Identification of Nucleolin as a Novel AEG-1-Interacting Protein in Breast Cancer via Interactome Profiling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transfection
2.3. Western Blotting
2.4. Antibodies
2.5. Interactome Profiling
2.6. In-Gel Digestion
2.7. Co-Immunoprecipitation (Co-IP)
2.8. Wound-Healing Assay
2.9. Transwell Migration & Invasion Assay
2.10. Colony Formation (Clonogenic) Assay
2.11. Phospho-Tyrosine RTK Array
2.12. Statistical Analysis
3. Results
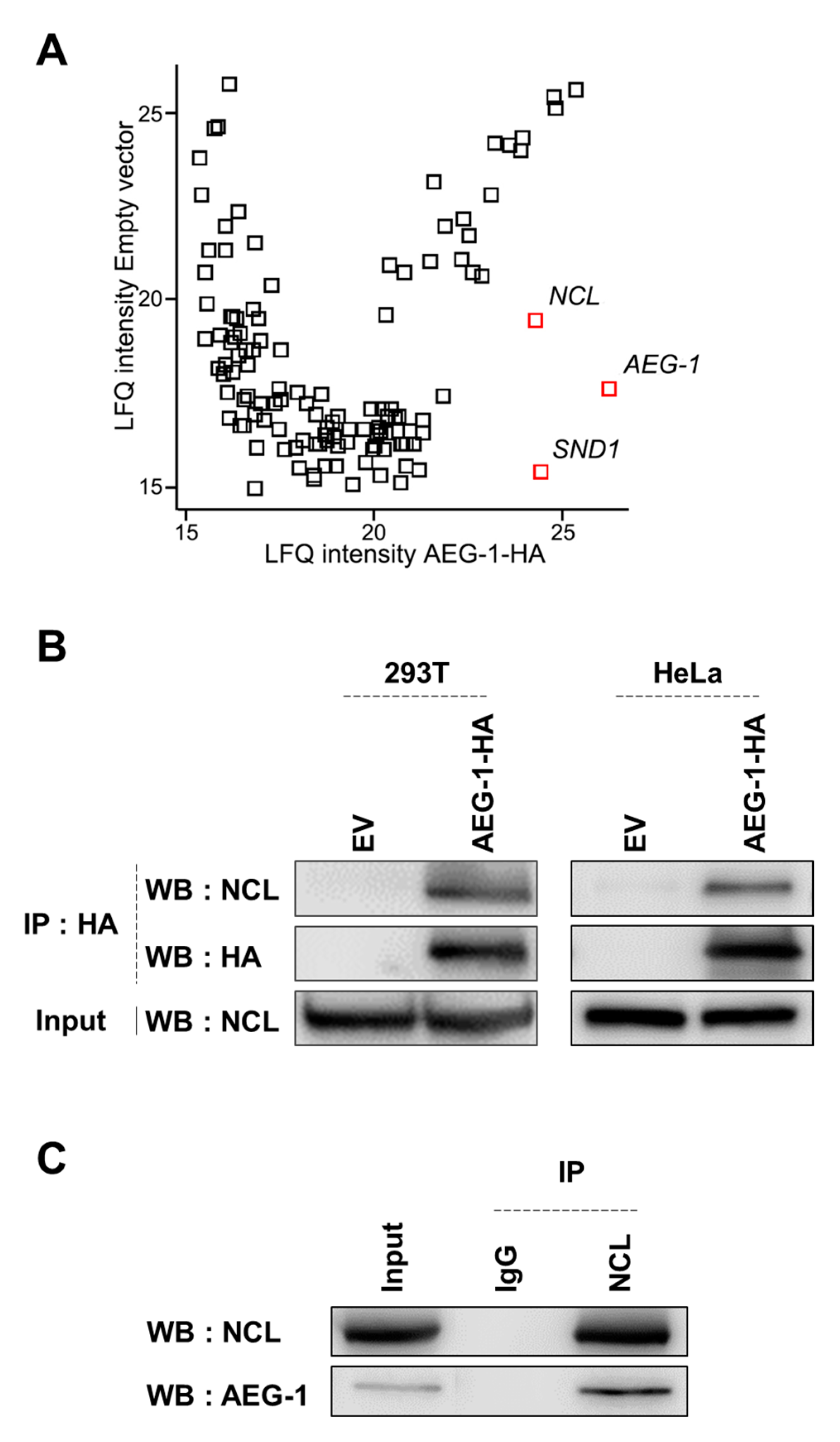
3.1. Identification of NCL as a Novel AEG-1-Interacting Protein
3.2. Silencing NCL Markedly Decreased AEG-1 Induced HeLa Cell Migration
3.3. AEG-1 Is Aberrantly Expressed in Breast Cancer and Is Associated with a Poor Prognosis
3.4. Silencing NCL Reduces AEG-1 Induced Proliferation, Migration, and Invasion in Breast Cancer
3.5. NCL Regulates the Signal of AEG-1 by Reducing the Phosphorylation of C-Met
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical diagnosis and management of breast cancer. J. Nucl. Med. 2016, 57, 9S–16S. [Google Scholar] [CrossRef] [PubMed]
- Cancernet. Available online: https://www.cancer.net/cancer-types/breast-cancer/statistics (accessed on 1 January 2021).
