Multiparametric Magnetic Resonance Imaging for Immediate Target Hit Assessment of CD13—Targeted Tissue Factor tTF-NGR in Advanced Malignant Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Treatment
2.3. MR Imaging
2.4. Immunohistochemistry (IHC) Staining
2.5. Statistical Analysis
3. Results
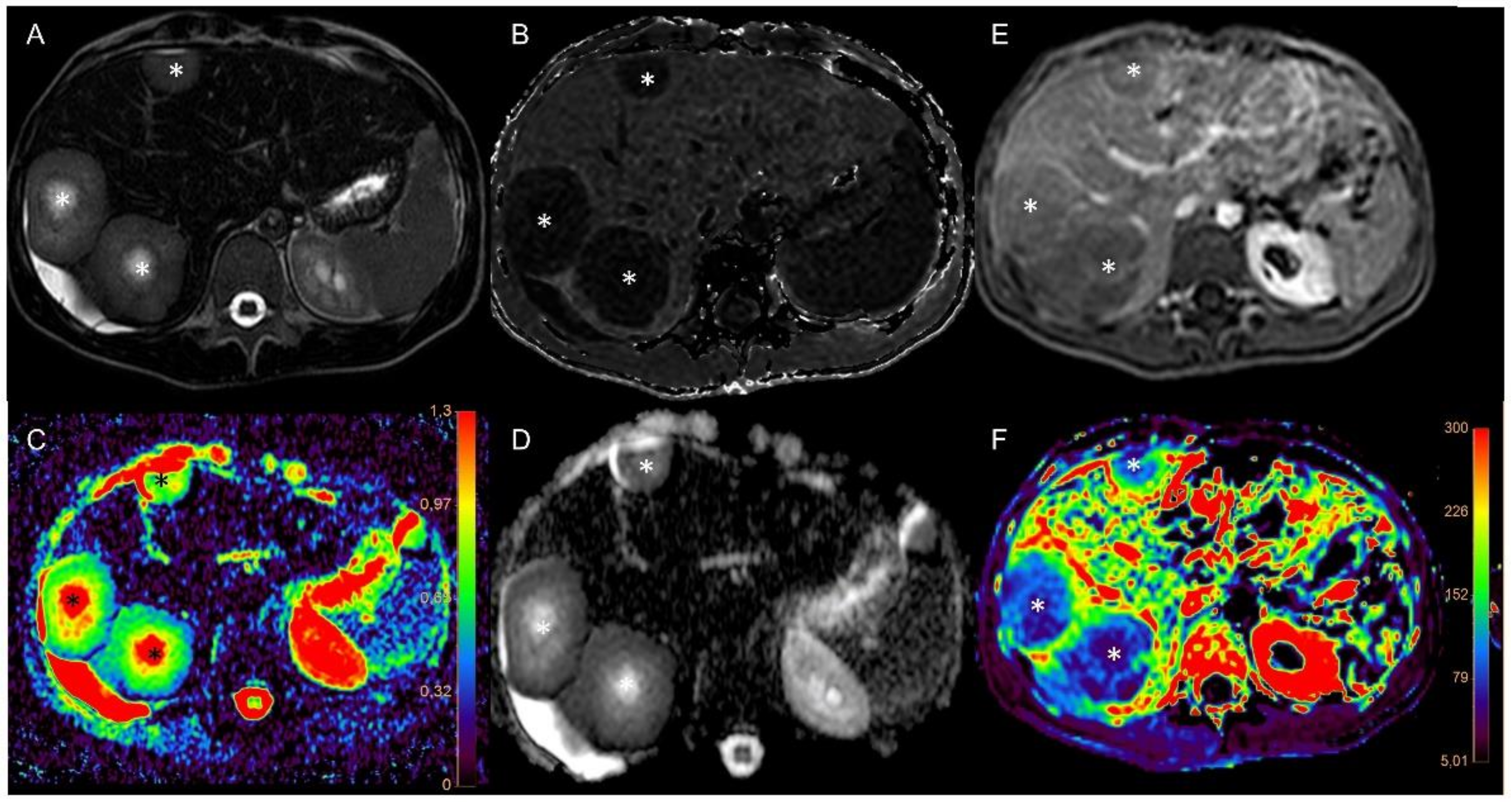
3.1. Imaging of Target Hit and Effectiveness
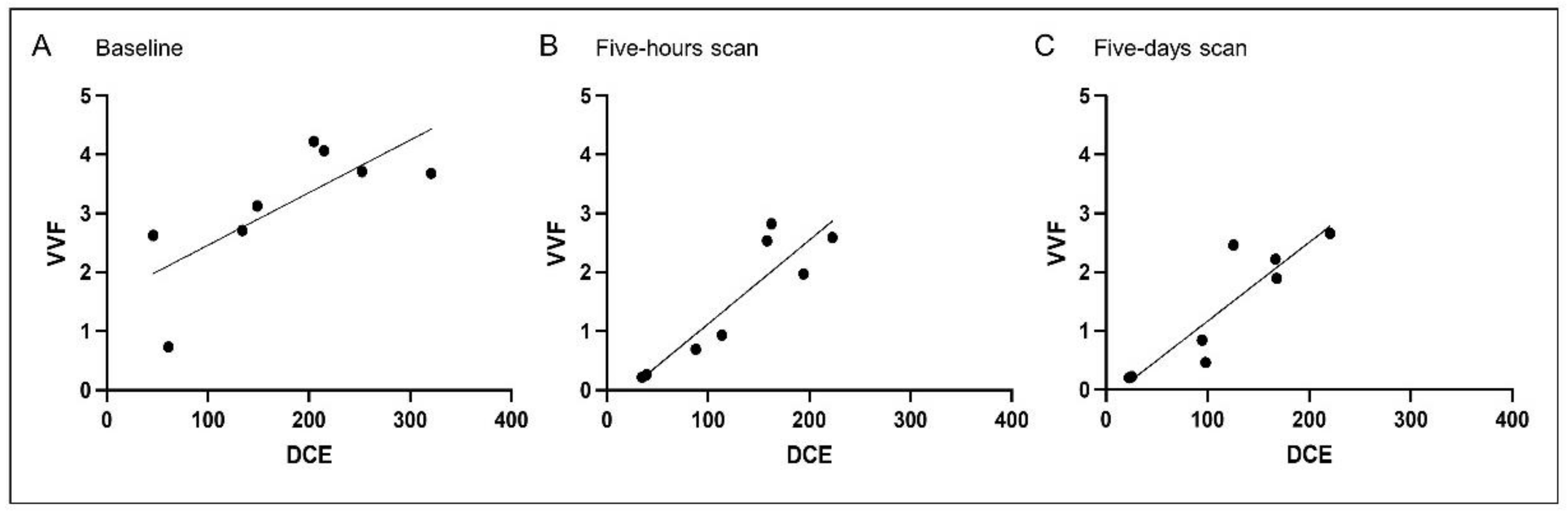
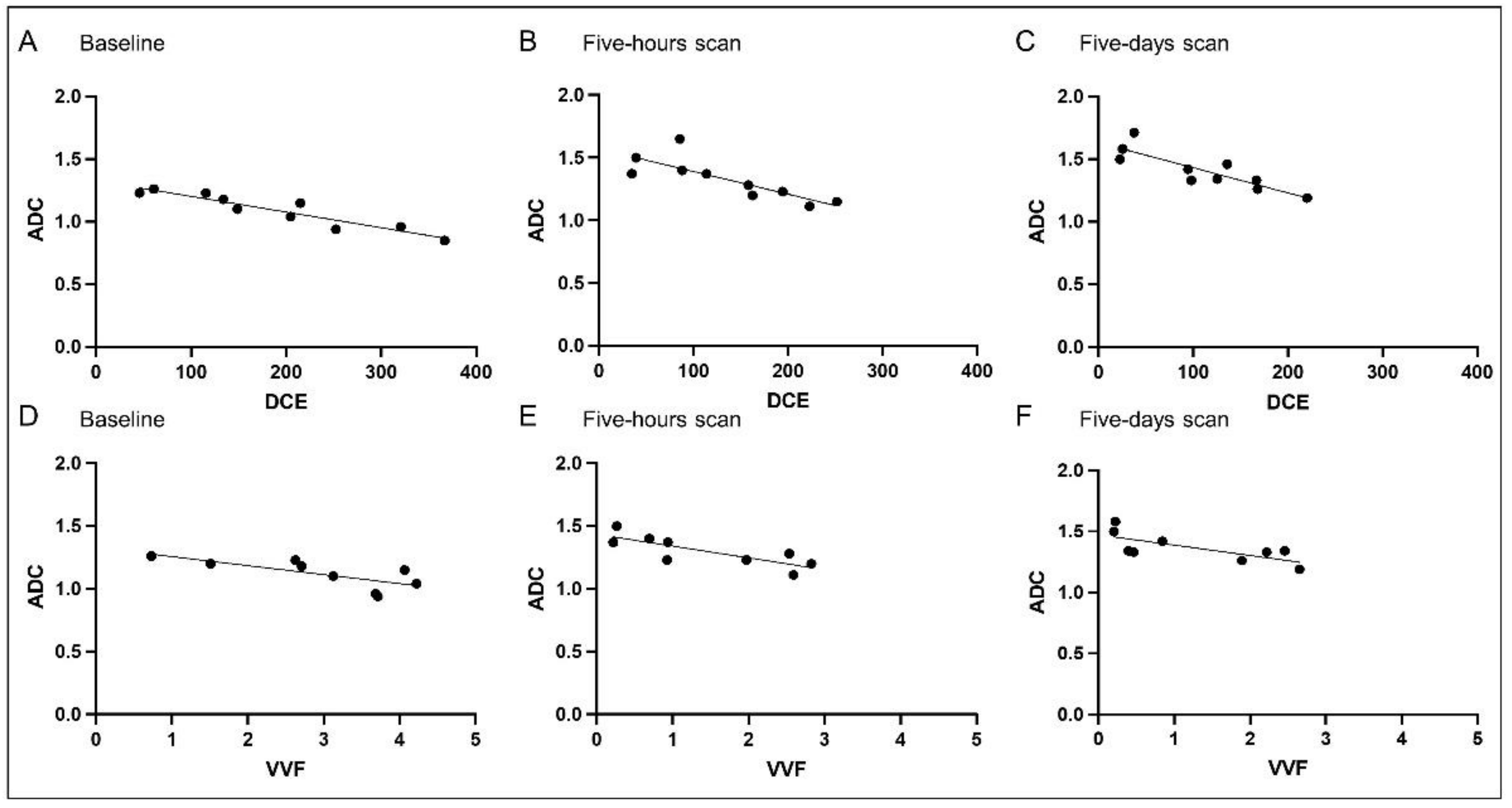
3.2. Correlation Analysis
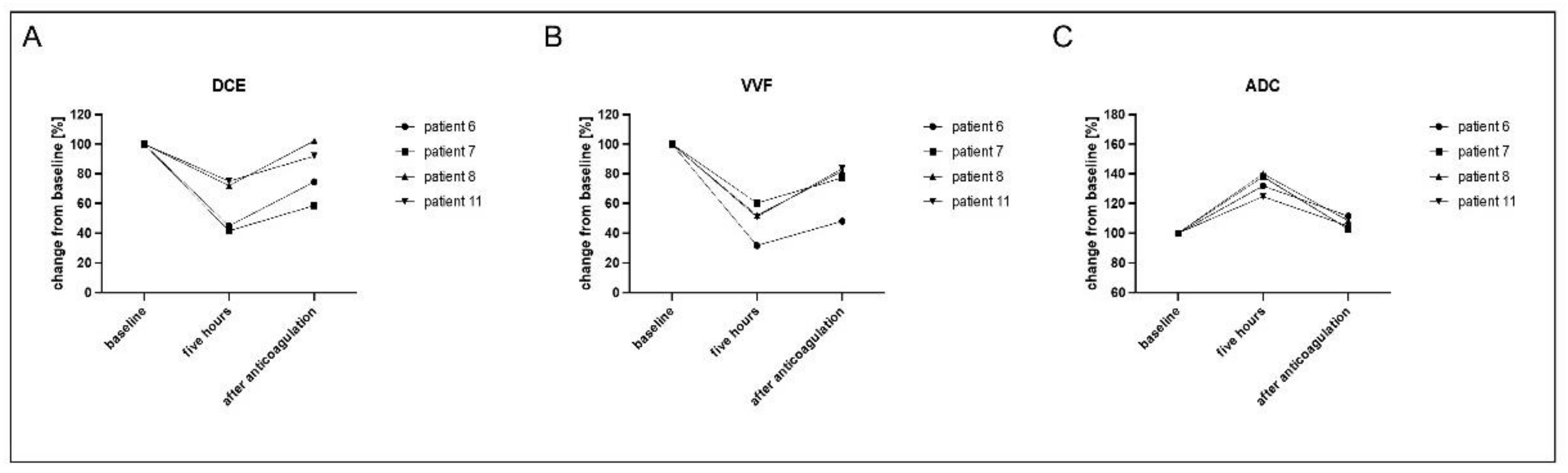
3.3. Evaluation of Reversed Effects Due to Therapeutic Anticoagulation
3.4. Correlation with Immunohistochemistry (IHC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerwing, M.; Herrmann, K.; Helfen, A.; Schliemann, C.; Berdel, W.E.; Eisenblatter, M.; Wildgruber, M. The beginning of the end for conventional RECIST—Novel therapies require novel imaging approaches. Nat. Rev. Clin. Oncol. 2019, 16, 442–458. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Noronha, V.; Joshi, A.; Muddu Krishna, V.; Juvekar, S.; Pantvaidya, G.; Chaturvedi, P.; Chaukar, D.; Arya, S.; Patil, A.; et al. Is There a Limitation of RECIST Criteria in Prediction of Pathological Response, in Head and Neck Cancers, to Postinduction Chemotherapy? ISRN Oncol. 2013, 2013, 259154. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.; Maitland, M.L.; Ratain, M.J. RECIST: No longer the sharpest tool in the oncology clinical trials toolbox—Point. Cancer Res. 2012, 72, 5145–5149, discussion 5150. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litiere, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Nishino, M.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Optimizing immune-related tumor response assessment: Does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J. Immunother. Cancer 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: Establishing a “common language” for the new arena of cancer treatment. J. Immunother. Cancer 2016, 4, 30. