A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy
Abstract
:Simple Summary
Abstract
1. Cancer: A Global Public Health Issue
2. Genome Instability: A Basic Mechanism in Cancer Development
3. Green Synthesized Metallic NPs: An Insight
4. NPs for Cancer Therapy
5. The Fate of Cancer Cells Exposed to NPs
6. Anti-Cancer Activities of Biosynthesized Metallic NPs
6.1. Applications of Biosynthesized Silver NPs (AgNPs) as Anti-Cancer Therapeutics
6.2. Applications of Biosynthesized Gold NPs (AuNPs) as Anti-Cancer Therapeutics
6.3. Applications of Biosynthesized Zinc and Zinc Oxide NPs (Zn/ZnO-NPs) as Anti-Cancer Therapeutics
6.4. Applications of Biosynthesized Copper/Copper Oxide NPs (Cu/CuO-NPs) as Anti-Cancer Therapeutics
7. Nano-Toxicity, the Concern/Bottleneck
8. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sufyani, A.; Moslah, N.; Hussien, N.A.; Hawsawi, Y.M. Characterization and Anticancer Potential of Silver Nanoparticles Biosynthesized from Olea chrysophylla and Lavandula dentata Leaf Extracts on HCT116 Colon Cancer Cells. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.; Shim, Y.Y.; Reaney, M.J.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized silver nanoparticles: A step forward for cancer theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef]
- Macdonald, J.S. Toxicity of 5-fluorouracil. Oncology 1999, 13, 33–34. [Google Scholar] [PubMed]
- Khan, S.; Parvez, S.; Chaudhari, B.; Ahmad, F.; Anjum, S.; Raisuddin, S. Ellagic acid attenuates bleomycin and cyclophosphamide-induced pulmonary toxicity in Wistar rats. Food Chem. Toxicol. 2013, 58, 210–219. [Google Scholar]
- Adamson, I. Pulmonary toxicity of bleomycin. Environ. Health Perspect. 1976, 16, 119–125. [Google Scholar] [CrossRef]
- Avilés, A.; Arévila, N.; Díaz Maqueo, J.C.; Nambo, M.J. Late cardiac toxicity of doxorubicin, epirubicin, and mitoxantrone therapy for Hodgkin′s disease in adults. Leuk. Lymphoma 1993, 11, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A. Alternative cancer cures:“unproven” or “disproven”? CA Cancer J. Clin. 2004, 54, 110–118. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zivyar, N.; Bagherzade, G.; Moudi, M.; Manzari Tavakoli, M. Evaluation of the green synthesis, characterization and antibacterial activity of silver nanoparticles from corm extract of Crocus sativus var. Haussknechtii. J. Hortic. Postharvest Res. 2021, 19–32. [Google Scholar] [CrossRef]
- Nguyen, K.T. Targeted nanoparticles for cancer therapy: Promises and challenge. Nanomed. Nanotechnol. 2011. [Google Scholar] [CrossRef] [Green Version]
- Leal-Esteban, L.C.; Fajas, L. Cell cycle regulators in cancer cell metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165715. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93. [Google Scholar] [CrossRef] [Green Version]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer. Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Lipsick, J. A history of cancer research: Tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2020, 12, a035907. [Google Scholar] [CrossRef] [PubMed]
- Kaptain, S.; Tan, L.K.; Chen, B. Her-2/neu and breast cancer. Diagn. Mol. Pathol. 2001, 10, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Hermeking, H. The MYC oncogene as a cancer drug target. Curr. Cancer Drug Targets 2003, 3, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Finn, R. Targeting Src in breast cancer. Ann. Oncol. 2008, 19, 1379–1386. [Google Scholar] [CrossRef]
- Janknecht, R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 2004, 564, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Liu, S.; Tao, Y. Regulating tumor suppressor genes: Post-translational modifications. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch repair pathway, genome stability and cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Ruggiano, A.; Ramadan, K. DNA–protein crosslink proteases in genome stability. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Ui, A.; Chiba, N.; Yasui, A. Relationship among DNA double-strand break (DSB), DSB repair, and transcription prevents genome instability and cancer. Cancer Sci. 2020, 111, 1443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Han, Y.; Ji, T.; Huang, X.; Gao, Q.; Ma, D. RAD54B potentiates tumor growth and predicts poor prognosis of patients with luminal a breast cancer. Biomed. Pharmacother. 2019, 118, 109341. [Google Scholar] [CrossRef]
- McAndrew, E.N.; McManus, K.J. The enigmatic oncogene and tumor suppressor-like properties of RAD54B: Insights into genome instability and cancer. Genes Chromosomes Cancer 2017, 56, 513–523. [Google Scholar] [CrossRef]
- Yasuhara, T.; Suzuki, T.; Katsura, M.; Miyagawa, K. Rad54B serves as a scaffold in the DNA damage response that limits checkpoint strength. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitot, H.C. The molecular biology of carcinogenesis. Cancer 1993, 72, 962–970. [Google Scholar] [CrossRef]