- Kang, D.-C.; Su, Z.-Z.; Sarkar, D.; Emdad, L.; Volsky, D.J.; Fisher, P.B. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 2005, 353, 8–15. [Google Scholar] [CrossRef]
- Brown, D.M.; Ruoslahti, E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 2004, 5, 365–374. [Google Scholar] [CrossRef]
- Sutherland, H.G.; Lam, Y.W.; Briers, S.; Lamond, A.I.; Bickmore, W.A. 3D3/lyric: A novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Exp. Cell Res. 2004, 294, 94–105. [Google Scholar] [CrossRef]
- Su, Z.-Z.; Kang, D.-C.; Chen, Y.; Pekarskaya, O.; Chao, W.; Volsky, D.J.; Fisher, P.B. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene 2002, 21, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.K.; Emdad, L.; Lee, S.-G.; Su, Z.-Z.; Santhekadur, P.; Chen, D.; Gredler, R.; Fisher, P.B.; Sarkar, D. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol. Ther. 2011, 130, 1–8. [Google Scholar] [CrossRef]
- Emdad, L.; Lee, S.G.; Su, Z.Z.; Jeon, H.Y.; Boukerche, H.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21300–21305. [Google Scholar] [CrossRef]
- Lee, S.-G.; Su, Z.-Z.; Emdad, L.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc. Natl. Acad. Sci. USA 2006, 103, 17390–17395. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Su, Z.; Emdad, L.; Sarkar, D.; Franke, T.; Fisher, P. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 2008, 27, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Emdad, L.; Sarkar, D.; Su, Z.-Z.; Randolph, A.; Boukerche, H.; Valerie, K.; Fisher, P.B. Activation of the nuclear factor κB pathway by astrocyte elevated gene-1: Implications for tumor progression and metastasis. Cancer Res. 2006, 66, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guan, H.; Li, Y.; Ying, Z.; Wu, J.; Zhu, X.; Song, L.; Li, J.; Li, M. Astrocyte elevated gene 1 interacts with acetyltransferase p300 and c-Jun to promote tumor aggressiveness. Mol. Cell. Biol. 2017, 37, e00456-16. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.K.; Nolte, H.; Sun, T.; Kaur, H.; Sreenivasan, K.; Looso, M.; Offermanns, S.; Krüger, M.; Swiercz, J.M. Quantitative analysis of the TNF-α-induced phosphoproteome reveals AEG-1/MTDH/LYRIC as an IKKβ substrate. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Hu, B.; Emdad, L.; Bacolod, M.D.; Kegelman, T.P.; Shen, X.-N.; Alzubi, M.A.; Das, S.K.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 interacts with Akt isoform 2 to control glioma growth, survival, and pathogenesis. Cancer Res. 2014, 74, 7321–7332. [Google Scholar] [CrossRef]
- Blanco, M.A.; Alečković, M.; Hua, Y.; Li, T.; Wei, Y.; Xu, Z.; Cristea, I.M.; Kang, Y. Identification of staphylococcal nuclease domain-containing 1 (SND1) as a Metadherin-interacting protein with metastasis-promoting functions. J. Biol. Chem. 2011, 286, 19982–19992. [Google Scholar] [CrossRef]
- Yoo, B.K.; Emdad, L.; Su, Z.-Z.; Villanueva, A.; Chiang, D.Y.; Mukhopadhyay, N.D.; Mills, A.S.; Waxman, S.; Fisher, R.A.; Llovet, J.M. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J. Clin. Investig. 2009, 119, 465–477. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Song, L.-B.; Liao, W.-T.; Jiang, L.-L.; Gong, L.-Y.; Wu, J.; Yuan, J.; Zhang, H.-Z.; Zeng, M.-S. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin. Cancer Res. 2008, 14, 3319–3326. [Google Scholar] [CrossRef]