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Punt, C.J.A.; Huiskens, J.; van Gulik, T.; Engelbrecht, M. Pseudoprogression on bevacizumab treatment: Tumor-dynamics in the modern era of systemic treatment for metastatic colorectal cancer. Acta Oncol. 2018, 57, 681–682. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.-P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. (Sudbury Mass.) 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Willett, C.G.; Boucher, Y.; di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V.; et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004, 10, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, C.; Gerwing, M.; Heinzow, H.; Harrach, S.; Schwöppe, C.; Wildgruber, M.; Hansmeier, A.A.; Angenendt, L.; Berdel, A.F.; Stalmann, U.; et al. First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study. Cancers 2020, 12, 1488. [Google Scholar] [CrossRef]
- Bieker, R.; Kessler, T.; Schwöppe, C.; Padró, T.; Persigehl, T.; Bremer, C.; Dreischalück, J.; Kolkmeyer, A.; Heindel, W.; Mesters, R.M.; et al. Infarction of tumor vessels by NGR-peptide-directed targeting of tissue factor: Experimental results and first-in-man experience. Blood 2009, 113, 5019–5027. [Google Scholar] [CrossRef] [PubMed]
- Berdel, A.F.; Schwöppe, C.; Brand, C.; Harrach, S.; Brömmel, K.; Hintelmann, H.; Lenz, G.; Liersch, R.; Heinzow, H.; Schliemann, C.; et al. Targeting Tissue Factor to Tumor Vasculature to Induce Tumor Infarction. Cancers 2021, 13, 2841. [Google Scholar] [CrossRef]
- Stucke-Ring, J.; Ronnacker, J.; Brand, C.; Höltke, C.; Schliemann, C.; Kessler, T.; Schmidt, L.H.; Harrach, S.; Mantke, V.; Hintelmann, H.; et al. Combinatorial effects of doxorubicin and retargeted tissue factor by intratumoral entrapment of doxorubicin and proapoptotic increase of tumor vascular infarction. Oncotarget 2016, 7, 82458–82472. [Google Scholar] [CrossRef] [PubMed]