- Chung, G.; Sundaresan, V.; Hasleton, P.; Rudd, R.; Taylor, R.; Rabbitts, P. Sequential molecular genetic changes in lung cancer development. Oncogene 1995, 11, 2591–2598. [Google Scholar]
- Haggman, M.J.; Macoska, J.A.; Wojno, K.J.; Oesterling, J.E. The relationship between prostatic intraepithelial neoplasia and prostate cancer: Critical issues. J. Urol. 1997, 158, 12–22. [Google Scholar] [CrossRef]
- Khan, S.A. Metal nanoparticles toxicity: Role of physicochemical aspects. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–11. [Google Scholar]
- Singh, J.; Singh, T.; Rawat, M. Green synthesis of silver nanoparticles via various plant extracts for anti-cancer applications. Nanomedicine 2017, 7, 1–4. [Google Scholar]
- Perveen, S.; Al-Taweel, A.M. Green Chemistry and Synthesis of Anticancer Molecule. Green Chem. 2018, 51–72. [Google Scholar] [CrossRef] [Green Version]
- Cyril, N.; George, J.B.; Joseph, L.; Raghavamenon, A.; VP, S. Assessment of antioxidant, antibacterial and anti-proliferative (lung cancer cell line A549) activities of green synthesized silver nanoparticles from Derris trifoliata. Toxicol. Res. 2019, 8, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Aziz, N.; Faraz, M.; Pandey, R.; Shakir, M.; Fatma, T.; Varma, A.; Barman, I.; Prasad, R. Facile algae-derived route to biogenic silver nanoparticles: Synthesis, antibacterial, and photocatalytic properties. Langmuir 2015, 31, 11605–11612. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Wong, C.; Yasumira, A. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E -J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H. Protein-assisted nanoparticle synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 464–470. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahal, A.; SAINI, A.; Chhillar, A.K.; SAINI, R. Natural antioxidants as defense system against cancer. Asian J. Pharm. Clin. Res. 2018, 11, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Hira, I.; Kumar, A.; Kumari, R.; Saini, A.K.; Saini, R.V. Pectin-guar gum-zinc oxide nanocomposite enhances human lymphocytes cytotoxicity towards lung and breast carcinomas. Mater. Sci. Eng. C 2018, 90, 494–503. [Google Scholar] [CrossRef]
- Likus, W.; Bajor, G.; Siemianowicz, K. Nanosilver-does it have only one face? Acta Biochim. Pol. 2013, 60. [Google Scholar] [CrossRef]
- Jurj, A.; Braicu, C.; Pop, L.-A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2871. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577. [Google Scholar] [CrossRef] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.L.; Bernas, L.M.; Rutt, B.K.; Foster, P.J.; Gillies, E.R. Enhanced cell uptake of superparamagnetic iron oxide nanoparticles functionalized with dendritic guanidines. Bioconjugate Chem. 2008, 19, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Telrandhe, R. Anti-Cancer Potential of Green Synthesized Silver Nanoparticles-A Review. Asian J. Pharm. Technol. 2019, 9, 260–266. [Google Scholar] [CrossRef]
- Nguyen, K.M. Key Receiver Circuits for Digital Beamforming in Millimeter-Wave Imaging. Mass. Inst. Technol. 2011. Available online: http://hdl.handle.net/1721.1/64587 (accessed on 10 March 2011).
- Saad, M.; Garbuzenko, O.B.; Ber, E.; Chandna, P.; Khandare, J.J.; Pozharov, V.P.; Minko, T. Receptor targeted polymers, dendrimers, liposomes: Which nanocarrier is the most efficient for tumor-specific treatment and imaging? J. Control. Release 2008, 130, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbiah, R.; Veerapandian, M.; S Yun, K. Nanoparticles: Functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [Green Version]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
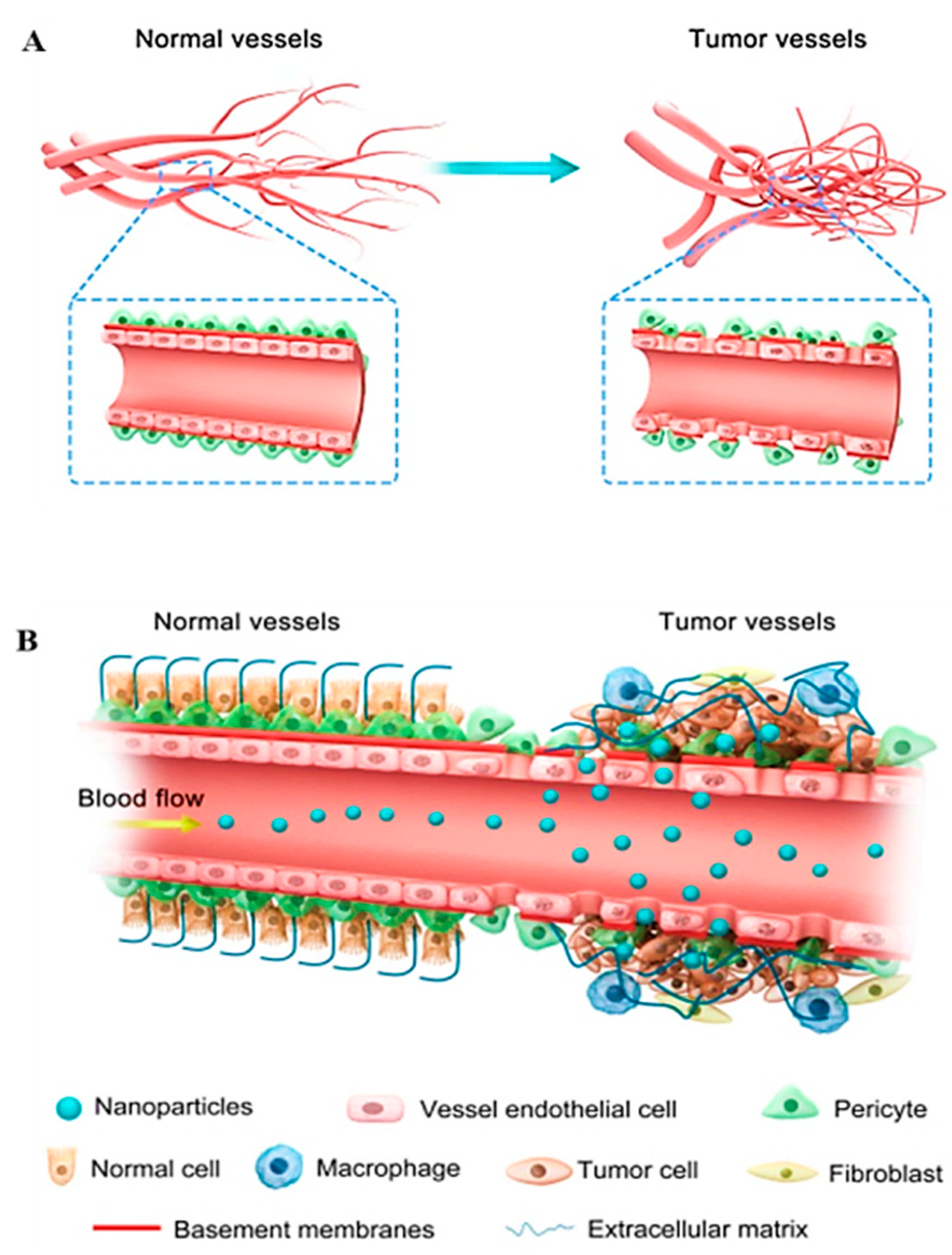
- Abdalla, A.M.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 2018, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Barabadi, H.; Alizadeh, A.; Ovais, M.; Ahmadi, A.; Shinwari, Z.K.; Saravanan, M. Efficacy of green nanoparticles against cancerous and normal cell lines: A systematic review and meta-analysis. IET Nanobiotechnol. 2018, 12, 377–391. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. Comparative therapeutic effects of plant-extract synthesized and traditionally synthesized gold nanoparticles on alcohol-induced inflammatory activity in SH-SY5Y cells in vitro. Biomedicines 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yan, Y.; Zhao, Y.; Guo, F.; Jiang, C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS ONE 2012, 7, e43442. [Google Scholar] [CrossRef]
- De Stefano, D.; Carnuccio, R.; Maiuri, M.C. Nanomaterials toxicity and cell death modalities. J. Drug Deliv. 2012, 2012, 167896. [Google Scholar] [CrossRef] [Green Version]