- Tokunaga, E.; Nakashima, Y.; Yamashita, N.; Hisamatsu, Y.; Okada, S.; Akiyoshi, S.; Aishima, S.; Kitao, H.; Morita, M.; Maehara, Y. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer 2014, 21, 341–349. [Google Scholar] [CrossRef]
- Kornegoor, R.; Moelans, C.B.; Verschuur-Maes, A.H.; Hogenes, M.C.; De Bruin, P.C.; Oudejans, J.J.; Marchionni, L.; Van Diest, P.J. Oncogene amplification in male breast cancer: Analysis by multiplex ligation-dependent probe amplification. Breast Cancer Res. Treat. 2012, 135, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Chong, R.A.; Yang, Q.; Wei, Y.; Blanco, M.A.; Li, F.; Reiss, M.; Au, J.L.-S.; Haffty, B.G.; Kang, Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell 2009, 15, 9–20. [Google Scholar] [CrossRef]
- Choi, K.M.; Cho, E.; Bang, G.; Lee, S.J.; Kim, B.; Kim, J.H.; Park, S.G.; Han, E.H.; Chung, Y.H.; Kim, J.Y.; et al. Activity-Based Protein Profiling Reveals Potential Dasatinib Targets in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 9276. [Google Scholar] [CrossRef]
- Srivastava, M.; Pollard, H.B. Molecular dissection of nucleolin’s role in growth and cell proliferation: New insights. FASEB J. 1999, 13, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Destouches, D.; El Khoury, D.; Hamma-Kourbali, Y.; Krust, B.; Albanese, P.; Katsoris, P.; Guichard, G.; Briand, J.P.; Courty, J.; Hovanessian, A.G. Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS ONE 2008, 3, e2518. [Google Scholar] [CrossRef]
- Teng, Y.; Girvan, A.C.; Casson, L.K.; Pierce, W.M., Jr.; Qian, M.; Thomas, S.D.; Bates, P.J. AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 2007, 67, 10491–10500. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hamhouyia, F.; Thomas, S.D.; Burke, T.J.; Girvan, A.C.; McGregor, W.G.; Trent, J.O.; Miller, D.M.; Bates, P.J. Inhibition of DNA replication and induction of S phase cell cycle arrest by G-rich oligonucleotides. J. Biol. Chem. 2001, 276, 43221–43230. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Yoon, B.-H.; Kim, S.-K.; Kim, S.-Y. GENT2: An updated gene expression database for normal and tumor tissues. BMC Med. Genom. 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Park, E.S.; Emdad, L.; Lee, S.-G.; Su, Z.-Z.; Fisher, P.B. Molecular basis of nuclear factor-κB activation by astrocyte elevated gene-1. Cancer Res. 2008, 68, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tsuge, H.; Imagawa, T.; Kise, D.; Hirano, K.; Beppu, M.; Takahashi, A.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Nucleolin as cell surface receptor for tumor necrosis factor-α inducing protein: A carcinogenic factor of Helicobacter pylori. J. Cancer Res. Clin. Oncol. 2010, 136, 911–921. [Google Scholar] [CrossRef]
- Hovanessian, A.G.; Soundaramourty, C.; El Khoury, D.; Nondier, I.; Svab, J.; Krust, B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS ONE 2010, 5, e15787. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhou, F.; Zhang, Q.; Sun, X.; Shi, X.; Liang, Y.; Wang, X.; Yue, L. Overexpression of nucleolin and different expression sites both related to the prognosis of gastric cancer. Apmis 2013, 121, 919–925. [Google Scholar] [CrossRef]