- Kesler, M.; Levine, C.; Hershkovitz, D.; Mishani, E.; Menachem, Y.; Lerman, H.; Zohar, Y.; Shibolet, O.; Even-Sapir, E. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: A prospective pilot study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 60, 185–191. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, N.; Zhang, J.; Lang, L.; Zhang, W.; Li, S.; Zhao, J.; Niu, G.; Li, F.; Zhu, Z.; et al. 68Ga-NOTA-PRGD2 PET/CT for Integrin Imaging in Patients with Lung Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1823–1827. [Google Scholar] [CrossRef]
- Nathan, P.; Zweifel, M.; Padhani, A.R.; Koh, D.-M.; Ng, M.; Collins, D.J.; Harris, A.; Carden, C.; Smythe, J.; Fisher, N.; et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 3428–3439. [Google Scholar] [CrossRef]
- Persigehl, T.; Ring, J.; Bremer, C.; Heindel, W.; Holtmeier, R.; Stypmann, J.; Claesener, M.; Hermann, S.; Schäfers, M.; Zerbst, C.; et al. Non-invasive monitoring of tumor-vessel infarction by retargeted truncated tissue factor tTF-NGR using multi-modal imaging. Angiogenesis 2014, 17, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Cyran, C.C.; Nikolaou, K.; Hirner, H.; Reiser, M.F.; Dietrich, O. Monitoring early response to anti-angiogenic therapy: Diffusion-weighted magnetic resonance imaging and volume measurements in colon carcinoma xenografts. PLoS ONE 2014, 9, e106970. [Google Scholar] [CrossRef]
- Fredrickson, J.; Serkova, N.J.; Wyatt, S.K.; Carano, R.A.; Pirzkall, A.; Rhee, I.; Rosen, L.S.; Bessudo, A.; Weekes, C.; de Crespigny, A. Clinical translation of ferumoxytol-based vessel size imaging (VSI): Feasibility in a phase I oncology clinical trial population. Magn. Reson. Med. 2017, 77, 814–825. [Google Scholar] [CrossRef]
- Flexman, J.A.; Yung, A.; Yapp, D.T.; Ng, S.S.; Kozlowski, P. Assessment of vessel size by MRI in an orthotopic model of human pancreatic cancer. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Daldrup, H.E.; Shames, D.M.; Husseini, W.; Wendland, M.F.; Okuhata, Y.; Brasch, R.C. Quantification of the extraction fraction for gadopentetate across breast cancer capillaries. Magn. Reson. Med. 1998, 40, 537–543. [Google Scholar] [CrossRef]
