.; Rovedatti, L.; Leakey, N.A.; Picariello, L.; Cazzola, P.; Monteleone, G.; Tonelli, F.; et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut 2009, 58, 777–789. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Mallikarjuna, G.U.; Dhanalakshmi, S.; Agarwal, C.; Agarwal, R. In vivo suppression of hormone-refractory prostate cancer growth by inositol hexaphosphate: Induction of insulin-like growth factor binding protein-3 and inhibition of vascular endothelial growth factor. Clin. Cancer Res. 2004, 10, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, G.; Penninkilampi, R.; Chaganti, J.; Montagnese, S.; Brew, B.J.; Danta, M. Meta-analysis of magnetic resonance spectroscopy in the diagnosis of hepatic encephalopathy. Neurology 2020, 94, e1147–e1156. [Google Scholar] [CrossRef]
- Pani, A.; Giossi, R.; Menichelli, D.; Fittipaldo, V.A.; Agnelli, F.; Inglese, E.; Romandini, A.; Roncato, R.; Pintaudi, B.; Del Sole, F.; et al. Inositol and Non-Alcoholic Fatty Liver Disease: A Systematic Review on Deficiencies and Supplementation. Nutrients 2020, 12, 3379. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D. Urinary Dimethylamine (DMA) and Its Precursor Asymmetric Dimethylarginine (ADMA) in Clinical Medicine, in the Context of Nitric Oxide (NO) and Beyond. J. Clin. Med. 2020, 9, 1843. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Tian, Q.W.; Li, H.; Zhang, B.B.; He, G.W.; Lun, L.M. Levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, and risk of coronary artery disease: A meta-analysis based on 4713 participants. Eur. J. Prev. Cardiol. 2016, 23, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, Q.; Li, X.; Chen, C.; Liu, J.; Ye, Y.; Ruan, Y.; Hei, Z. Asymmetric dimethylarginine and all-cause mortality: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44692. [Google Scholar] [CrossRef]
| Parameter Name | Description | Unit |
|---|---|---|
| Creatine | Concentration of serum creatine | µmol/L |
| Creatinine | Concentration of serum creatinine | µmol/L |
| Dimethylamine | Concentration of serum dimethylamine | µmol/L |
| Dimethylsulfone | Concentration of serum dimethyl sulfone | µmol/L |
| Glycerol | Concentration of serum glycerol | µmol/L |
| Isoleucine | Concentration of serum isoleucine | µmol/L |
| Myo-Inositol | Concentration of serum myo-inositol | µmol/L |
| Valine | Concentration of serum valine | µmol/L |
| GFR(NMR) | Glomerular filtration rate estimated from metabolite constellation | mL/min/1.73 m² |
| LVLDL-P | Concentration of large VLDL particles | nmol/L |
| LDL-P | Concentration of LDL particles | nmol/L |
| LLDL-P | Concentration of large LDL particles | nmol/L |
| SLDL-P | Concentration of small LDL particles | nmol/L |
| HDL-P | Concentration of HDL particles | nmol/L |
| LHDL-P | Concentration of large HDL particles | nmol/L |
| SHDL-P | Concentration of small HDL particles | nmol/L |
| VLDL-s | Mean diameter of VLDL particles | nm |
