Hyperspectral Imaging (HSI)—A New Tool to Estimate the Perfusion of Upper Abdominal Organs during Pancreatoduodenectomy
Abstract
Simple Summary
Abstract
1. Introduction
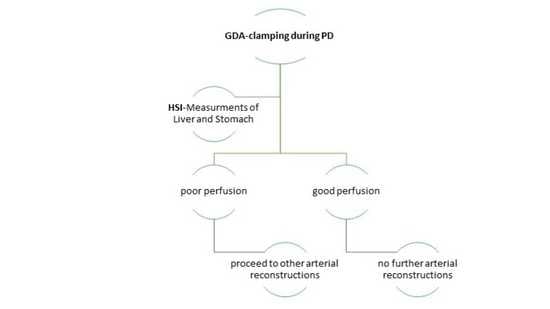
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Hyperspectral Imaging
2.4. Pre- and Intraoperative Assessment
2.5. Follow-Up and Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Laboratory Tests
3.3. Intraoperative HSI-GDA Test
3.4. Patients with CAS
4. Discussion
- i.
- The GDA-clamping test is a routine test performed at the beginning of PD and before GDA division. The weakness of hepatic arterial flow by palpation may reflect the necessity of arterial reconstruction, such as MAL division.
- ii.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSI | hyperspectral imaging |
| StO2 | oxygen saturation |
| NIR-PI | Near-Infrared Perfusion Index |
| PD | pancreatoduodenectomy |
| GDA | gastroduodenal artery |
| CAS | celiac artery stenosis |
| MAL | median arcuate ligament |
| SMA | superior mesenteric artery |
| OHI | organ hemoglobin index |
| ROI | region of interest |
| FOV | field of view |
| EASL | European Association for the Study of the Liver |
| PT | Prothrombin time |
| PPPD | pylorus preserved pancreatoduodenectomy |
| ALT | Alanin Aminotransferase |
| CT | computertompgraphy |
| US | ultrasonography |
References
- Kornblith, P.L.; Boley, S.J.; Whitehouse, B.S. Anatomy of the Splanchnic Circulation. Surg. Clin. N. Am. 1992, 72, 1–30. [Google Scholar] [CrossRef]
- Bron, K.M.; Redman, H.C. Splanchnic artery stenosis and occlusion: Incidence; arteriographic and clinical manifestations. Radiology 1969, 92, 323–328. [Google Scholar] [CrossRef]
- Reuter, S.R.; Olin, T. Stenosis of the Celiac Artery. Radiology 1965, 85, 617–627. [Google Scholar] [CrossRef]
- Bertelli, E.; di Gregorio, F.; Bertelli, L.; Mosca, S. The arterial blood supply of the pancreas: A review. I. The superior pancreaticoduodenal and the anterior superior pancreaticoduodenal arteries. An anatomical and radiological study. Surg. Radiol. Anat. 1995, 17, 97–106. [Google Scholar] [CrossRef]
- Donatini, B. A systematic study of the vascularisation of the pancreas. Surg. Radiol. Anat. 1990, 12, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Berney, T.; Pretre, R.; Chassot, G.; Morel, P. The role of revascularization in celiac occlusion and pancreatoduodenectomy. Am. J. Surg. 1998, 176, 352–356. [Google Scholar] [CrossRef]
- Thompson, N.W.; Eckhauser, F.E.; Talpos, G.; Cho, K.J. Pancreaticoduodenectomy and Celiac Occlusive Disease. Ann. Surg. 1981, 193, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Derrick, J.R.; Pollard, H.S.; Moore, R.M. The Pattern of Arteriosclerotic Narrowing of the Celiac and Superior Mesenteric Arteries. Ann. Surg. 1959, 149, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Sarr, M.G.; Peros, G. Celiac Artery Stenosis: An Underappreciated and Unpleasant Surprise in Patients Undergoing Pancreaticoduodenectomy. J. Am. Coll. Surg. 2008, 206, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, M.; Frank-Moldzio, L.; Klauß, M.; Mayer, P.; Bruckner, T.; Khajeh, E.; Golriz, M.; Mehrabi, A.; Knebel, P.; Diener, M.K.; et al. Intraoperative evaluation of hepatic artery blood flow during pancreatoduodenectomy (HEPARFLOW): Protocol of an exploratory study. Int. J. Surg. Protoc. 2020, 21, 21–26. [Google Scholar] [CrossRef]
- Gaujoux, S.; Sauvanet, A.; Vullierme, M.P.; Cortes, A.; Dokmak, S.; Sibert, A.; Vilgrain, V.; Belghiti, J. Ischemic complications after pancreaticoduodenectomy: Incidence, prevention, and management. Ann. Surg. 2009, 249, 111–117. [Google Scholar] [CrossRef]