- Galbraith, S.M.; Lodge, M.A.; Taylor, N.J.; Rustin, G.J.; Bentzen, S.; Stirling, J.J.; Padhani, A.R. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: Comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002, 15, 132–142. [Google Scholar] [CrossRef]
- Kiessling, F.; Jugold, M.; Woenne, E.C.; Brix, G. Non-invasive assessment of vessel morphology and function in tumors by magnetic resonance imaging. Eur. Radiol. 2007, 17, 2136–2148. [Google Scholar] [CrossRef]
- Persigehl, T.; Wall, A.; Kellert, J.; Ring, J.; Remmele, S.; Heindel, W.; Dahnke, H.; Bremer, C. Tumor blood volume determination by using susceptibility-corrected DeltaR2* multiecho MR. Radiology 2010, 255, 781–789. [Google Scholar] [CrossRef]
- Koh, T.S.; Hennedige, T.P.; Thng, C.H.; Hartono, S.; Ng, Q.S. Understanding K (trans): A simulation study based on a multiple-pathway model. Phys. Med. Biol. 2017, 62, N297–N319. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, X.; Shen, B. Contrast agents in dynamic contrast-enhanced magnetic resonance imaging. Oncotarget 2017, 8, 43491–43505. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.J.M.; Padhani, A.R. T1-W DCE-MRI: T1-Weighted Dynamic Contrast-Enhanced MRI. In Quantitative MRI of the Brain; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 341–364. [Google Scholar]
- Khalifa, F.; Soliman, A.; El-Baz, A.; Abou El-Ghar, M.; El-Diasty, T.; Gimel'farb, G.; Ouseph, R.; Dwyer, A.C. Models and methods for analyzing DCE-MRI: A review. Med. Phys. 2014, 41, 124301. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Quaas, A.; Quantius, J.; Loser, H.; Meinel, J.; Peifer, M.; Wagner, S.; Gattenlohner, S.; Wittekindt, C.; von Knebel Doeberitz, M.; et al. Deep Learning Predicts HPV Association in Oropharyngeal Squamous Cell Carcinomas and Identifies Patients with a Favorable Prognosis Using Regular H&E Stains. Clin. Cancer Res. 2021, 27, 1131–1138. [Google Scholar] [CrossRef]