- Halamoda Kenzaoui, B.; Chapuis Bernasconi, C.; Guney-Ayra, S.; Juillerat-Jeanneret, L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem. J. 2012, 441, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaid, P.; Raizada, P.; Saini, A.K.; Saini, R.V. Biogenic silver, gold and copper nanoparticles-A sustainable green chemistry approach for cancer therapy. Sustain. Chem. Pharm. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Mishra, A.; Mehdi, S.J.; Irshad, M.; Ali, A.; Sardar, M.; Moshahid, M.; Rizvi, A. Effect of biologically synthesized silver nanoparticles on human cancer cells. Sci. Adv. Mater. 2012, 4, 1200–1206. [Google Scholar] [CrossRef]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, K.; Krishnamoorthy, K.; Alsagaby, S.A.; Singaravelu, G.; Premanathan, M. Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnol. 2015, 9, 325–330. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.; Antony, J.; lakkannan, S.K.; Thangam, R.; Gunasekaran, P.; Krishnan, M.L.; Achiraman, S. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochemistry 2012, 47, 273. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B Biointerfaces 2013, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Thanki, K.; Jain, S.; Banerjee, U.C. Comparative studies of anticancer and antimicrobial potential of bioinspired silver and silver-selenium nanoparticles. J. Mater. Nanosci. 2016, 3, 22–27. [Google Scholar]
- Nakkala, J.R.; Mata, R.; Gupta, A.K.; Sadras, S.R. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur. J. Med. Chem. 2014, 85, 784–794. [Google Scholar] [CrossRef]
- Rajkuberan, C.; Sudha, K.; Sathishkumar, G.; Sivaramakrishnan, S. Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 924–930. [Google Scholar] [CrossRef]
- Vijistella Bai, G. Green synthesis of silver nanostructures against human cancer cell lines and certain pathogens. Int. J. Pharm. Chem. Biol. Sci. 2014, 4, 101–111. [Google Scholar]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R. Br.) Asch. & Magnus mediated silver nanoparticles. J. Photochem. Photobiol. B Biol. 2015, 153, 145–152. [Google Scholar]
- Devi, J.S.; Bhimba, B.V.; Ratnam, K. In vitro anticancer activity of silver nanoparticles synthesized using the extract of Gelidiella sp. Int. J. Pharm. Pharm. Sci. 2012, 4, 710–715. [Google Scholar]
- Kuppusamy, P.; Ichwan, S.J.; Al-Zikri, P.N.H.; Suriyah, W.H.; Soundharrajan, I.; Govindan, N.; Maniam, G.P.; Yusoff, M.M. In vitro anticancer activity of Au, Ag nanoparticles synthesized using Commelina nudiflora L. aqueous extract against HCT-116 colon cancer cells. Biol. Trace Elem. Res. 2016, 173, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Shawkey, A.M.; Rabeh, M.A.; Abdulall, A.K.; Abdellatif, A.O. Green nanotechnology: Anticancer activity of silver nanoparticles using Citrullus colocynthis aqueous extracts. Adv. Life Sci. Technol. 2013, 13, 60–70. [Google Scholar]
- Prabhu, D.; Arulvasu, C.; Babu, G.; Manikandan, R.; Srinivasan, P. Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo L. induce growth-inhibitory effect on human colon cancer cell line HCT15. Process. Biochem. 2013, 48, 317–324. [Google Scholar] [CrossRef]
- Manikandan, R.; Manikandan, B.; Raman, T.; Arunagirinathan, K.; Prabhu, N.M.; Basu, M.J.; Perumal, M.; Palanisamy, S.; Munusamy, A. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 120–129. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kanipandian, N.; Thirumurugan, R. A feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypium hirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Ind. Crop. Prod. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Subramanian, V.; Tumala, A.; Vellaichamy, E. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac. J. Trop. Med. 2014, 7, S294–S300. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, K.; Rather, H.; Rajagopal, K.; Shanthi, M.; Sheriff, K.; Illiyas, M.; Rather, R.; Manikandan, E.; Uvarajan, S.; Bhaskar, M. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, P.; Sathishkumar, G.; Sankar, R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 885–890. [Google Scholar] [CrossRef]
- Sre, P.R.; Reka, M.; Poovazhagi, R.; Kumar, M.A.; Murugesan, K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 1137–1144. [Google Scholar]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Evaluation of antibacterial activity and cytotoxicity of green synthesized silver nanoparticles using Scoparia dulcis. Nano Biomed. Nano Biomed Eng. 2015, 7, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Shandiz, S.A.S.; Ghanbar, F.; Darvish, M.R.; Ardestani, M.S.; Mirzaie, A.; Jafari, M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016, 11, 1835. [Google Scholar]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of silver nanoparticles using Taxus yunnanensis callus and their antibacterial activity and cytotoxicity in human cancer cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef]
- Firdhouse, J.; Lalitha, P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 2015, 4, 113–121. [Google Scholar]