- Berger, C.M.; Gaume, X.; Bouvet, P. The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Farin, K.; Di Segni, A.; Mor, A.; Pinkas-Kramarski, R. Structure-function analysis of nucleolin and ErbB receptors interactions. PLoS ONE 2009, 4, e6128. [Google Scholar] [CrossRef] [PubMed]
- Di Segni, A.; Farin, K.; Pinkas-Kramarski, R. Identification of nucleolin as new ErbB receptors-interacting protein. PLoS ONE 2008, 3, e2310. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, E.; Solomon, S.; Schmukler, E.; Goldshmit, Y.; Pinkas-Kramarski, R. Nucleolin and ErbB2 inhibition reduces tumorigenicity of ErbB2-positive breast cancer. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, E.; Goldenberg, M.; Solomon, S.; Frishberg, A.; Pinkas-Kramarski, R. Nucleolin-binding by ErbB2 enhances tumorigenicity of ErbB2-positive breast cancer. Oncotarget 2016, 7, 65320. [Google Scholar] [CrossRef]
- Matteucci, E.; Ridolfi, E.; Desiderio, M. Hepatocyte growth factor differently influences Met-E-cadherin phosphorylation and downstream signaling pathway in two models of breast cells. Cell. Mol. Life Sci. CMLS 2006, 63, 2016–2026. [Google Scholar] [CrossRef]
- Hung, C.-M.; Kuo, D.-H.; Chou, C.-H.; Su, Y.-C.; Ho, C.-T.; Way, T.-D. Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells. J. Agric. Food Chem. 2011, 59, 9683–9690. [Google Scholar] [CrossRef]
- Hiscox, S.; Parr, C.; Nakamura, T.; Matsumoto, K.; Mansel, R.E.; Jiang, W.G. Inhibition of HGF/SF-induced breast cancer cell motility and invasion by the HGF/SF variant, NK4. Breast Cancer Res. Treat. 2000, 59, 245–254. [Google Scholar] [CrossRef]
- Lee, S.-G.; Kang, D.-C.; DeSalle, R.; Sarkar, D.; Fisher, P.B. AEG-1/MTDH/LYRIC, the beginning: Initial cloning, structure, expression profile, and regulation of expression. Adv. Cancer Res. 2013, 120, 1–38. [Google Scholar] [PubMed]
- Parada, C.A.; Roeder, R.G. A novel RNA polymerase II-containing complex potentiates Tat-enhanced HIV-1 transcription. EMBO J 1999, 18, 3688–3701. [Google Scholar] [CrossRef] [PubMed]
- Ginisty, H.; Sicard, H.; Roger, B.; Bouvet, P. Structure and functions of nucleolin. J. Cell Sci. 1999, 112, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, A.; Song, F.; Reed, K.R.; Jenkins, R.E.; Meniel, V.S.; Watson, A.J.; Pritchard, D.M.; Clarke, A.R.; Jenkins, J.R. Proteomic profiling of a mouse model of acute intestinal Apc deletion leads to identification of potential novel biomarkers of human colorectal cancer (CRC). Biochem. Biophys. Res. Commun. 2013, 440, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Palmieri, D.; De Luca, L.; Consiglio, J.; You, J.; Rocci, A.; Talabere, T.; Piovan, C.; Lagana, A.; Cascione, L.; et al. In vivo NCL targeting affects breast cancer aggressiveness through miRNA regulation. J. Exp. Med. 2013, 210, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, Y.; Xue, C.; Chen, Y.; Hou, X.; Guo, Y.; Zhao, L.; Hu, Z.; Huang, Y.; Luo, Y.; et al. Prognostic significance of the combined score of endothelial expression of nucleolin and CD31 in surgically resected non-small cell lung cancer. PLoS ONE 2013, 8, e54674. [Google Scholar] [CrossRef]