| LDL-s | Mean diameter of LDL particles | nm |
| HDL-s | Mean diameter of HDL particles | nm |
| VLDL-c | Cholesterol concentration in VLDL class | mg/dL |
| IDL-c | Cholesterol concentration in IDL class | mg/dL |
| LDL-c | Cholesterol concentration in LDL class | mg/dL |
| LDL.A-c | Cholesterol concentration in LDL subclass A (large particles) | mg/dL |
| LDL.B-c | Cholesterol concentration in LDL subclass B (medium-sized particles) | mg/dL |
| LDL.C-c | Cholesterol concentration in LDL subclass C (small particles) | mg/dL |
| HDL.A-c | Cholesterol concentration in HDL subclass A (large particles) | mg/dL |
| HDL.B-c | Cholesterol concentration in HDL subclass B (medium-sized particles) | mg/dL |
| HDL.C-c | Cholesterol concentration in HDL subclass C (small particles) | mg/dL |
| Total-Cholesterol | Concentration of total cholesterol in serum | mg/dL |
| LDL-Cholesterol | Concentration of LDL-cholesterol in serum | mg/dL |
| HDL-Cholesterol | Concentration of HDL-cholesterol in serum | mg/dL |
| Triglycerides | Concentration of total triglycerides in serum | mg/dL |
| Alanine | Concentration of alanine in serum | µmol/L |
| Leucine | Concentration of leucine in serum | µmol/L |
| Variables | Total | Early HCC | Advanced HCC | p-Value |
|---|---|---|---|---|
| (n = 60) | (n = 30) | (n = 30) | ||
| Gender | 0.1287 | |||
| Male | 52 (86.7) | 24 (80.0) | 28 (93.3) | |
| Female | 8 (13.3) | 6 (20.0) | 2 (6.7) | |
| Age | 0.6095 | |||
| Mean (SD) | 66.4 (8.2) | 67.0 (8.2) | 65.9 (8.4) | |
| Median (IQR) | 66.5 (12.5) | 65.5 (12.0) | 67.5 (14.0) | |
| Min–Max | 41.0–83.0 | 53.0–83.0 | 41.0–79.0 | |
| Age | 1.0000 | |||
| < 65 | 22 (36.7) | 11 (36.7) | 11 (36.7) | |
| ≥ 65 | 38 (63.3) | 19 (63.3) | 19 (63.3) | |
| BMI | 0.6930 | |||
| Mean (SD) | 27.9 (4.1) | 27.7 (3.2) | 28.1 (4.8) | |
| Median (IQR) | 27.2 (4.8) | 27.1 (4.2) | 27.3 (5.1) | |
| Min–Max | 19.5–38.0 | 22.8–35.3 | 19.5–38.0 | |
| BMI | 0.8752 | |||
| Normal | 15 (25.0) | 7 (23.3) | 8 (26.7) | |
| Overweight | 30 (50.0) | 16 (53.3) | 14 (46.7) | |
| Obese | 15 (25.0) | 7 (23.3) | 8 (26.7) | |
| Etiology: HBV | 0.1611 | |||
| Yes | 5 (8.3) | 1 (3.3) | 4 (13.3) | |
| No | 55 (91.7) | 29 (96.7) | 26 (86.7) | |
| Etiology: HCV | 1.0000 | |||
| Yes | 14 (23.3) | 7 (23.3) | 7 (23.3) | |
| No | 46 (76.7) | 23 (76.7) | 23 (76.7) | |
| Etiology: Alcohol | 0.1213 | |||
| Yes | 30 (50.0) | 18 (60.0) | 12 (40.0) | |
| No | 30 (50.0) | 12 (40.0) | 18 (60.0) | |
| Child Pugh | 0.7386 | |||
| A | 49 (81.7) | 25 (83.3) | 24 (80.0) | |
| B | 11 (18.3) | 5 (16.7) | 6 (20.0) | |
| BCLC | < 0.0001 | |||
| A | 30 (50.0) | 30 (100.0) | ||
| B | 8 (13.3) | 8 (26.7) | ||
| C | 22 (36.7) | 22 (73.3) | ||
| Liver Dominant Disease | 1.0000 | |||
| Yes | 58 (96.7) | 29 (96.7) | 29 (96.7) | |
| No | 2 (3.3) | 1 (3.3) | 1 (3.3) | |
| Extrahepatic Metastases | 0.0049 | |||
| Yes | 7 (11.7) | 7 (23.3) | ||