- Elangovan, K.; Elumalai, S.; Anupriya, S.; Shenbhagaraman, R.; Kaleena, P.K.; Murugesan, K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographis echioides and its bio-efficacy on anticancer and antibacterial activities. J. Photochem. Photobiol. B Biol. 2015, 151, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Johnson, P.; Raji, V.; Abdull, R.M.Y.; Fuad, A.; Sadhasivam, S.; Ramasamy, B.; Thayumanavan, P. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol. B Biol. 2016, 163, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Debabrata, C.; Rajesh, K.; Sujata, P. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Jayachandra, D.; Nagoor, V.; Rani, M.; Sudha, S.R. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Sharma, D.; Lalita, L.; Nitu, B. Antimicrobial and cytotoxic potential of silver nanoparticles synthesized using Rheum emodi roots extract. New Front. Chem. 2015, 24, 121. [Google Scholar]
- Devi, J.S.; Bhimba, B.V.; Ratnam, K. Anticancer activity of silver nanoparticles synthesized by the seaweed Ulva lactuca in vitro. Sci. Rep. 2012, 1, 242. [Google Scholar]
- Baharara, J.; Farideh, N.; Tayebe, R.; Marzieh, M.; Rosfarizan, M. Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: Apoptosis induction in MCF-7 cells via caspase activation and regulation of Bax and Bcl-2 gene expression. Molecules 2015, 20, 2693–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathiravan, V.; Ravi, S.; Ashokkumar, S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; Mubarak, A.D.; Rajesh, M.; Arun, R.; Kapildev, G. Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B Biointerfaces 2013, 106, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 1–8. [Google Scholar]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Toxicity Study of Silver Nanoparticles Synthesized from Suaeda monoica on Hep-2 Cell Line. Avicenna J. Med. Biotechnol. 2012, 4, 35. [Google Scholar] [PubMed]
- Kumar, B.; Kumari, S.; Rachid, S.; Karen, B.; Marcelo, G.; Luis, C. In vitro evaluation of silver nanoparticles cytotoxicity on Hepatic cancer (Hep-G2) cell line and their antioxidant activity: Green approach for fabrication and application. J. Photochem. Photobiol. B Biol. 2016, 159, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Sudip, M.; Ayan, K.B.; Anirban, G.; Bojja, S.; Chitta, R.P. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef]
- He, Y.; Zhiyun, D.; Shijing, M.; Yue, L.; Dongli, L.; Huarong, H.; Sen, J. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomed. 2016, 11, 1879. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Yogamoorthy, A.; Sundarapandian, S.M. Green synthesis of gold nanoparticles and evaluation of its cytotoxic property against colon cancer cell line. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 1–17. [Google Scholar]
- Wang, L.; Jianwei, X.; Ye, Y.; Han, L.; Thiruventhan, K.; Feng, L. Green synthesis of gold nanoparticles from Scutellaria barbata and its anticancer activity in pancreatic cancer cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortie, M.B.; David, L.; Cortie Victoria, T. Heat transfer from nanoparticles for targeted destruction of infectious organisms. Int. J. Hyperth. 2018, 34, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Uma Suganya, K.S.; Govindaraju, K.; Prabhu, D.; Arulvasu, C.; Karthick, V.; Niranjan, C. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 2016, 371, 415–424. [Google Scholar]
- Arunkumar, P.; Hemamalini, V.; Kumpati, P. Rapid bioreduction of trivalent aurum using banana stem powder and its cytotoxicity against MCF-7 and HEK-293 cell lines. J. Nanoparticle Res. 2013, 15, 1–8. [Google Scholar] [CrossRef]
- Balasubramani, G.; Rajendira, R.; Narayanaswamy, K.; Annamalai, P.; Thillainathan, N.; Rajamani, S.; Pachiappan, P. Structural characterization, antioxidant and anticancer properties of gold nanoparticles synthesized from leaf extract (decoction) of Antigonon leptopus Hook. & Arn. J. Trace Elem. Med. Biol. 2015, 30, 83–89. [Google Scholar]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.D.; Kalaichelvan, P.T. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef] [Green Version]
- El-Kassas, H.Y.; El-Sheekh, M.M. Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 4311–4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banu, H.; Renuka, N.; Faheem, S.M.; Ismail, R.; Singh, V.; Saadatmand, Z.; Khan, S.S.; Narayanan, K.; Raheem, A.; Premkumar, K.; et al. Gold and silver nanoparticles biomimetically synthesized using date palm pollen extract-induce apoptosis and regulate p53 and Bcl-2 expression in human breast adenocarcinoma cells. Biol. Trace Elem. Res. 2018, 186, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, K.; Mathew, N.L.; Nellore, J.; Siddarth, C.R.V.; Kumar, J. Facile synthesis of biocompatible gold nanoparticles from Vites vinefera and its cellular internalization against HBL-100 cells. Cancer Nanotechnol. 2011, 2, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagalingam, M.; Kalpana, V.N.; Panneerselvam, A. Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible gold nanoparticles from Alternanthera bettzickiana. Biotechnol. Rep. 2018, 19, 268. [Google Scholar]