- Nara, S.; Sakamoto, Y.; Shimada, K.; Sano, T.; Kosuge, T.; Takahashi, Y.; Onaya, H.; Yamamoto, J. Arterial Reconstruction during Pancreatoduodenectomy in Patients with Celiac Axis Stenosis—Utility of Doppler Ultrasonography. World J. Surg. 2005, 29, 885–889. [Google Scholar] [CrossRef] [PubMed]
- BBalakrishnan, S.; Kapoor, S.; Vijayanath, P.; Singh, H.; Nandhakumar, A.; Venkatesulu, K.; Shanmugam, V. An innovative way of managing coeliac artery stenosis during pancreaticoduodenectomy. Ann. R. Coll. Surg. Engl. 2018, 100, e168–e170. [Google Scholar] [CrossRef] [PubMed]
- Sucher, R.; Athanasios, A.; Köhler, H.; Wagner, T.; Brunotte, M.; Lederer, A.; Gockel, I.; Seehofer, D. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int. J. Surg. Case Rep. 2019, 62, 108–111. [Google Scholar] [CrossRef]
- Köhler, H.; Jansen-Winkeln, B.; Maktabi, M.; Barberio, M.; Takoh, J.; Holfert, N.; Moulla, Y.; Niebisch, S.; Diana, M.; Neumuth, T.; et al. Evaluation of hyperspectral imaging (HSI) for the measurement of ischemic conditioning effects of the gastric conduit during esophagectomy. Surg. Endosc. 2019, 33, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef]
- Michels, N.A. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am. J. Surg. 1966, 112, 337–347. [Google Scholar] [CrossRef]
- Jansen-Winkeln, B.; Germann, I.; Köhler, H.; Mehdorn, M.; Maktabi, M.; Sucher, R.; Barberio, M.; Chalopin, C.; Diana, M.; Moulla, Y.; et al. Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections—a comparative study. Int. J. Color. Dis. 2021, 36, 283–291. [Google Scholar] [CrossRef]
- Holmer, A.; Marotz, J.; Wahl, P.; Dau, M.; Kämmerer, P.W. Hyperspectral imaging in perfusion and wound diagnostics—Methods and algorithms for the determination of tissue parameters. Biomed. Tech. Eng. 2018, 63, 547–556. [Google Scholar] [CrossRef]
- Sugae, T.; Fujii, T.; Kodera, Y.; Kanzaki, A.; Yamamura, K.; Yamada, S.; Sugimoto, H.; Nomoto, S.; Takeda, S.; Nakao, A. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery 2012, 151, 543–549. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Samuel, D.; Simpson, K.J.; Yaron, I.; Bernardi, M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar]
- Punzalan, C.S.; Barry, C.T. Acute Liver Failure: Diagnosis and Management. J. Intensive Care Med. 2016, 31, 642–653. [Google Scholar] [CrossRef]
- Yamamoto, M.; Itamoto, T.; Oshita, A.; Matsugu, Y. Celiac axis stenosis due to median arcuate ligament compression in a patient who underwent pancreatoduodenectomy; intraoperative assessment of hepatic arterial flow using Doppler ultrasonography: A case report. J. Med Case Rep. 2018, 12, 92. [Google Scholar] [CrossRef]
- Lindner, H.H.; Kemprud, E. A Clinicoanatomical Study of the Arcuate Ligament of the Diaphragm. Arch. Surg. 1971, 103, 600–605. [Google Scholar] [CrossRef]
- Miyazaki, K.; Morine, Y.; Saito, Y.; Yamada, S.; Tokuda, K.; Ikemoto, T.; Imura, S.; Shimada, M. Pancreatoduodenectomy co-morbid with celiac axis compression syndrome: A report of three cases. Surg. Case Rep. 2020, 6, 113. [Google Scholar] [CrossRef]
- Kurosaki, I.; Hatakeyama, K.; Nihei, K.-E.; Oyamatsu, M. Celiac axis stenosis in pancreaticoduodenectomy. J. Hepato-Biliary-Pancreat. Surg. 2004, 11, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, J.; Adam, U.; Drognitz, O.; Kröger, J.C.; Makowiec, F.; Schareck, W.; Hopt, U.T. Celiac axis stenosis in pancreatic head resection for chronic pancreatitis. Langenbeck’s Arch. Surg. 2002, 387, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-Y.; Chung, J.W.; Kwon, J.W.; Joh, J.H.; Shin, S.J.; Kim, H.B.; Park, J.H. Collateral Pathways in Patients with Celiac Axis Stenosis: Angiographic–Spiral CT Correlation. Radiographics 2002, 22, 881–893. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Harihara, Y.; Nakatsuka, T.; Kawarasaki, H.; Takayama, T.; Kubota, K.; Kimura, W.; Kita, Y.; Tanaka, H.; Ito, M.; et al. Rescue of liver grafts from hepatic artery occlusion in living-related liver transplantation. BJS 2002, 86, 886–889. [Google Scholar] [CrossRef]