| No | 53 (88.3) | 30 (100) | 23 (76.7) | |
| Number of Liver Lesions | < 0.0001 | |||
| 1 | 30 (50.0) | 26 (86.7) | 4 (13.3) | |
| 2 | 8 (13.3) | 4 (13.3) | 4 (13.3) | |
| 3–10 | 8 (13.3) | 8 (26.7) | ||
| Diffuse Disease | 14 (23.3) | 14 (46.7) | ||
| PVI | 0.0015 | |||
| Missing | 3 (5.0) | 2 (6.7) | 1 (3.3) | |
| Yes | 12 (21.1) | 1 (3.6) | 11 (37.9) | |
| No | 45 (78.9) | 27 (96.4) | 18 (62.1) | |
| Bilirubin (mg/dL) | 0.0234 | |||
| Mean (SD) | 16.1 (7.8) | 18.3 (8.1) | 13.8 (7.0) | |
| Median (IQR) | 14.7 (11.0) | 17.4 (10.4) | 11.6 (8.6) | |
| Min–Max | 6.0–36.4 | 7.4–36.4 | 6.0–35.0 | |
| Albumin (g/dL) | 0.0966 | |||
| Mean (SD) | 37.0 (9.6) | 39.1 (5.3) | 34.9 (12.3) | |
| Median (IQR) | 39.6 (9.5) | 39.7 (7.5) | 39.2 (11.4) | |
| Min–Max | 0.5–47.0 | 27.4–47.0 | 0.5–46.9 | |
| ALBI Score | 0.6821 | |||
| Mean (SD) | −2.5 (0.6) | −2.5 (0.5) | −2.4 (0.7) | |
| Median (IQR) | −2.6 (0.9) | −2.5 (0.7) | −2.6 (1.2) | |
| Min–Max | −3.3–0.2 | −3.2–1.3 | −3.3–0.2 | |
| ALBI Grade | 1.0000 | |||
| Mean (SD) | 28 (46.7) | 14 (46.7) | 14 (46.7) | |
| Median (IQR) | 30 (50.0) | 15 (50.0) | 15 (50.0) | |
| Min–Max | 2 (3.3) | 1 (3.3) | 1 (3.3) |
| Group | Intention to Treat (ITT) | Actually Received Treatment | n | Treatment Group as Displayed in Manuscript |
|---|---|---|---|---|
| Early HCC | RFA + sorafenib | RFA + sorafenib | 15 | Early HCC with RFA and sorafenib |
| Early HCC | RFA + sorafenib | RFA (no sorafenib received) | 2 | Early HCC with RFA but no sorafenib |
| Early HCC | RFA + placebo | RFA + placebo | 13 | |
| Advanced HCC | SIRT/sorafenib | SIRT/sorafenib | 12 | Advanced HCC with SIRT and sorafenib |
| Advanced HCC | SIRT/sorafenib | Sorafenib (no SIRT received) | 2 | Advanced HCC without SIRT |
| Advanced HCC | Sorafenib | Sorafenib | 14 | |
| Advanced HCC | SIRT/sorafenib | (No study treatment) | 2 |
| Variables | Early HCC | Advanced HCC | p-Value |
|---|---|---|---|
| (n = 30) | (n = 30) | ||
| Creatine (µmol/L) | 32.2 (24.4) | 33.0 (25.2) | 0.9003 |
| Creatinine (µmol/L) | 95.8 (28.6) | 95.1 (32.4) | 0.9396 |
| Dimethylamine (µmol/L) | 4.3 (0.6) | 4.4 (0.7) | 0.4523 |
| Dimethylsulfone (µmol/L) | 12.2 (5.1) | 12.1 (6.4) | 0.9339 |
| Glycerol (µmol/L) | 173.0 (65.4) | 183.6 (58.8) | 0.5181 |
| Isoleucine (µmol/L) | 83.2 (21.5) | 85.5 (16.7) | 0.6527 |
| Myo-Inositol (µmol/L) | 71.3 (28.8) | 69.1 (17.3) | 0.7285 |
| Valine (µmol/L) | 282.5 (72.6) | 287.0 (54.4) | 0.7914 |
| GFR(NMR) (mL/min/1.73 m²) | 76.7 (21.4) | 79.8 (19.6) | 0.5711 |
| LVLDL-P (nmol/L) | 3.7 (2.9) | 3.0 (2.2) | 0.3076 |
| LDL-P (nmol/L) | 1132.1 (477.7) | 1291.1 (586.4) | 0.2575 |
| LLDL-P (nmol/L) | 632.8 (215.0) | 713.8 (300.7) | 0.2377 |
| SLDL-P (nmol/L) | 506.4 (343.5) | 581.9 (329.2) | 0.3922 |
| HDL-P (nmol/L) | 20,798.6 (7999.7) | 21,304.6 (8719.5) | 0.8171 |
| LHDL-P (nmol/L) | 6039.9 (2806.9) | 5140.3 (2189.9) | 0.1763 |
| SHDL-P (nmol/L) | 15,910.8 (7652.0) | 17,444.8 (7993.8) | 0.4663 |