- Klein, S.; Mauch, C.; Brinker, K.; Noh, K.W.; Knez, S.; Buttner, R.; Quaas, A.; Helbig, D. Tumor infiltrating lymphocyte clusters are associated with response to immune checkpoint inhibition in BRAF V600(E/K) mutated malignant melanomas. Sci. Rep. 2021, 11, 1834. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Tong, H.; Wang, S.; Yang, Y.; Liu, G.; Zhang, W. Clinical Applications of Contrast-Enhanced Perfusion MRI Techniques in Gliomas: Recent Advances and Current Challenges. Contrast Media Mol. Imaging 2017, 2017, 7064120. [Google Scholar] [CrossRef]
- Hayes, C.; Padhani, A.R.; Leach, M.O. Assessing changes in tumour vascular function using dynamic contrast-enhanced magnetic resonance imaging. NMR Biomed. 2002, 15, 154–163. [Google Scholar] [CrossRef]
- Ehling, J.; Lammers, T.; Kiessling, F. Non-invasive imaging for studying anti-angiogenic therapy effects. Thromb. Haemost. 2013, 109, 375–390. [Google Scholar] [CrossRef]
- Winfield, J.M.; Payne, G.S.; Weller, A.; de Souza, N.M. DCE-MRI, DW-MRI, and MRS in Cancer: Challenges and Advantages of Implementing Qualitative and Quantitative Multi-parametric Imaging in the Clinic. Top. Magn. Reson. Imaging 2016, 25, 245–254. [Google Scholar] [CrossRef]
- Höink, A.; Persigehl, T.; Kwiecien, R.; Balthasar, M.; Mesters, R.; Berdel, W.; Heindel, W.; Bremer, C.; Schwöppe, C. Gadofosveset-enhanced MRI as simple surrogate parameter for real-time evaluation of the initial tumour vessel infarction by retargeted tissue factor tTF-NGR. Oncol. Lett. 2019, 17, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Iima, M.; Honda, M.; Sigmund, E.E.; Ohno Kishimoto, A.; Kataoka, M.; Togashi, K. Diffusion MRI of the breast: Current status and future directions. J. Magn. Reson. Imaging 2020, 52, 70–90. [Google Scholar] [CrossRef]
- Theilmann, R.J.; Borders, R.; Trouard, T.P.; Xia, G.; Outwater, E.; Ranger-Moore, J.; Gillies, R.J.; Stopeck, A. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 2004, 6, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Yan, C.; Zhu, R.; Wang, J.; Wang, Y.; Wang, Y.; Wang, R.; Feng, F.; Ma, W. Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas. Neuroimage Clin. 2018, 20, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, M.; Konaktchieva, M.; Weisel, K.; Kraus, S.; Claussen, C.D.; Horger, M. Response assessment in patients with multiple myeloma during antiangiogenic therapy using arterial spin labeling and diffusion-weighted imaging: A feasibility study. Acad. Radiol. 2010, 17, 1326–1333. [Google Scholar] [CrossRef]