- Ramalingam, V.; Revathidevi, S.; Shanmuganayagam, T.; Muthulakshmi, L.; Rajaram, R. Biogenic gold nanoparticles induce cell cycle arrest through oxidative stress and sensitize mitochondrial membranes in A549 lung cancer cells. RSC Adv. 2016, 6, 20598–20608. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Pavagadhi, S.; Mahadevan, A.; Balasubramanian, R. Biosynthesis of gold nanoparticles and related cytotoxicity evaluation using A549 cells. Ecotoxicol. Environ. Saf. 2015, 114, 232–240. [Google Scholar] [CrossRef]
- Yang, N.; WeiHong, L.; Hao, L. Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells. Mater. Lett. 2014, 134, 67–70. [Google Scholar] [CrossRef]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J. Med Biotechnol. 2016, 8, 75. [Google Scholar]
- Saikia, I.; Sonowal, S.; Pal, M.; Boruah, P.K.; Das, M.R.; Tamuly, C. Biosynthesis of gold decorated reduced graphene oxide and its biological activities. Mater. Lett. 2016, 178, 239–242. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Camacho, J.; Hernández-Gallegos, E.; de Guadalupe Chávez-López, M.; Grijalva, M.; Andrade, K. One pot phytosynthesis of gold nanoparticles using Genipa americana fruit extract and its biological applications. Mater. Sci. Eng. C 2016, 62, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, N.; Jamshidi, F. Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: Synthesis, characterization, and evaluation of anticancer and antibacterial activity. J. Appl. Biomed. 2016, 14, 235–245. [Google Scholar] [CrossRef]
- Rajan, A.; Vilas, V.; Philip, D. Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J. Mol. Liq. 2015, 212, 331–339. [Google Scholar] [CrossRef]
- Mishra, P.; Ray, S.; Sinha, S.; Das, B.; Khan, M.I.; Behera, S.K.; Yun, S.I.; Tripathy, S.K.; Mishra, A. Facile bio-synthesis of gold nanoparticles by using extract of Hibiscus sabdariffa and evaluation of its cytotoxicity against U87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016, 105, 264–272. [Google Scholar] [CrossRef]
- Wani, K.; Choudhari, A.; Chikate, R.; Kaul-Ghanekar, R. Synthesis and characterization of gold nanoparticles using Ficus religiosa extract. Carbon Sci. Technol. 2013, 5, 203–210. [Google Scholar]
- Geetha, R.; Ashokkumar, T.; Tamilselvan, S.; Govindaraju, K.; Sadiq, M.; Singaravelu, G. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol. 2013, 4, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathishkumar, G.; Bharti, R.; Jha, P.K.; Selvakumar, M.; Dey, G.; Jha, R.; Jeyaraj, M.; Mandal, M.; Sivaramakrishnan, S. Dietary flavone chrysin (5, 7-dihydroxyflavone ChR) functionalized highly-stable metal nanoformulations for improved anticancer applications. RSC Adv. 2015, 5, 89869–89878. [Google Scholar] [CrossRef]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [Green Version]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis—antiproliferative effect against prostate cancer cells. Cancer Nanotechnol. 2013, 4, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.W.; Weber, P.K.; Martin, M.C.; Gilbert, B.; Hutcheon, I.D.; Banfield, J.F. Extracellular proteins limit the dispersal of biogenic nanoparticles. Science 2007, 316, 1600–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, G.M.; Dringen, R.; Robinson, S.R. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free. Radic. Biol. Med. 2007, 42, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Gazaryan, I.G.; Krasnikov, B.F.; Ashby, G.A.; Thorneley, R.N.; Kristal, B.S.; Brown, A.M. Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J. Biol. Chem. 2002, 277, 10064–10072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.S. Physiological significance of metallothionein in oxidative stress. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2007, 127, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Park, Y.C.; Lee, S.W.; Jeong, M.S.; Yu, K.N.; Jung, H.; Lee, J.K.; Kim, J.S.; Cho, M.H. Comparing the toxic mechanism of synthesized zinc oxide nanomaterials by physicochemical characterization and reactive oxygen species properties. Toxicol. Lett. 2011, 207, 197–203. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, L.; Bian, X.; Kong, J.; Yang, P.; Liu, B. pH-Controlled delivery of doxorubicin to cancer cells, based on small mesoporous carbon nanospheres. Small 2012, 8, 2715–2720. [Google Scholar] [CrossRef]
- Wang, L.; Bowman, L.; Lu, Y.; Rojanasakul, Y.; Mercer, R.R.; Castranova, V.; Ding, M. Essential role of p53 in silica-induced apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L488–L496. [Google Scholar] [CrossRef]