| VLDL-s (nm) | 50.5 (4.8) | 49.7 (3.5) | 0.4531 |
| LDL-s (nm) | 21.3 (0.4) | 21.5 (0.5) | 0.1125 |
| HDL-s (nm) | 9.3 (0.5) | 9.2 (0.4) | 0.2851 |
| VLDL-c (mg/dL) | 24.2 (7.4) | 25.2 (10.4) | 0.6794 |
| IDL-c (mg/dL) | 43.8 (14.6) | 47.9 (17.4) | 0.3253 |
| LDL-c (mg/dL) | 97.3 (35.3) | 109.8 (38.8) | 0.2143 |
| LDL.A-c (mg/dL) | 32.3 (10.9) | 37.9 (9.7) | 0.045 |
| LDL.B-c (mg/dL) | 14.9 (10.5) | 20.4 (12.2) | 0.0767 |
| LDL.C-c(mg/dL) | 4.1 (2.1) | 4.0 (1.8) | 0.8177 |
| HDL.A-c (mg/dL) | 17.5 (6.1) | 18.5 (4.4) | 0.5037 |
| HDL.B-c (mg/dL) | 15.4 (2.3) | 15.2 (1.8) | 0.6692 |
| HDL.C-c (mg/dL) | 9.2 (5.9) | 10.6 (6.7) | 0.4451 |
| Total-Cholesterol (mg/dL) | 159.0 (53.8) | 181.1 (47.2) | 0.0997 |
| LDL-Cholesterol (mg/dL) | 95.1 (47.1) | 116.2 (44.3) | 0.0807 |
| HDL-Cholesterol (mg/dL) | 42.1 (11.5) | 42.0 (10.8) | 0.9912 |
| Triglycerides (mg/dL) | 124.4 (53.0) | 117.2 (54.6) | 0.6096 |
| Alanine (µmol/L) | 508.4 (112.1) | 471.8 (93.1) | 0.1789 |
| Leucine (µmol/L) | 158.4 (44.0) | 150.7 (36.2) | 0.4832 |
| Early and Advanced HCC | Correlation of Parameters with Overall Survival (OS) | |||
|---|---|---|---|---|
| Pearson Correlation | Spearman Correlation | |||
| Correlation Coefficient r | p-Value | Correlation Coefficient r | p-Value | |
| Creatine | −0.00129 | 0.9924 | −0.04675 | 0.7299 |
| Creatinine | 0.14623 | 0.2777 | 0.23309 | 0.0810 |
| Dimethylamine | 0.27911 | 0.0339 | 0.25011 | 0.0583 |
| Dimethylsulfone | 0.17080 | 0.1999 | 0.17478 | 0.1894 |
| Glycerol | −0.02346 | 0.8612 | 0.00386 | 0.9770 |
| Isoleucine | 0.04831 | 0.7187 | 0.13595 | 0.3089 |
| Myo-Inositol | 0.33071 | 0.0112 | 0.10792 | 0.4200 |
| Valine | 0.00968 | 0.9425 | 0.14008 | 0.2943 |
| GFR(NMR) | −0.20704 | 0.1223 | −0.24733 | 0.0636 |
| LVLDL-P | 0.00781 | 0.9532 | 0.06300 | 0.6355 |
| LDL-P | −0.36050 | 0.0050 | −0.32044 | 0.0134 |
| LLDL-P | −0.33219 | 0.0102 | −0.28946 | 0.0262 |
| SLDL-P | −0.31139 | 0.0164 | −0.29027 | 0.0257 |
| HDL-P | 0.02843 | 0.8308 | 0.11318 | 0.3934 |
| LHDL-P | −0.04053 | 0.7605 | 0.03888 | 0.7700 |
| SHDL-P | −0.04230 | 0.7569 | 0.02053 | 0.8806 |
| VLDL-s | −0.18036 | 0.1716 | −0.13655 | 0.3024 |
| LDL-s | −0.16874 | 0.2014 | −0.11896 | 0.3695 |
| HDL-s | −0.03146 | 0.8130 | 0.02049 | 0.8776 |
| VLDL-c | −0.23989 | 0.0672 | −0.12459 | 0.3471 |
| IDL-c | −0.28479 | 0.0288 | −0.26278 | 0.0444 |
| LDL-c | −0.30483 | 0.0224 | −0.27454 | 0.0406 |
| LDL.A-c | −0.32746 | 0.0114 | −0.28946 | 0.0262 |
| LDL.B-c | −0.35541 | 0.0072 | −0.30513 | 0.0222 |
| LDL.C-c | −0.07026 | 0.5969 | −0.03362 | 0.8005 |
| HDL.A.c | −0.33777 | 0.0089 | −0.29831 | 0.0217 |
| HDL.B-c | −0.13888 | 0.2942 | −0.09813 | 0.4597 |
| HDL.C-c | −0.00008 | 0.9995 | 0.02121 | 0.8790 |
| Total-cholesterol | −0.36757 | 0.0042 | −0.32705 | 0.0115 |
| LDL-cholesterol | −0.35939 | 0.0052 | −0.35565 | 0.0057 |
| HDL-cholesterol | −0.08681 | 0.5132 | −0.04480 | 0.7362 |