- Crossin, J.D.; Muradali, D.; Wilson, S.R. US of Liver Transplants: Normal and Abnormal. Radiographics 2003, 23, 1093–1114. [Google Scholar] [CrossRef] [PubMed]









| CAS by MAL Compression | Type A | Type B | Type C |
|---|---|---|---|
| Stenosis rate, % | ≤50 | 50–80 | ≥80 |
| Stenosis length, mm | ≤3 | 3–8 | ≥8 |
| Distance from aorta, mm | ≥5 | ≥5 | small |
| Time of GDA Clamping | Selected Measurement |
|---|---|
| t0 = Before GDA Clamping | HSI + Lactate Measurement |
| t1 = directly after GDA clamping | HSI |
| t2 = 15 min after GDA clamping | HSI |
| t3 = 30 min after GDA clamping | HSI + Lactate Measurement |
| Characteristics | Number of Cases (%) |
|---|---|
| Entity of pancreatic lesions | |
| Malignant | 14 (70%) |
| Benign | 6 (30%) |
| Surgical procedure | |
| PPPD | 14 (70%) |
| Whipple’s procedure | 2 (10%) |
| Total pancreatectomy | 4 (20%) |
| pTNM stage (UICC, 8th Edition) | |
| IA | 2 (10%) |
| IB | 3 (15%) |
| IIA | 2 (10%) |
| IIB | 4 (20%) |
| III | 1 (5%) |
| IV * | 2 (10%) |
| No malignancy | 6 (30%) |
| pR-classification | |
| R0 | 19 (95%) |
| R1 (pancreas < 1 mm) | 1 (5%) |
| Co-morbidities | |
| Mild liver disease ** | 3 (15%) |
| Diabetes mellitus type II | 10 (50%) |
| Arterial hypertension | 10 (50%) |
| COPD/Asthma | 2 (10%) |
| Auricular fibrillation | 3 (15%) |
| Coronary heart diseases | 2 (10%) |
| Patient No. | Before GDA Clamping | 30 min after GDA Clamping | ||||||
|---|---|---|---|---|---|---|---|---|
| Nr. | StO2 in % | OHI (0–100) | Lac in mmol/L | StO2 in % | OHI (0–100) | Lac. in mmol/L | Additional Surgical Procedure | |
| Pat. No. 1 Liver Stomach | Type A | 63 89 | 18 33 | 0.6 | 79 83 | 42 58 | 0.7 | none |
| Pat. No. 2 Liver Stomach | Type A | 70 91 | 39 48 | 0.8 | 75 94 | 42 44 | 0.8 | none |
| Pat. No. 3 * Liver Stomach | Type B | 78 98 | 82 35 | 1.1 | 61 92 | 85 79 | 2.3 | dissection of MAL |
| Pat. No. 4 Liver Stomach | Type C | 67 91 | 74 60 | 1.2 | 59 91 | 59 70 | 1.2 | dissection of MAL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulla, Y.; Buchloh, D.C.; Köhler, H.; Rademacher, S.; Denecke, T.; Meyer, H.-J.; Mehdorn, M.; Lange, U.G.; Sucher, R.; Seehofer, D.; et al. Hyperspectral Imaging (HSI)—A New Tool to Estimate the Perfusion of Upper Abdominal Organs during Pancreatoduodenectomy. Cancers 2021, 13, 2846. https://doi.org/10.3390/cancers13112846
Moulla Y, Buchloh DC, Köhler H, Rademacher S, Denecke T, Meyer H-J, Mehdorn M, Lange UG, Sucher R, Seehofer D, et al. Hyperspectral Imaging (HSI)—A New Tool to Estimate the Perfusion of Upper Abdominal Organs during Pancreatoduodenectomy. Cancers. 2021; 13(11):2846. https://doi.org/10.3390/cancers13112846
Chicago/Turabian StyleMoulla, Yusef, Dorina Christin Buchloh, Hannes Köhler, Sebastian Rademacher, Timm Denecke, Hans-Jonas Meyer, Matthias Mehdorn, Undine Gabriele Lange, Robert Sucher, Daniel Seehofer, and et al. 2021. "Hyperspectral Imaging (HSI)—A New Tool to Estimate the Perfusion of Upper Abdominal Organs during Pancreatoduodenectomy" Cancers 13, no. 11: 2846. https://doi.org/10.3390/cancers13112846
APA StyleMoulla, Y., Buchloh, D. C., Köhler, H., Rademacher, S., Denecke, T., Meyer, H.-J., Mehdorn, M., Lange, U. G., Sucher, R., Seehofer, D., Jansen-Winkeln, B., & Gockel, I. (2021). Hyperspectral Imaging (HSI)—A New Tool to Estimate the Perfusion of Upper Abdominal Organs during Pancreatoduodenectomy. Cancers, 13(11), 2846. https://doi.org/10.3390/cancers13112846