- Saud Alarifi, D.A.; Alkahtani, S.; Verma, A.; Ahamed, M.; Ahmed, M.; Alhadlaq, H.A. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int. J. Nanomed. 2013, 8, 983. [Google Scholar]
- Gong, K.W.; Zhao, W.; Li, N.; Barajas, B.; Kleinman, M.; Sioutas, C.; Horvath, S.; Lusis, A.J.; Nel, A.; Araujo, J.A. Air-pollutant chemicals and oxidized lipids exhibit genome-wide synergistic effects on endothelial cells. Genome Biol. 2007, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Q.; Hein, S.; Misra, R.D.K. New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: Synthesis, characterization and in vitro drug delivery response. Acta Biomater. 2010, 6, 2732–2739. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Talalay, P.; Dinkova-Kostova, A.T.; Holtzclaw, W.D. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzym. Regul. 2003, 43, 121–134. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C. Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Colloids Surf. B Biointerfaces 2006, 47, 140–145. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Tian, M.; Li, C. Copper-based nanomaterials for cancer imaging and therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017, 10, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. JBIC J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro study. Biotechnol. Prog. 2018, 34, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239. [Google Scholar] [CrossRef]
- Harne, S.; Sharma, A.; Dhaygude, M.; Joglekar, S.; Kodam, K.; Hudlikar, M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf. B Biointerfaces 2012, 95, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.R.; Kanchi, S.; Naidoo, E.B. In-vitro evaluation of copper nanoparticles cytotoxicity on prostate cancer cell lines and their antioxidant, sensing and catalytic activity: One-pot green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 375–382. [Google Scholar] [CrossRef]
- Wang, X.; Reece, S.P.; Brown, J.M. Immunotoxicological impact of engineered nanomaterial exposure: Mechanisms of immune cell modulation. Toxicol. Mech. Methods 2013, 23, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.C.; Chan, W.C. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef]
- Wahab, R.; Kaushik, N.K.; Kaushik, N.; Choi, E.H.; Umar, A.; Dwivedi, S.; Musarrat, J.; Al-Khedhairy, A.A. ZnO nanoparticles induces cell death in malignant human T98G gliomas, KB and non-malignant HEK cells. J. Biomed. Nanotechnol. 2013, 9, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef]
- Handy, R.D.; Von der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Plant | Plant Part Used | Morphology/Size (nm) | Exposure Time | Cancer Type/Cell Line | IC50 Value | Ref. |
|---|---|---|---|---|---|---|
| Moringa olifera | Stem bark | Spherical/38–40 | 24 h | Cervical Cancer/HeLa | Dose dependent | [78] |
| Sargassum vulgare | Whole plant | Spherical/10 | 3 h | Cervical Cancer/HeLa | Dose dependent | [79] |
| Melia azedarach | Leaf | Spherical, cubical/78 | 10 min | Cervical Cancer/HeLa | 300μg/mL (LD50) | [80] |
| Podophyllum hexandrum | Leaf | Spherical/14 | 30–150 min | Cervical Cancer/HeLa | 20 μg/mL | [81] |
| Syzygium cumini | Leaf | Spherical/<40 | 6 h | Cervical Cancer/HeLa | Dose dependent | [82] |
| Azadiracht a indica | Leaf | Hexagonal, triangular/2–18 | - | Cervical cancer/Siha | ≤4.25 μg/mL | [77] |
| Acorous calamus | Rhizome | Spherical/31.86 | 20 h | Cervical cancer/Siha | Dose dependent | [83] |
| Calotropis gigantea | Latex | Spherical/5–30 | 24 h | Cervical cancer/Siha | Dose dependent | [84] |
| Heliotropium indicum | Leaf | Spherical/80–120 | 2 h | Cervical cancer/Siha | 20 μg/mL | [85] |
| Cymodocea serrulata | Whole plant | Spherical/17–29 | 2 h | Cervical cancer/Siha | 107.7 (GI50) | [86] |
| Ulva lactuca (algae) | Whole plant | Spherical/56 | 10 min | Colon Cancer/HT29 | 49 μg/mL | [87] |
| Commelina nudiflora L. | Whole plant | Spherical, triangular/24–80 | 24 h | Colon Cancer/HCT-116 | 100 μg/mL | [88] |
| Citrullus colocynthis | Leaf | Spherical/13.37 | 24 h | Colon Cancer/HCT-116 | >30 μg/mL | [89] |
| Citrullus colocynthis | Seeds | Spherical/16.57 | 24 h | Colon Cancer/HCT-116 | >30 μg/mL | [89] |
| Citrullus colocynthis | Fruit | Spherical/19.26 | 24 h | Colon Cancer/HCT-116 | 21.2 μg/mL | [89] |
| Vitex negundo | Leaf | Spherical/22 | 4 h | Colon Cancer/HCT 15 | 20 μg/mL | [90] |
| Rosa indica | Petal | Spherical/23.52–60.83 | 1 h | Colon Cancer/HCT 15 | 30 μg/mL | [91] |
| Artemisia princeps | Leaf | Spherical/20 | 15 min | Lung cancer/A549 | Time dependent | [92] |
| Gossypium hirsutum | Leaf | Spherical/13–40 | 3 min | Lung cancer/A549 | 40 μg/mL | [93] |
| Origanum vulgare | Leaf | Spherical/136 ± 10.09 | Temp. dependent | Lung cancer/A549 | 100 μg/mL (LD50) | [94] |
| Rosa damascene | Petal | Spherical/15–27 | 0–25 min | Lung cancer/A549 | 80 μg/mL | [95] |