| Triglycerides | −0.05797 | 0.6627 | −0.03840 | 0.7728 |
| Alanine | −0.11190 | 0.3988 | −0.07233 | 0.5861 |
| Leucine | 0.05495 | 0.6931 | 0.12933 | 0.3513 |
| Parameter | Category | DF | Parameter | Standard | Chi-Squared | p-Value | Hazard | 95% Hazard Ratio Confidence | |
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Error | Ratio | |||||||
| Group | Advanced HCC (vs. ref.: Early HCC) | 1 | 3.343 | 0.677 | 24.348 | < 0.0001 | 28.296 | 7.500 | 106.746 |
| Age | ≥ 65 years (vs. ref.: < 65 years) | 1 | 0.754 | 0.481 | 2.457 | 0.117 | 2.126 | 0.828 | 5.461 |
| BMI | Obese (BMI > 30) (vs. ref.: normal) | 1 | −0.384 | 0.582 | 0.434 | 0.51 | 0,681 | 0.218 | 2.133 |
| BMI | Overweight (BMI 25–30) (vs. ref.: normal) | 1 | −0.881 | 0.478 | 3.398 | 0.0653 | 0.414 | 0.162 | 1.057 |
| Alcohol Etiology | Yes (vs. ref.: No) | 1 | 1.632 | 0.547 | 8.898 | 0.0029 | 5.115 | 1.750 | 14.947 |
| Child Pugh | Child Pugh B (vs. ref.: Child Pugh A) | 1 | 1.023 | 0.596 | 2.949 | 0.0859 | 2.782 | 0.865 | 8.943 |
| Dimethylamine | Continuous | 1 | 0.028 | 0.302 | 0.009 | 0.9264 | 1.028 | 0.569 | 1.859 |
| Myo-Inositol | Continuous | 1 | −0.031 | 0.012 | 7.117 | 0.0076 | 0.969 | 0.947 | 0.992 |
| LDL-P | Continuous | 1 | 0.001 | 0.000 | 4.581 | 0.0323 | 1.001 | 1.000 | 1.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geyer, T.; Rübenthaler, J.; Alunni-Fabbroni, M.; Schinner, R.; Weber, S.; Mayerle, J.; Schiffer, E.; Höckner, S.; Malfertheiner, P.; Ricke, J. NMR-Based Lipid Metabolite Profiles to Predict Outcomes in Patients Undergoing Interventional Therapy for a Hepatocellular Carcinoma (HCC): A Substudy of the SORAMIC Trial. Cancers 2021, 13, 2787. https://doi.org/10.3390/cancers13112787
Geyer T, Rübenthaler J, Alunni-Fabbroni M, Schinner R, Weber S, Mayerle J, Schiffer E, Höckner S, Malfertheiner P, Ricke J. NMR-Based Lipid Metabolite Profiles to Predict Outcomes in Patients Undergoing Interventional Therapy for a Hepatocellular Carcinoma (HCC): A Substudy of the SORAMIC Trial. Cancers. 2021; 13(11):2787. https://doi.org/10.3390/cancers13112787
Chicago/Turabian StyleGeyer, Thomas, Johannes Rübenthaler, Marianna Alunni-Fabbroni, Regina Schinner, Sabine Weber, Julia Mayerle, Eric Schiffer, Sebastian Höckner, Peter Malfertheiner, and Jens Ricke. 2021. "NMR-Based Lipid Metabolite Profiles to Predict Outcomes in Patients Undergoing Interventional Therapy for a Hepatocellular Carcinoma (HCC): A Substudy of the SORAMIC Trial" Cancers 13, no. 11: 2787. https://doi.org/10.3390/cancers13112787
APA StyleGeyer, T., Rübenthaler, J., Alunni-Fabbroni, M., Schinner, R., Weber, S., Mayerle, J., Schiffer, E., Höckner, S., Malfertheiner, P., & Ricke, J. (2021). NMR-Based Lipid Metabolite Profiles to Predict Outcomes in Patients Undergoing Interventional Therapy for a Hepatocellular Carcinoma (HCC): A Substudy of the SORAMIC Trial. Cancers, 13(11), 2787. https://doi.org/10.3390/cancers13112787