- Thoeny, H.C.; De Keyzer, F.; Chen, F.; Vandecaveye, V.; Verbeken, E.K.; Ahmed, B.; Sun, X.; Ni, Y.; Bosmans, H.; Hermans, R.; et al. Diffusion-weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a-4 phosphate after repeated administration. Neoplasia 2005, 7, 779–787. [Google Scholar] [CrossRef]
- Wybranski, C.; Zeile, M.; Lowenthal, D.; Fischbach, F.; Pech, M.; Rohl, F.W.; Gademann, G.; Ricke, J.; Dudeck, O. Value of diffusion weighted MR imaging as an early surrogate parameter for evaluation of tumor response to high-dose-rate brachytherapy of colorectal liver metastases. Radiat. Oncol. 2011, 6, 43. [Google Scholar] [CrossRef]
- Halappa, V.G.; Bonekamp, S.; Corona-Villalobos, C.P.; Li, Z.; Mensa, M.; Reyes, D.; Eng, J.; Bhagat, N.; Pawlik, T.M.; Geschwind, J.F.; et al. Intrahepatic cholangiocarcinoma treated with local-regional therapy: Quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology 2012, 264, 285–294. [Google Scholar] [CrossRef]
- Shaghaghi, M.; Aliyari Ghasabeh, M.; Ameli, S.; Ghadimi, M.; Hazhirkarzar, B.; Rezvani Habibabadi, R.; Khoshpouri, P.; Pandey, A.; Pandey, P.; Kamel, I.R. Post-TACE changes in ADC histogram predict overall and transplant-free survival in patients with well-defined HCC: A retrospective cohort with up to 10 years follow-up. Eur. Radiol. 2021, 31, 1378–1390. [Google Scholar] [CrossRef]
- Hamm, B.; Staks, T.; Taupitz, M.; Maibauer, R.; Speidel, A.; Huppertz, A.; Frenzel, T.; Lawaczeck, R.; Wolf, K.J.; Lange, L. Contrast-enhanced MR imaging of liver and spleen: First experience in humans with a new superparamagnetic iron oxide. J. Magn. Reson. Imaging 1994, 4, 659–668. [Google Scholar] [CrossRef]
- Haegele, J.; Duschka, R.L.; Graeser, M.; Schaecke, C.; Panagiotopoulos, N.; Ludtke-Buzug, K.; Buzug, T.M.; Barkhausen, J.; Vogt, F.M. Magnetic particle imaging: Kinetics of the intravascular signal in vivo. Int. J. Nanomed. 2014, 9, 4203–4209. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef]