| Syzygium aromaticum | Fruit | Spherical/5–20 | 20 min | Lung cancer/A549 | 70 μg/mL | [96] |
| Acorous calamus | Rhizome | Spherical/31.86 | 20 h | Lung cancer/A549 | Dose dependent | [77] |
| Cymodocea serrulate | Leaf | Spherical/29.28 | 1 h | Lung cancer/A549 | 100 μg/mL (LD50) | [97] |
| Olax scandens | Leaf | Spherical/30–60 | 2 h | Lung cancer/A549 | Dose dependent | [98] |
| Scoparia dulcis | Leaf | Spherical/15–25 | 1 h | Lung cancer/A549 | Dose dependent | [99] |
| Artemisia marschalliana | Shoots | Spherical/5–50 | 5 min | Gastric cancer/AGS | 21.05 μg/mL | [100] |
| Taxus yunnanensis | Callus | Spherical/6.4–27.2 | 10 min | Intestinal cancer/SMMC-7721 | 27.75 μg/mL | [101] |
| Cucurbita maxima | Petal | Spherical, cuboidal/76 | 5–60 min | Epidermoid cancer/A431 | 82.39 μg/mL | [102] |
| Acorus calamus | Rhizome | Spherical, cuboidal/59 | 5–60 min | Epidermoid cancer/A431 | 78.58 μg/mL | [102] |
| Alternanthera sessilis | Shoots/Aerial parts | Spherical/10–30 | 6 h | Breast cancer/MCF-7 | 3.04 μg/mL | [103] |
| Andrographis echioides | Leaf | Pentagonal, cubic, hexagonal/68.06 | 12 h | Breast cancer/MCF-7 | 31.5 μg/mL | [104] |
| Butea monosperma | Leaf | Spherical/20–80 | 2 h | Breast cancer/MCF-7 | Dose dependent | [105] |
| Citrullus colocynthis | Roots | Spherical/7.39 | 24 h | Breast cancer/MCF-7 | 2.4 μg/mL | [89] |
| Citrullus colocynthis | Fruit | Spherical/19.26 | 24 h | Breast cancer/MCF-7 | >30 μg/mL | [89] |
| Citrullus colocynthis | Leaf | Spherical/13.37 | 24 h | Breast cancer/MCF-7 | >30 μg/mL | [89] |
| Citrullus colocynthis | Seeds | Spherical/16.57 | 24 h | Breast cancer/MCF-7 | >30 μg/mL | [89] |
| Erythrina indica | Root | Spherical/20–118 | Overnight | Breast cancer/MCF-7 | - | [98] |
| Olax scandens | Leaf | Spherical/30–60 | 2 h | Breast cancer/MCF-7 | Dose dependent | [106] |
| Piper longum | Fruit | Spherical/46 | 24 h | Breast cancer/MCF-7 | 67 μg/mL | [107] |
| Rheum emodi | Root | Spherical/27.5 | 24 h | Breast cancer/MCF-7 | Dose dependent | [108] |
| Syzygium cumini | Flower | Spherical/40 | 6 h | Breast cancer/MCF-7 | Dose dependent | [82] |
| Taxus baccata | Needles | Spherical/56 | 10 min | Breast cancer/MCF-7 | 37 μg/mL | [109] |
| Syzygium aromaticum | Fruit | Spherical/5–20 | 20 min | Breast cancer/MCF-7 | 70 μg/mL | [96] |
| Ulva lactuca | Whole plant | Spherical/56 | 10 min | Breast cancer/MCF-7 | 37 μg/mL | [109] |
| Achillea biebersteinii | Flower | Spherical, pentagonal/12 | 3 h | Breast cancer/MCF-7 | 20 μg/mL | [110] |
| Azadirachta indica | Leaf | Spherical/<40 | 6 h | Breast cancer/MCF-7 | Dose dependent | [82] |
| Melia dubia | Leaf | Irregular/7.3 | 15 min | Breast cancer/MCF-7 | 31.2 μg/mL | [111] |
| Sesbania grandiflora | Leaf | Spherical/22 | 24 h | Breast cancer/MCF-7 | 20 μg/mL | [112] |
| Citrullus colocynthi s | Callus | Spherical/31 | 24 h | Laryngeal Cancer/Hep-2 | 3.42 μg/mL | [113] |
| Suaeda monoica | Leaf | Spherical/31 | 5 h | Laryngeal Cancer/Hep-2 | 500 nM, AgNPs conc. | [114] |
| Ulva lactuca (algae) | Whole plant | Spherical/56 | 10 min | Laryngeal Cancer/Hep-2 | 12.5 μg/mL | [109] |
| Rubus glaucus Benth | Root | Quasi-spherical/12–50 | 48 h | Hepatic cancer/Hep-G2 | Dose dependent | [115] |
| Citrullus colocynthis | Root | Spherical/7.39 | 24 h | Hepatic cancer/Hep-G2 | 17.2 μg/mL | [116] |
| Citrullus colocynthis | Fruit | Spherical/19.26 | 24 h | Hepatic cancer/Hep-G2 | 22.4 μg/mL | [116] |
| Citrullus colocynthis | Leaf | Spherical/13.37 | 24 h | Hepatic cancer/Hep-G2 | 10.02 μg/mL | [116] |
| Sargassum vulgare | Whole plant | Spherical/10 | 3 h | Leukemia cancer/HL-60 | Dose dependent | [79] |
| Dimocarpus longan | Peel | Spherical/8–22 | 2 h | Leukemia cancer/H1299 | 5.33 μg/mL | [117] |
| Azadirachta indica | Leaf | Spherical/< 40 | 6 h | Kidney cancer/Hek-293 | Dose dependent | [82] |
| Plant | Plant Part Used | Morphology/Size (nm) | Exposure Time | Cancer Type/Cell Line Used | IC50 Value | Ref. |
|---|---|---|---|---|---|---|
| Azadirachta indica | Leaf | Spherical, triangular, hexagonal | 48 h | Cervical cancer/HeLa | No toxicity | [133] |
| Genipa americana L. | Fruit | Spherical/30.4 ± 14.9 | 48 h | Cervical cancer/HeLa | No toxicity | [134] |
| Dracocephalum kotschyi | Leaf | Spherical/11 | 24 h, 48 h, 72 h | Cervical cancer/HeLa | 152.16 µg/mL | [135] |
| Zataria multiflora | Leaf | Pentagon, triangular/10–50 | 48 h | Cervical cancer/HeLa | 100 µg/m | [132] |
| Areca catechu | Nut | Spherical/22.2 | 24 h | Cervical cancer/HeLa | 25.17 µg/mL | [136] |
| Mimosa pudica | Leaf | Spherical/12 | 24 h, 48 h | Breast cancer/MCF-7 | 6 µg/mL | [121] |
| Musa paradisiaca (banana) | Stem | Spherical/30 | 24 h | Breast cancer/MCF-7 | Low toxicity | [122] |
| Antigonon letopus Hook. and Arn. | Aerial part | Spherical, triangular/13–28 | 48 h | Breast cancer/MCF-7 | 257.8 μg/mL | [123] |