- Bywater, M.J.; Pearson, R.B.; McArthur, G.A.; Hannan, R.D. Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nat. Rev. Cancer 2013, 13, 299–314. [Google Scholar] [CrossRef]
- Uribe, D.J.; Guo, K.; Shin, Y.J.; Sun, D. Heterogeneous nuclear ribonucleoprotein K and nucleolin as transcriptional activators of the vascular endothelial growth factor promoter through interaction with secondary DNA structures. Biochemistry 2011, 50, 3796–3806. [Google Scholar] [CrossRef]
- Shang, Y.; Kakinuma, S.; Nishimura, M.; Kobayashi, Y.; Nagata, K.; Shimada, Y. Interleukin-9 receptor gene is transcriptionally regulated by nucleolin in T-cell lymphoma cells. Mol. Carcinog. 2012, 51, 619–627. [Google Scholar] [CrossRef]
- Grinstein, E.; Wernet, P.; Snijders, P.J.; Rosl, F.; Weinert, I.; Jia, W.; Kraft, R.; Schewe, C.; Schwabe, M.; Hauptmann, S.; et al. Nucleolin as activator of human papillomavirus type 18 oncogene transcription in cervical cancer. J. Exp. Med. 2002, 196, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Farin, K.; Schokoroy, S.; Haklai, R.; Cohen-Or, I.; Elad-Sfadia, G.; Reyes-Reyes, M.E.; Bates, P.J.; Cox, A.D.; Kloog, Y.; Pinkas-Kramarski, R. Oncogenic synergism between ErbB1, nucleolin, and mutant Ras. Cancer Res. 2011, 71, 2140–2151. [Google Scholar] [CrossRef]
- Lee, H.; Kim, T.H.; Park, D.; Jang, M.; Chung, J.J.; Kim, S.H.; Kim, S.-H.; Lee, K.H.; Jung, Y.; Oh, S.J. Combinatorial Inhibition of Cell Surface Receptors Using Dual Aptamer-Functionalized Nanoconstructs for Cancer Treatment. Pharmaceutics 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Palka, H.L.; Park, M.; Tonks, N.K. Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J. Biol. Chem. 2003, 278, 5728–5735. [Google Scholar] [CrossRef] [PubMed]



| Uniprot ID | Protein Name | Peptide Count |
|---|---|---|
| Q86UE4 | LYRIC | 25 |
| Q7KZF4 | SND1 | 17 |
| P19338 | NCL | 19 |
| Q02878 | RL6 | 5 |
| Q9UQ80 | PA2G4 | 4 |
| P18124 | RL7 | 3 |
| P47914 | RL29 | 2 |
| P07900 | HS90A | 3 |
| Q15233 | NONO | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Choi, K.-M.; Bang, G.; Park, S.-G.; Kim, E.-B.; Choi, J.-W.; Chung, Y.-H.; Kim, J.; Lee, S.-G.; Kim, E.; et al. Identification of Nucleolin as a Novel AEG-1-Interacting Protein in Breast Cancer via Interactome Profiling. Cancers 2021, 13, 2842. https://doi.org/10.3390/cancers13112842
Lee S-J, Choi K-M, Bang G, Park S-G, Kim E-B, Choi J-W, Chung Y-H, Kim J, Lee S-G, Kim E, et al. Identification of Nucleolin as a Novel AEG-1-Interacting Protein in Breast Cancer via Interactome Profiling. Cancers. 2021; 13(11):2842. https://doi.org/10.3390/cancers13112842
Chicago/Turabian StyleLee, Seong-Jae, Kyoung-Min Choi, Geul Bang, Seo-Gyu Park, Eun-Bi Kim, Jin-Woong Choi, Young-Ho Chung, Jinyoung Kim, Seok-Geun Lee, Eunjung Kim, and et al. 2021. "Identification of Nucleolin as a Novel AEG-1-Interacting Protein in Breast Cancer via Interactome Profiling" Cancers 13, no. 11: 2842. https://doi.org/10.3390/cancers13112842
APA StyleLee, S.-J., Choi, K.-M., Bang, G., Park, S.-G., Kim, E.-B., Choi, J.-W., Chung, Y.-H., Kim, J., Lee, S.-G., Kim, E., & Kim, J.-Y. (2021). Identification of Nucleolin as a Novel AEG-1-Interacting Protein in Breast Cancer via Interactome Profiling. Cancers, 13(11), 2842. https://doi.org/10.3390/cancers13112842