| Parameter | Baseline | Five-Hours Scan | Five-Days Scan |
|---|---|---|---|
| DCE | r = 0.1084 p = 0.838 | r = 0.1539 p = 0.771 | r = 0.08016 p = 0.88 |
| VVF | r = −0.5441 p = 0.4559 | r = −0.1657 p = 0.8343 | r = −0.2757 p = 0.7243 |
| ADC | r = −0.1724 p = 0.7439 | r = −0.2192 p = 0.6765 | r = −0.3883 p = 0.5184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerwing, M.; Krähling, T.; Schliemann, C.; Harrach, S.; Schwöppe, C.; Berdel, A.F.; Klein, S.; Hartmann, W.; Wardelmann, E.; Heindel, W.L.; et al. Multiparametric Magnetic Resonance Imaging for Immediate Target Hit Assessment of CD13—Targeted Tissue Factor tTF-NGR in Advanced Malignant Disease. Cancers 2021, 13, 5880. https://doi.org/10.3390/cancers13235880
Gerwing M, Krähling T, Schliemann C, Harrach S, Schwöppe C, Berdel AF, Klein S, Hartmann W, Wardelmann E, Heindel WL, et al. Multiparametric Magnetic Resonance Imaging for Immediate Target Hit Assessment of CD13—Targeted Tissue Factor tTF-NGR in Advanced Malignant Disease. Cancers. 2021; 13(23):5880. https://doi.org/10.3390/cancers13235880
Chicago/Turabian StyleGerwing, Mirjam, Tobias Krähling, Christoph Schliemann, Saliha Harrach, Christian Schwöppe, Andrew F. Berdel, Sebastian Klein, Wolfgang Hartmann, Eva Wardelmann, Walter L. Heindel, and et al. 2021. "Multiparametric Magnetic Resonance Imaging for Immediate Target Hit Assessment of CD13—Targeted Tissue Factor tTF-NGR in Advanced Malignant Disease" Cancers 13, no. 23: 5880. https://doi.org/10.3390/cancers13235880
APA StyleGerwing, M., Krähling, T., Schliemann, C., Harrach, S., Schwöppe, C., Berdel, A. F., Klein, S., Hartmann, W., Wardelmann, E., Heindel, W. L., Lenz, G., Berdel, W. E., & Wildgruber, M. (2021). Multiparametric Magnetic Resonance Imaging for Immediate Target Hit Assessment of CD13—Targeted Tissue Factor tTF-NGR in Advanced Malignant Disease. Cancers, 13(23), 5880. https://doi.org/10.3390/cancers13235880