| Corallina officinalis | Aqueous Extract | Spherical/14.6 | NA | Breast Cancer/MCF-7 | NA | [125] |
| Phoenix dactylifera | flower | Near spherical/95 | 24 h | Breast Cancer/MCF-7 | 4.76 μg/mL | [126] |
| Vites vinefera | Aqueous Extract | Spherical/20–45 | 24 h | Breast Cancer/HBL- 100 | NA | [127] |
| Acalypha indica | Leaf | Spherical/20–30 | 30 min | Breast Cancer/MDA- MB-231 | NA | [124] |
| Alternanthera bettzickiana | Leaf | Spherical and aggregated/80–120 | 10 min | Lung Cancer/A549 | NA | [128] |
| Sesuvium portulacastrum | Leaf | Mostly Spherical/35–40 | 0–8 h | Lung Cancer/A549 | 14 μg/mL | [129] |
| Star anise (Illicium verum) | Pod | Hexagonal, triangular/20–150 | 48 h | Lung cancer/A549 | Low toxicity | [130] |
| Star anise (Illicium verum) | Pod | Hexagonal, triangular/20–50 | 48 h | Lung cancer/A549 | Low toxicity at 200 nM | [130] |
| Musa paradisiaca (banana) | Stem | Spherical/30 | 24 h | Kidney cancer/HEK293 | >80 nM | [122] |
| Ficus religiosa | Bark | Spherical/20–30 | 24 h | Kidney cancer/HEK 293 | No toxicity | [138] |
| Hibiscus sabdariffa | Leaf, stem | Near spherical/10–60 | 48 h | Kidney cancer/HEK 293 | 2 ng/mL | [137] |
| Couroupita guianensis | Flower | Polydispersed, spherical, triangular, tetragonal/7–48 | 5 min | Leukaemia/HL-60 | NA | [139] |
| Cajanus cajan | Seed coat | Spherical/9–41 | 24 h | Liver cancer/HepG2 | 6 µg/mL | [140] |
| Plant | Plant Part Used | Morphology/Size (nm) | Exposure Time | Cancer Type/Cell Line | IC50 Value | Ref. |
|---|---|---|---|---|---|---|
| Abutilon indicum | Leaf | Spherical/35.2 ± 2.3 | 2–3 h | Lung cancer/Calu-6 | 9.34 ± 0.4 μg/mL | [143] |
| Calotropis gigantea | Leaf | Spherical/30–35 | 3 h | Lung cancer/Calu-6 | 11.6 ± 0.9 μg/mL | [144] |
| Laurus nobilis | Leaf | Hexagonal/47.27 | 4 h | Lung cancer/A549 | 11.3 ± 0.9 μg/mL | [142] |
| Cannabis sativa | Leaf | Hexagonal/40 ± 1.5 | 3 h | Lung cancer/A549 | 18.3 ± 1.3 μg/mL | [145] |
| Calotropis procera | Leaf | Spherical/5–40 | 4 h | Lung cancer/A549 | 15.2 ± 1.6 μg/mL | [146] |
| Withania Somnifera | Leaf | Hexagonal/51.34 | 2–3 h | Leukemia/WEHI-3 | 12.4 ± 1.6 μg/mL | [147] |
| Sargassum muticum | Leaf | Spherical/22.5 ± 3.5 | 3–4 h | Leukemia/WEHI-3 | 2.25 ± 0.4 μg/mL | [148] |
| Tabernaemontana divaricate | Leaf | Spherical/36 ± 5 | 3 h | Breast cancer/MCF-7 | 30.6 μg/mL | [149] |
| Tabernaemontana divaricate | Leaf | Spherical/36 ± 5 | 4 h | Breast cancer/MCF-7 | 30.6 μg/mL | [150] |
| Tabernaemontana | Leaf | Spherical/36 ± 5 | 3–4 h | Breast cancer/MCF-7 | 30 μg/mL | [151] |
| Borassus flabellifer | Leaf | Spherical/55 | 3 h | Breast cancer/MCF-7 | 0.125 μg/mL | [152] |
| Embelia ribes | Root | Spherical/130–150 | 2 h | Breast cancer/MCF-7 | 9.62 ± 1.9 μg/mL | [153] |
| Saccharum officinarum | Juice | Spherical/19 ± 2.3 | 4 h | Breast cancer/MCF-7 | 16.7 ± 0.5 μg/mL | [106] |
| Anabaena variabilis | Phyco-bili pigment | Spherical/42 ± 3 | 5–6 h | Breast cancer/MCF-7 | 16.5 1.6 μg/mL | [154] |
| Atropa belladonna | Leaf | Hexagonal/34 ± 3.2 | 2 h | Breast cancer/MCF-7 | 12 ±0.9 μg/mL | [160] |
| Plant | Plant Part Used | Morphology/Size (nm) | Exposure Time | CancerType/Cell Line | IC50 Value | Ref. |
|---|---|---|---|---|---|---|
| Azadirachta indica | Leaf | Spherical/12 | 1 h | Cervical Cancer/HeLa | 0.89 μg/mL | [164] |
| Phaseolus vulgaris | Seed | Spherical/26.6 | 7–8 h | Cervical Cancer/HeLa | NA | [163] |
| Calotropis procera L. | Latex | Spherical/5–30 | 24 h | Cervical Cancer/HeLa | No toxicity | [168] |
| Azadirachta indica | Leaf | Spherical/12 | 1 h | Breast cancer/MCF-7 | 27.4, 45.3, 37μg/mL | [164] |
| Olea europaea | - | Spherical/20–50 | 24 h | Breast cancer/AMJ-13 | 1.47 μg/mL | [166] |
| Acalypha indica | Leaf | Spherical/26–30 | 48 h | Breast cancer/MCF-7 | 56.16 μg/mL | [165] |
| Ficus religiosa | Leaf | Spherical/577 | 24 h | Lung cancer/A549 | 200 μg/mL | [167] |
| Calotropis procera L. | Latex | Spherical/55 | 24 h | Lung cancer/A549 | No toxicity | [168] |
| Azadirachta indica | Leaf | Spherical//12 | 1 h | Lung cancer/A549 | 26.7, 21.6,μg/mL | [164] |
| Olea europaea | - | Spherical/20–50 | 24 h | Ovarian cancer/SKOV-3 | 2.27 μg/mL | [166] |
| Broccoli | Whole plant | Spherical/∼4.8 | 2 h | prostate cancer/PC-3 | No toxicity | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. https://doi.org/10.3390/cancers13112818
Andleeb A, Andleeb A, Asghar S, Zaman G, Tariq M, Mehmood A, Nadeem M, Hano C, Lorenzo JM, Abbasi BH. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers. 2021; 13(11):2818. https://doi.org/10.3390/cancers13112818
Chicago/Turabian StyleAndleeb, Anisa, Aneeta Andleeb, Salman Asghar, Gouhar Zaman, Muhammad Tariq, Azra Mehmood, Muhammad Nadeem, Christophe Hano, Jose M. Lorenzo, and Bilal Haider Abbasi. 2021. "A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy" Cancers 13, no. 11: 2818. https://doi.org/10.3390/cancers13112818









